Abstract
The growth of Cu2ZnSnS4 thin films was optimized via sulfurization free chemical spray pyrolysis technique. The sulfate based precursors were used to prepare the CZTS films with varying sulfur contents ranging from 0.08 to 0.12 M to maintain the stoichiometric and avoid sulfurization or H2S gas. The formation of secondary phases on the CZTS films was minimized by controlling the sulfur concentrations with the 2- methoxyethanol solvent. The CZTS kesterite structure with preferential orientation along (112) plane was confirmed by X-Ray diffraction at the sulfur content of 0.12 M. From the Raman analysis, we observed that the addition of the higher sulfur concentration to the precursor solution resulted in a pure phase CZTS peak at 336 cm−1 and reduces the secondary phase segregation. Scanning electron microscopy confirms that higher sulfur concentrations facilitate the growth of CZTS films with larger grain size and achieved Cu-poor and Zn-rich compositions for 0.12 M sulfur concentration from energy dispersive spectra. The UV analysis revealed the maximum absorption peak in the visible region with increasing sulfur concentration and the measurement of the Hall effect affirmed p-type conductivity with carrier concentrations ranging from 1014 to 1016 cm−3 as sulfur concentration increased. The charge transport property of the deposited films was characterized by electrochemical impedance spectroscopy where higher sulfur concentrations resulted in lower internal charge transfer resistance, which is the ideal absorber layer in thin film photovoltaic cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The Cu2ZnSnS4 quaternary p-type semiconductor material has acquired distinctive photovoltaic absorber properties such as non-toxic nature, earth-abundant elements, optimal direct band gap in the visible region of 1.4–1.6 eV, and a large absorption coefficient (104–105 cm−1) that allows for a theoretical conversion efficiency of 32% according to the Shockley–Queisser limit [1, 2]. Hence, the researchers are motivated to find suitable deposition methods for obtaining the pure phase CZTS thin films and therefore several techniques are employed to optimize the CZTS absorber layer such as thermal evaporation [3], sputtering [4], chemical bath deposition [5], Sol–gel [6] and spray pyrolysis [7, 8]. Till date, the incorporation of pure sulphide Cu2ZnSnS4 absorbers into thin film solar cells has resulted in a power conversion efficiency of 8.4% using a vacuum-based process [9]. At the laboratory scale, the CZTS device efficiency is still significantly lower than the commercialized CIGS thin film solar cell efficiency of 21.7% [10]. The disparity could be explained by the complexity of the material composition that includes secondary phases, flaws and interfacial recombination all of which pose significant obstacles to enhancing the performance of the device [11]. Therefore, a precise control of the material composition and preparation method is highly required for the achievement of high photovoltaic performance of CZTS based solar cells.
Remarkably, the spray pyrolysis technique does not require any high quality targets and possess the advantage of controlling stoichiometry and easiness to deposit thin films over a large scale area [12]. According to the literature, there are many papers that reported the preparation of CZTS thin films using spray pyrolysis technique and optimized various parameters like concentration [13], doping [14] and solvent [15]. The preparation of precursor solution is one of the key roles for the deposition of CZTS films. Due to the precipitate formation in the preparation and pyrolysis stages of the precursor solution, the deposition becomes inhomogeneous that leads to the formation of secondary phases in the resulting film. On the other hand, the fast evaporation of sulfur is yet another complication in spray deposition. To overcome the problems of secondary phase segregations on CZTS surface and deficit of sulfur elements, the researchers are focusing on the sulfurization process in an inert or vacuum atmosphere. However, the process of sulfurization in CZTS films requires long processing time, expensive routes and also causes deleterious effects on the environment. Due to this consideration, the researchers aim at developing the pure phase CZTS absorber material in a cost-effective manner. A few researchers reported the deposition of CZTS using sulfurization free method and optimized the different molar concentrations of sulfur using aqueous solvents. Diwate et al. reported the influence of sulfur concentration on CZTS thin films prepared through chemical spray pyrolysis method and studied the structural, optical, morphological and electrical properties of the optimized material [16]. Jeganath et al. investigated the near-optimal composition of spray pyrolyzed CZTS thin film by varying copper and thiourea molar concentrations in the precursor solution and found that 0.016 M and 0.12 M had appropriate absorber layer properties with Cu/(Zn + Sn) = 0.81 and Zn/Sn = 1.26, respectively [17]. However, the effect of sulfur concentrations on CZTS formation using spray pyrolysis technique with sulfate precursors are not yet reported. In our previous work, we optimized the CZTS films with 2-methoxyethanol solvent and studied the effect of temperature and solvent variations [18, 19].
