Abstract
The characteristics of large surface area, high conductivity and mechanical flexibility performances make graphene suitable for high power next-generation energy storage devices. However, it only stores charge through double-layer capacitors, which limits its energy storage mechanism, resulting in a limited specific capacitance of graphene. In this paper, black Ti3+ self-doped nano titanium dioxide rods (TiOxNRs) were introduced into graphene nanosheets to improve capacitor performance. The results manifest that when 20 wt% TiOxNRs were added into the TiOxNRs/rGO composite, the capacitance performance is the best and the specific capacitance value is 149.5 F g−1 at the scanning speed of 40 mV s−1. In addition, the specific capacitance of the electrode prepared with the TiOxNRs/rGO composite still maintains the original 89.57% after 2000 charge–discharge cycles and has a good cycle life at a current density of 1 A g−1. In short, the TiOxNRs/rGO composite is a prospect and potential material for supercapacitor electrodes in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Supercapacitors, also known as electrochemical capacitors, are energy storage devices between traditional capacitors and traditional batteries. It is a good auxiliary power source and can be well applied in many power plants due to its advantages, such as long life, low cycle cost, good reversibility, fast charge and discharge, small internal resistance, high cycle efficiency and large output power [1,2,3,4,5].
Graphene is the thinnest material and has extraordinary characteristics due to its unique structure [6,7,8]. The excellent performances include good electrical conductivity, high mechanical strength, good light transmittance, good thermal conductivity and thermal expansion coefficient, which have attracted special attention of researchers [9,10,11,12,13,14]. Therefore, graphene is widely used in fields such as physics, chemistry, biology, supercapacitors and microwave absorption materials [15,16,17]. In particular, the high conductivity and large specific surface area of graphene are appropriate as electrode materials for supercapacitors. However, due to the defects of the energy storage mechanism, the specific capacitance is limited [18,19,20,21].
Titanium oxide (TiO2) is a typical metal oxide. TiO2 has many excellent properties such as high natural abundance, stable electrochemical property, non-toxicity, low cost, good pseudocapacitance performance, high photocatalytic efficiency and good biocompatibility. Thus, it can be widely used in sensors, photocatalysis, dye-sensitized solar cells, biomedicine, lithium-ion batteries and supercapacitors, etc. [22,23,24,25]. Attentions on primary nano-TiO2 structures of researchers are those of nanoparticles, one-dimensional TiO2 and TiO2 thin films. Among them, one-dimensional structure TiO2 has been concerned and extensively studied by researchers attributing to its larger specific surface area, structure particularity and charge transfer efficiency, etc. [26,27,28,29]. As is known, one-dimensional structure of nano-TiO2 mainly includes nanorods, nanotubes, nanobelts, nanowires. He [30] et al. synthesized TiO2NRs with electrospinning technology, and then heated in KOH solution for 1 h to finally obtain KOH-treated TiO2NRs. The results showed that TiO2NRs can be used to prepare flexible supercapacitor electrodes, with a specific capacitance of 65.84 F g−1 at a scan rate of 1 mV s−1. Selvakumar et al. [31] prepared activated carbon nanocomposite electrodes and TiO2 nanoparticles by a microwave method. The results manifested that the specific capacitance of the p/p symmetric supercapacitor is 122 F g−1. Meanwhile, at current densities of 2 mA cm−2, 4 mA cm−2, 6 mA cm−2, and 7 mA cm−2, the specific capacitance of the supercapacitor stabilized at 5000 cycles. Lu [23] et al. reported that anodized TiO2 was calcined in hydrogen to obtain hydrogenated TiO2 (H-TiO2) with a scanning rate of 100 mV s−1. The maximum specific capacity of H-TiO2 prepared at 400 °C was 3.24 mF cm−2, which is 40 times that of an air-annealed TiO2 capacitors under the same conditions. The initial specific capacitance is only 3.1% reduction after 10,000 cycles absolutely ascribed to its good long-term cycle stability.
In this study, in view of the disadvantages of graphene nanosheets that easy to agglomerate in the charging and discharging process of supercapacitors, black Ti3+ self-doped TiO2 nanoparticles were introduced into graphene nanosheets to solve the above problems. So as to achieve the purpose of enhancing the specific surface area and the capacitance performance.
2 Experimental section
2.1 Preparation of TiOx nanoparticles
10 M NaOH solution was slowly poured into the mixture of butyl titanate and isopropanol (volume ratio 1:2), reacted for 0.5 h under magnetic stirring. Then the mixed solution was reacted in a 100 mL polytetrafluoroethylene hydrothermal kettle at 180 °C for 20 h. Natural cooling to room temperature, washed the precipitate with deionized water several times and then washed with 0.1 M HCl solution until the pH value was close to 7. Then, replaced the sodium ions with hydrochloric acid of the above concentration for 10 h. Finally, washed with deionized water until the pH value was close to 7, filtered and dried at 80 °C for 24 h [32].
The above product was thoroughly mixed with NaBH4 with a mass fraction of 1:2 and ground at room temperature for 30 min. Then, the mixture was transferred to a porcelain boat and placed in a tubular furnace for 2 h at a heating rate of 5 °C/min from room temperature to 350 °C in an Air environment. After natural cooling to room temperature, TiOx nanorods were obtained, washed with deionized water and anhydrous ethanol several times, dried at 80 °C for 24 h. In the absence of NaBH4, TiO2 nanorods were synthesized under the same condition for comparison [33,34,35].