As a progressive work towards the economical way, our present focus is to optimize the CZTS thin films via chemical spray pyrolysis technique without using sulfurization by H2S gas or post annealing treatments under high temperatures in sulfur atmosphere. Here, the influence of various sulfur concentrations on CZTS films is optimized using sulfate-based precursor materials. Furthermore, the 2-methoxyethanol solvent is used in place of the aqueous solvent to avoid precipitation, and the effects of sulfur on the growth of CZTS films are investigated in detail.
1.1 Experimental technique
The precursors of Cu2ZnSnS4 solution were prepared with 0.015 M of Copper sulfate pentahydrate (CuSO4.5H2O -98%, Merck), 0.010 M of Zinc sulfate heptahydrate (ZnSO4.7H2O- 98%, Merck), 0.010 M of Tin chloride pentahydrate (SnCl4.5H2O- 98%,Merck) and with different ratios of 0.08, 0.10 and 0.12 M Thiourea (CHN2S-98%, Merck). The 2-methoxyethanol was used as a solvent to dissolve the precursor solution without form of any precipitation and the prepared solution was sprayed on to the preheated glass substrates at the temperature of 350 °C. The solution spray rate was maintained at 6 ml/min and the pressure was fixed as 1.2 kg/cm2 using compressed air as a carrier gas. The samples were denoted as CZTS0.08, CZTS0.10 and CZTS0.12 for the films deposited with different sulfur ratios of 0.08, 0.10 and 0.12 M respectively in the following discussions.
The structural characterization of the deposited films was done by Glancing Incidence X-Ray Diffraction (Rigakui- High Resolution X-Ray Diffractometer) and Raman spectroscopy with the excitation wavelength of 785 nm. The morphological studies of the prepared films were analyzed using Scanning Electron Microscopy (SEM, EVO-18, CAREL ZEISS) and the surface roughness of the deposited films were studied using Atomic Force Microscopy (AFM, Model BT02218, Nanosurf). The elemental composition analysis of the CZTS films was characterized through Energy Dispersive Spectroscopy (EDS). In order to study the optical properties, the thin films were examined by UV–Vis spectrophotometer and Spectroflurometer (Perkin Elmer LS 45) with Xenon lamp source. The electrical conductivity of the deposited CZTS absorber layers was characterized using Hall measurement (Agilent B1500A semiconductor device analyzer). The thickness of the deposited films was calculated using stylus profilometer (Bruker Singapore Pte. Ltd., Singapore, DXT-15–1022). The Electrochemical impedance was measured for deposited CZTS films using FTO as a front electrode and aluminum as a back electrode with the configuration of FTO/CZTS/Al and it was recorded in the frequency range between 105 and 0.1 Hz.
2 Results and discussion
2.1 Structural studies
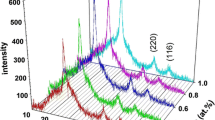
The X-ray diffraction patterns of the CZTS films with different sulfur concentrations are shown in Fig. 1a. As the sulfur concentration increases from 0.08 to 0.12 M in the spraying solution, the deposited films improve the crystallinity with the high intense diffraction peaks at angles (2θ) in 28.4°, 47.5° and 56.2° corresponds to the (112), (220) and (312) planes of the kesterite crystal structure (JCPDS No.26–0575) [20]. This result reveals that all the spray coated films exhibit the impurity-free formation of tetragonal kesterite phase. The average crystallite size (D) and strain (ɛ) on the deposited films are estimated from the breadth of the peak \(\beta^{*}_{hkl}\) and planar spacing \(d^{*}_{hkl}\) related to each reflected plane using the Halder-Wagner method [21, 22]
where \(\beta^{*}_{hkl} = \frac{{\beta_{hkl} \cos \theta }}{\lambda }\) and \(d^{*}_{hkl} = \frac{2\sin \theta }{\lambda }\), λ is the wavelength of the target CuKα1 (= 1.54051), and θ is the position of respective Bragg peaks. The average crystallite size values of the deposited CZTS0.08, CZTS0.10 and CZTS0.12 films are determined from the slope inverse of the linearly fitted data and the micro-strains that have caused in the deposited films are calculated from root of the y intercept as represented in Fig. 1b. As the size of the crystallites in the CZTS films increases, the strain values that are occurred in the films decrease, and the estimated values are summarized in Table 1. Additionally, the dislocation density revealed the quantity of the defects that are developed in the surface of the films and it is calculated by the following formula [23]
Figure 1c. shows that the dislocation density of the deposited films are decreased with the increasing sulfur concentrations in the range between 0.37 × 1012 lines/cm2 and 0.20 × 1012 lines/cm2. Thus, the reduction of dislocation density at higher sulfur concentrations shows good crystallinity.
Due to the similar XRD patterns of tetragonal structured Cu2SnS3 and cubic structured ZnS and existence of other secondary and ternary phases (ZnS, CuS, SnS, Cu2S, SnS2 and Cu2SnS3), the single-phase quaternary compound Cu2ZnSnS4 and the crystal quality can be identified using Raman spectroscopy in Fig. 2. In increased in sulfur concentration, the Raman high intense peaks of the deposited films shift towards the higher wavenumbers between 326 and 336 cm−1. The predominant peak present at 336 cm−1 in CZTS0.12 reveals A1-mode symmetry of CZTS kesterite (I-4) structure which originates from the vibration of S atoms [24]. The following sulfur ratios of 0.10 and 0.08 M exhibit the major peaks at 330 and 326 cm−1 which indicate the disorder of Cu–Zn cations in the kesterite phase. The weak shoulder peak at 374 cm−1 in 0.12 M and the small ascend peaks identified at 366 cm−1 in film with 0.10 M sulfur ratios further confirm the B(LO) and E(LO) vibration modes of CZTS phase. In addition this, there is less intense secondary peaks are existing from CZTS0.08 and CZTS0.10 films in the range between 450 and 475 cm−1 which may be attributed to Cu–S and CuxS phases [25]. Hence there is no emergence of secondary phases with increasing concentration of sulfur atoms in CZTS sample. The minimization of secondary formation in the CZTS film is the main source for the enhancement in device performance.
2.2 Morphological studies
The surface morphology of the CZTS films with different sulfur concentrations is presented in Fig. 3. All the deposited films have uniform density particles without any voids or cracks on the surface and the films exhibit the sphere-like structure with good interconnected grains as shown in the higher magnification (inset) of SEM images. A higher amount of spherical with less agglomerated grains are seen in the films with 0.08 and 0.10 M sulfur concentrations and is associated with Cu-rich (CuxS) secondary phase[17]. Whereas, the significant reduction in the number of spherical grains are observed for the deposited film with 0.12 M sulfur concentration which is the characteristic of the diminution of secondary phases and the results are confirmed in Raman and EDS studies. In addition, the growth of grains became larger and the density of the film surface is also improved as the amount of sulfur atoms increases in the film. The grain size of the films lies in the range between 50 and 400 nm. The larger grains that exhibited in the CZTS with 0.12 M sulfur concentration contribute to the lower recombination centers due to the reduction of grain boundaries. According to the literature, the Cu-poor condition enhances the grain size of the absorber layer in order to control the stoichiometry of the films and to improve the device efficiency [26].
The surface roughness of the deposited CZTS films is characterized using atomic force microscopy. The 2D and 3D AFM is carried out to examine the effect of sulfur concentrations on CZTS and are shown in Fig. 4. The spherical grains are observed for all deposited films in 2D micrographs. The film with 0.08 M sulfur concentration shows more compact films with a dense uniform surface and the flat homogenous column structure is detected in the 3D image Fig. 4a. Whereas, 0.10 M concentration of sulfur exhibits the distinct grains with aggregated particles as depicted in Fig. 4b. Moreover, the larger grains are identified for 0.12 M sulfur concentration of CZTS film which tends to grow by coalescing together as it is shown in Fig. 4c. The average surface roughness of coated CZTS0.08, CZTS0.10 and CZTS0.12 films is 84.1, 91.7 and 92.7 nm respectively. Likewise, the root mean square roughness is found to be 107.1, 121.6 and 133 nm respectively. The CZTS with higher sulfur ratios exhibits more surface roughness with larger grains as compared to the films prepared at lower molar concentrations which will lead to a lower recombination rate and enhances the PV performance [27]. The average spherical grains of all deposited films are in the range between 50 and 300 nm. Thereby the observed AFM results are in good agreement with the SEM analysis.
2.3 Compositional studies
The EDS measurement is used to analyze the elemental composition of kesterite Cu2ZnSnS4 films and the quantitative atomic percentage of elements are indicated in Fig. 5a. As the concentration of sulfur is further added to the spraying solution, it is observed that sulfur atoms in the films are increased from 57 to 61 atomic percentage and the results are represented in Table 2. The increased amount of copper present in CZTS0.08 and CZTS0.10 samples are the reason for copper-related secondary phase which is also confirmed in Raman studies. Likewise, the CZTS0.12 film with a lesser amount of copper suppresses the formation of the CuxS secondary phase and enhances the sulfur-rich properties. The composition CZTS0.12 having Cu/(Zn + Sn) is of ratio 0.59 and with Zn/Sn the ratio is 1.03 which indicates Cu-poor and Zn rich properties is about 0.8 and 1.1 that are preferable for high efficiency PV performance. The estimated values of Cu/(Zn + Sn) and Zn/Sn atomic content ratio is decreased than the expected values and this could be the existence of disorder between Cu and Zn on the cation position in the CZTS crystal structure. However, the Zn/Sn atomic ratio should be more than 1 and Cu/(Zn + Sn) ratio lower than 1 in order to prevent the defect clusters and improve the conductivity of p-type CZTS films [28].
Additionally, the optical microscopic images of the CZTS films with various sulfur concentrations are shown in Fig. 5b. Here, it can observe that changing in color of coating from light brown to dark brown as concentration of sulfur in the films increases from 0.08 to 0.12 M in the CZTS films. Evidently, the loss of sulfur during pyrolysis can be seen from the micrographs for CZTS0.08 and CZTS0.10 films and all the deposited films are uniformly coated without any cracks.
2.4 Optical studies
Figure 6a represents that all coated films exhibit UV absorption peaks in the visible region. As a result, the film optimized from a solution with 0.12 M sulfur concentration has attained maximum absorption as compared with 0.08 M and 0.10 M. The optical bandgap of the prepared films is determined by the Tauc plot as derived from the formula [29],
where α is the absorption coefficient, h is Planck’s constant, υ is the photon frequency, A is a constant, Eg is the band gap energy and n = ½ for direct allowed transitions. Figure 6b indicates the bandgap plots of CZTS0.08, CZTS0.10 and CZTS0.12 that are found to be 1.36, 1.68 and 1.58 eV respectively. According to the theoretical perspective the optimum bandgap values of CZTS are between 1.4 and 1.5 eV for the kesterite structure [30]. The exhibited bandgap of 1.58 eV for CZTS film with 0.12 M sulfur concentration showed a suitable bandgap value for the absorber layer in photovoltaics which revealed the good crystallinity film with the absence of secondary phases. Besides, there is slightly higher bandgap value is observed for the CZTS0.10 sample which is due to the detection of the secondary phase of CuS and the lower bandgap value attained for the CZTS0.08 sample might be the reason for the poor formation of CZTS kesterite structure as stated in Raman analysis. The absorption coefficient α is calculated from the relation [31],
where, d is the thickness of the film and A is the absorption. Figure 6c illustrates that the deposited films acquire an absorption coefficient in the order between 105 and 106 cm−1 with different sulfur concentrations which permits for the efficient absorption of incident photons in the absorber layers. Figure 6d shows the room temperature fluorescence spectra of deposited films and the broad emission peaks detected at around 1.40–1.41 eV corresponding NIR wavelength range of 879–885 nm for CZTS0.08, CZTS0.10 and CZTS0.12 films. The observed peak values are ascribed to the band-to-band transition which is closely related to the bandgap of the CZTS material and the other secondary phases of CuS, SnS2 and SnS and did not inhibit since the bandgap of such materials are above 1.6 and 1.3 eV respectively [32]. More excitingly the deposited CZTS with 0.12 M sulfur concentration has shown a less intense emission peak than CZTS0.08, CZTS0.10 which has lower recombination of electron–hole pairs, resulting in the better performance in solar cells.
2.5 Electrical studies
The Van der Pauw Hall measurement is used to study the deposited CZTS samples with a magnetic field intensity of 0.5 T and the carrier concentration, resistivity and hole mobility values of the deposited films from 0.08 to 0.12 M sulfur concentrations that are listed in Table 3. All the deposited films showed p-type conductivity which is suitable for absorber layer in photovoltaic applications. The CZTS films with p-type conductivity owing to the lower formation energy of acceptor defects than that of donor defects which are created by self-doped intrinsic defects in the material [33]. The carrier concentration and mobility increase with increasing sulfur ratios from 1014 to 1016 cm−3 and 1.64 to 9.28 cm2 V−1 s−1 respectively. The increased hole concentration of CZTS films is caused by the reduction of dislocation density and increase of particle size of the deposited samples. Whereas, the resistivity of the prepared films decreases with increasing sulfur concentrations as shown in Fig. 7 and this can be defined by the increment of carrier concentration as well as the improvement of crystallite size of the absorber layer. The poor mobility values are observed for CZTS with 0.08 and 0.10 M sulfur concentrations. This may be ascribed to the small particle size of the films which leads to the higher recombination between electron–hole pairs [34] and also the lower thickness found in the optimized samples. The deposited samples showed higher resistivity values which may be due to the strain present in the films. In general, the electrical properties strongly depend on the quality of the deposited films. In accordance with the structural and morphological studies, the crystalline quality, larger particle sizes and thickness of the deposited films increased with sulfur concentration. Furthermore, the estimated thickness of the films is 0.8, 1.1 and 1.2 µm for CZTS0.08, CZTS0.10 and CZTS0.12 samples using an optical profilometer. The variation of films thickness for different sulfur concentrations are might be the evaporation of sulfur during the spray method. Moreover, the CZTS film with 0.12 M sulfur concentration showed an optimum thickness of 1.2 µm which provides the greater minority carrier lifetime that will lead to enhanced PV performance.
Electrochemical impedance spectroscopy is a powerful technique for further characterizing the electrical properties and electron transport phenomenon of the materials. Figure 8 indicates the impedance spectra in the complex plane which is generally named as a Nyquist plot representing the real (Z’) and imaginary (Z”) impedance and it is measured for deposited CZTS films with varying sulfur concentration. The deposited films exhibit a semicircle behavior consisting of the sheet resistance (Rs) of electrodes which is intercepted on the X- axis at high frequency region, the charge transfer resistance (Rct) and the constant phase element (CPE) [19]. The obtained plots are well fitted with an equivalent circuit and the corresponding values are listed in Table 4.
The diameter of the semicircle decreases as sulfur concentration increases which denotes a lower charge transport resistance and an efficient electron–hole separation in the CZTS thin films. Furthermore, the sheet resistance (Rs) and the total internal charge transfer resistance which is determined using the equation RT = Rs + Rct of the deposited films are reduced with the higher sulfur concentration. The calculated total resistance of the films is decreased from 75.30 to 24.12 kΩ indicating the lower charge transfer resistance (Rct) and higher capacitance of CZTS0.12 film shows good electronic conduction and efficient exciton separation as a promising absorbing material.
3 Conclusion
The CZTS thin films with different sulfur concentrations were deposited using a versatile chemical spray pyrolysis technique without the influence of any sulfurization method or high vacuum annealing. The effect of sulfate based precursors on the growth of CZTS thin films in 2-methoxyethanol solvent was investigated and the quality of the films was studied through structural, morphological, optical and electrical characterizations. The film deposited at 0.12 M sulfur concentration exhibited a high crystallinity at (112) plane kesterite structure compared to other deposited films. The phase pure CZTS peak at 336 cm−1 and diminished secondary phase formation in CZTS films were examined by Raman spectroscopy with an increase in sulfur composition. The morphological studies revealed that the films became dense and improved the particle size with high surface roughness as the sulfur concentration was increased. Moreover, the higher composition of sulfur in CZTS thin films showed the Cu-poor and Zn-rich properties that support the reduction of secondary phases with a near optimal composition ratio. From the optical analysis, the high optical absorption with low emission suggested that the deposited films with a higher sulfur ratio will act as a good candidate for absorber material. Furthermore, when the sulfur content was increased on the CZTS films, the carrier concentration and mobility values were enhanced. The charge transfer resistance and total internal resistance of the deposited films were reduced as the sulfur content increased. Hence, the obtained results indicate that CZTS films with 0.12 M sulfur concentration to be a promising absorber layer in thin film solar cells.
Data availability
All data investigated through this study are included in this manuscript as figures and tables.
References
H. Katagiri, K. Saitoh, T. Washio, H. Shinohara, T. Kurumadani, S. Miyajima, Development of thin film solar cell based on Cu2ZnSnS4 thin films. Sol. Energy Mater. Sol. Cells 65(1–4), 141–148 (2001). https://doi.org/10.1016/S0927-0248(00)00088-X
W. Shockley, Problems related to p-n junctions in silicon. Solid State Electron 2, 35–67 (1961). https://doi.org/10.1016/0038-1101(61)90054-5
S. Alamri, M. Khushaim, S. Alamri, Preparation and characterization of Cu2ZnSnS4 thin films with various compositions deposited by a dual thermal evaporation technique. J. Alloys Compd. 870, 159392 (2021). https://doi.org/10.1016/j.jallcom.2021.159392
M.A. Olgar, A. Altuntepe, S. Erkan, R. Zan, Fabrication of Cu-rich CZTS thin films by two-stage process: effect of gas flow-rate in sulfurization process. J. Mol. Struct. 1230, 129922 (2021). https://doi.org/10.1016/j.molstruc.2021.129922
Y. Jayasree, Y.B.K. Kumar, G.S. Babu, P.U. Bhaskar, Growth of Cu2ZnSnS4 thin films by hybrid chemical approach. Phys. Condens. Matter. 618, 413199 (2021). https://doi.org/10.1016/j.physb.2021.413199
V.G. Sajeesh, I.P. Selvam, M.S.D. Bharati, G.M. Rao, S.N. Potty, CZTS solar cell with non-toxic buffer layer: a study on sulphurization temperatures and absorber layer thickness. Sol. Energy 207, 419–427 (2020). https://doi.org/10.1016/j.solener.2020.06.103
S. Thiruvenkadam, D. Jovina, A.L. Rajesh, The influence of deposition temperature in the photovoltaic properties of spray deposited CZTS thin films. Sol. Energy 106, 166–170 (2014). https://doi.org/10.1016/j.solener.2014.02.041
P. Prabeesh, V.G. Sajeesh, I.P. Selvam, S.N. Potty, Influence of thiourea in the precursor solution on the structural, optical and electrical properties of CZTS thin films deposited via spray coating technique. J. Mater. Sci. Mater. Electron 32(4), 4146–4156 (2021). https://doi.org/10.1007/s10854-020-05156-y
B. Shin, O. Gunawan, Y. Zhu, N.A. Bojarczuk, S.J. Chey, S. Guha, Thin film solar cell with 8.4% power conversion efficiency using an earth-abundant Cu2ZnSnS4 absorber. Prog. Photovolt 21, 72–76 (2013). https://doi.org/10.1002/pip.1174
Press release dated 22nd September 2014, Accessed on 24th November 2014, (http://www.pv-magazine.com/news/details/beitrag/zsw-sets-217-thin-film-efficiency%20record 100016505/#axzz3IAXN9AUI).
M. He, C. Yan, J. Li, M.P. Suryawanshi, J. Kim, M.A. Green, X. Hao, Kesterite solar cells: insights into current strategies and challenges. Adv. Sci. 8, 2004313 (2021). https://doi.org/10.1002/advs.202004313
D. Perednis, L.J. Gauckler, Thin Film Deposition Using Spray Pyrolysis. J. Electroceram 14, 103–111 (2005). https://doi.org/10.1007/s10832-005-0870-x
F. Ynineb, M. Khammar, S. Guitouni, Copper concentration effect on physical properties of ultrasonically sprayed Cu2ZnSnS4 absorber thin films for solar cell applications. Appl. Phys. 127, 148 (2021). https://doi.org/10.1007/s00339-021-04290-6
Q. Zhao, H. Shen, K. Gao, Y. Xu, X. Wang, Y. Li, Effect of sodium doping on crystal growth and band matching of the heterojunction in flexible CZTS solar cells. J. Mater. Chem. 9, 17531–17541 (2021). https://doi.org/10.1039/D1TC03957A
S.K. Muhamaad, A.H.O. Alkhayatt, J. Phys. Conf. Ser. (2021). https://doi.org/10.1088/1742-6596/1999/1/012059
K. Diwate, K. Mohite, M. Shinde, S. Rondiya, A. Pawbake, A. Date, H. Pathan, S. Jadkar, Synthesis and characterization of chemical spray pyrolysed CZTS thin films for solar cell applications. Energy Procedia 110, 180–187 (2017). https://doi.org/10.1016/j.egypro.2017.03.125
K. Jeganath, Y. Raviprakash, Near-optimal composition of CZTS thin film via exploration of copper and thiourea molar concentration in spray pyrolysis technique. Mater. Res. Expr. 8, 116404 (2021)
G.G. Silvena, B. John, R.A.S. Christinal, M.C.S. Kumar, S. Chakravarty, A.L. Rajesh, Solution processed p-type Cu2ZnSnS4 thin films for absorber layer. J. Inorg Organomet Polym. Mater. 27, 1556–2562 (2017). https://doi.org/10.1007/s10904-017-0616-7
G.G. Silvena, B. John, R.A.S. Christinal, S. Chakravarty, A.L. Rajesh, Solution enhancement for the liable preparation of Cu2ZnSnS4 thin films. J. Mater. Sci. Mater. Electron 29, 6113–6118 (2018). https://doi.org/10.3390/ma7042833
M. Valdés, G. Santoro, M. Vázquez, D. Electroquímica, F. De Ingeniería, C.N. De Mar, J.B. Justo, B.M. Plata, Spray deposition of Cu2ZnSnS4 thin films. J. alloy compd. 585, 776–782 (2014). https://doi.org/10.1016/j.jallcom.2013.10.009
A.R. Stokes, A.J.C. Wilson, The Diffraction of X rays by distorted crystal aggregates-I. IOP conf. series 56(3), 174–181 (1944). https://doi.org/10.1088/0959-5309/56/3/303
A. Gholizadeh, A. Malekzadeh, M. Ghiasi, M, Structural, magnetic and catalytic properties of Co substituted manganite nano-perovskites. Bulg. Chem. Commun. 48, 430–439 (2016)
S. Mahjoubi, N. Bitri, M. Abaab, I. Ly, Effect of copper concentration on the characteristics of Cu2ZnSnS4 (CZTS) thin films. Mattr. Lett. 216, 154–157 (2018). https://doi.org/10.1016/j.matlet.2018.01.004
M. Kumar, A. Dubey, N. Adhikari, S. Venkatesan, Strategic review of secondary phases, defects and defect-complexes in kesterite CZTS-Se solar cells. Energy Environ. Sci. 8, 3134–3159 (2015). https://doi.org/10.1039/C5EE02153G
A. Ali, J. Jacob, K. Mehboob, S. Hussain, A. Ashfaq, K. Mahmood, S. Ikram, N. Amin, U. Rehman, S. Akbar, S.H. Siyal, Correlation of thermoelectric properties of Cu2ZnSnS4 thin films grown by the chemical solution method with sulfur concentration. J. Phys. hem. Solids 148, 109677 (2021). https://doi.org/10.1016/j.jpcs.2020.109677
A. Tumbult, F. Aslan, I.H. Mutlu, F. Aslan, I.H. Mutlu, All solution processed superstrate type Cu2ZnSnS4 (CZTS) thin film solar cell: effect of absorber layer thickness. J. alloy compd. 781, 280–288 (2019). https://doi.org/10.1016/j.jallcom.2018.12.012
T. Özdal, T. Chtouki, H.A. Kavak, V. Figa, D. Guichaoua, H. Erguig, J. Mysliwiec, B. Sahraoui, Effect of annealing temperature on morphology and optoelectronics properties of spin-coated CZTS thin films. J. Inorg. Organomet. Polym. Mater. 31, 89–99 (2021). https://doi.org/10.1007/s10904-020-01646-y
M.Y. Yeh, P.H. Lei, S.H. Lin, C.D. Yang, Copper-zinc-tin-sulfur thin film using spin-coating technology. Materials 9(7), 526 (2016). https://doi.org/10.3390/ma9070526
N.M. Shinde, R.J. Deokate, C.D. Lokhande, Properties of spray deposited Cu2ZnSnS4 (CZTS) thin films. J. Anal. Appl. Pyrolysis 100, 12–16 (2013). https://doi.org/10.1016/j.jaap.2012.10.018
M. Baid, A. Hashmi, B. Jain, A.K. Singh, Md.A.B.H. Susan, M. Aleksandrova, A comprehensive review on Cu2ZnSnS4 (CZTS) thin film for solar cell: forecast issues and future anticipation. Opt. Quant. Electron. 53, 656 (2021). https://doi.org/10.1007/s11082-021-03272-5
S. Thiruvenkadam, S. Prabhakaran, S. Chakravarty, V. Ganesan, V. Sathe, M.C.S. Kumar, A.L. Rajesh, Effect of Zn/Sn molar ratio on the microstructural and optical properties of Cu2Zn1-xSnxS4 thin films prepared by spray pyrolysis technique. Phys. Condens. Matter. 533, 22–27 (2018). https://doi.org/10.1016/j.physb.2017.12.065
M. Grossberg, T. Raadik, J. Raudoja, J. Krustok, Photoluminescence study of defect clusters in Cu2ZnSnS4 polycrystals. Curr. Appl. Phys. (2014). https://doi.org/10.1016/j.cap.2013.12.029
H. Ahmoum, P. Chelvanathan, M.S. Su’ait, M. Boughrara, G. Li, R. Gebauer, K. Sopian, M. Kerouad, N. Amin, Q. Wang, Sol-gel prepared Cu2ZnSnS4 (CZTS) semiconductor thin films: Role of solvent removal processing temperature. Mater. Sci. Semicond. Process 132, 105874 (2021). https://doi.org/10.1016/j.mssp.2021.105874
A. Krishnan, K.R. Ali, G. Vishnu, P. Kannan, Towards phase pure CZTS thin films by SILAR method with augmented Zn adsorption for photovoltaic applications. Mater. Renew. Sustain. Energy 8, 16 (2019). https://doi.org/10.1007/s40243-019-0152-1
Acknowledgements
One of the authors R. Anne Sarah Christinal would like to thank UGC-DAE Consortium for Scientific Research, Kalpakkam Node, Tamilnadu, India for the collaborative research work and for providing the financial support (CSR-KN/CRS-77/2015-16/804).
Funding
Author R. Anne Sarah Christinal and A. Leo Rajesh have received research funding from UGC-DAE Consortium for Scientific Research, Kalpakkam Node, Tamilnadu, India (CSR-KN/CRS-77/2015–16/804). Author Sujay Chakravarty is the project collaborator from UGC-DAE Consortium for Scientific Research, Kalpakkam Node, Tamilnadu. Author G. Genifer Silvena has no financial interests.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. RASC: Material preparation, data collection and analysis were performed. RASC: The first draft of the manuscript was written and all other authors analyzed and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have not disclosed any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Christinal, R.A.S., Silvena, G.G., Chakravarty, S. et al. Sulfurization free spray deposited kesterite Cu2ZnSnS4 absorber layer for photovoltaic applications. J Mater Sci: Mater Electron 33, 22361–22373 (2022). https://doi.org/10.1007/s10854-022-09014-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-022-09014-x