2.2 Preparation of TiOx/rGO composite
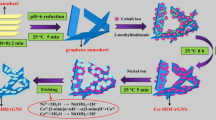
Firstly, graphene oxide (GO) was synthesized by a modified Hummers’ method [36]. A certain amount of prepared GO was dispersed in deionized water to form a 4 mg/mL suspension. Then a certain mass of TiOx was added in proportion with ultrasonic treatment for 30 min. After that, TiOx/GO composite was poured into a three-point flask, and then certain amount of hydrazine hydrate (the ratio of TiOx/GO composite to hydrazine hydrate was 1:10) was added into the above mixture under reflux condensation at 95 °C for 24 h. Finally, the obtained composite was washed with deionized water and absolute ethyl alcohol several times, dried at 80 °C for 24 h. The preparation process of the TiOxNRs/rGO composite is shown in Fig. 1.
2.3 Preparation and electrochemical measurement of working electrode
A three-electrode system was selected as the electrochemical test. The preparation of working electrode was as follows: Polyvinylidene fluoride) (PVDF) powder and acetylene black were chosen as the binder and conductive agent, respectively. The TiOxNRs/rGO composite was mixed with PVDF and acetylene black in a mass ratio of 8:2:1, appropriate amount of NMP solution was added into the above mixture and stirred to make the mixture into a slurry. After that, the slurry was placed in an agate mortar and grind it clockwise to a mushy. The polished paste was coated into the positive and negative electrode areas with an area of 1 cm × 1 cm at one end of a nickel foam, which was pretreated with 3 M HCl with an area of 1 cm × 2 cm, dried at 80 °C for 24 h. Finally, the foam was pressed into a sheet under a pressure of 8 MPa to obtain a working electrode.
2.4 Characterization
FTIR spectrometer (FTIR 650, Shimadzu, Japan) was used to analyze the structure of the samples. The crystal structure of the sample was determined by X-ray diffraction (XRD, Philips X' Pert, Holland) and X-ray Photoelectron Spectroscopy (XPS, Thermo Scientific ESCALAB 250, USA) was applied to analyze the elements on the surface of the composite. The main parameters of rGO were represented by Raman spectrometer (Lab RAM HR, HJY, France). The morphology of the sample was observed by Field Emission Scanning Electron Microscopy (FESEM, FEI Sirion-200, USA). The electrochemistry workstation for laboratory (RST 5000, SHIRUISI Instrument, Zhengzhou, China) can be used to test conduct cyclic voltammetry, charge and discharge and electrochemical impedance in electrode materials. A three-electrode system was selected to test, with 4 M KOH solution as the electrolyte. The saturated calomel electrode was used as the reference electrode, and the platinum wire was used as the counter electrode. The test voltage ranges of CV and GCD is − 0.1 to 0.4 V, the current density of GCD test is 1 A/g, and the frequency of EIS test is 10 MHz ~ 100 kHz and the amplitude is 7 mV. Before testing, the electrode was soaked for 30 min for electrochemical test.
3 Results and discussion
3.1 FTIR analysis
The FTIR spectrum of the TiOxNRs/rGO composite is observed in Fig. 2. The broad peak appears at 3174 cm−1 attributing to the O–H stretching vibration of the hydroxyl group. It is noteworthy that there is a wide peak of the TiOxNRs/rGO composite near 1633 cm−1, which is considered to be the characteristic peak of the in-plane vibration of graphene nanosheets. The peak near 913 cm−1 is mainly due to the bending vibration absorption peak of the C–H bond on the benzene ring. There are two small peaks below 900 cm−1, which are consistent with the tensile vibration peak of the Ti–O–Ti bond.
3.2 XRD analysis
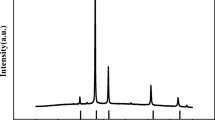
It can be seen from Fig. 3 that rGO has a broad peak at 25.48°, corresponding to the characteristic peak of rGO, which indicates that GO is largely reduced to rGO. As shown in Fig. 3, TiOxNRs and TiOxNRs/rGO composites have basically the same diffraction peaks at 25.3°, 37.8°, 48.1°, 53.9° and 55.1°. These peaks can be judged by the XRD standard pattern (ICSD No. 01-086-1156). In addition, the XRD curve of TiOx contains TiO crystal form (ICSD No. 01-073-9742) and TiO1.25 (ICSD No. 01-078-5811) crystal form, which is a manifestation of TiO2 being reduced. Only the diffraction peak of TiOx exists in the TiOxNRs/rGO composite, and the broad peak of rGO disappears. This is because the diffraction peaks of TiOx nanorods and rGO both appear at about 25°; however, the peak intensity of the TiOx nanorods is so high that the broad weak peak of rGO cannot be observed.
3.3 Raman spectrum analysis
Figure 4 shows the Raman spectra of rGO and TiOx/rGO composite, which contains 20% TiOx nanorods. A series peaks (112 cm−1, 363 cm−1, 492 cm−1, 619 cm−1) less than 1000 cm−1 in the TiOx/rGO composite are Raman characteristic peaks of TiOx. The two peaks appearing at 1349 cm−1 and 1585 cm−1 are D peak reflecting the structural defects of graphene and the G peak indicating the in-plane vibration of the sp2 carbon atom, respectively. The ratio of peak intensity (ID: IG) less than 1 manifests that GO is reduced to rGO throughly [37]. In summary, the TiOx/rGO composite is composed of TiOx nanorods and rGO, the result of which is consistent with the XRD pattern.
3.4 XPS spectra analysis
Figure 5 displays the XPS spectra of the TiOx/rGO composite. In Fig. 5a, the C 1s, Ti 2p and O 1s are observed in the XPS wide scan spectra of the TiOx/rGO composite. There are no other impurity peaks observed in the spectrum proving that the composite is pure. The C 1s spectrum of the TiOx/rGO composite are revealed in Fig. 5b, the binding energy of C–C, C=C and C–H bonds appears at 284.6 eV. The chemical shift to greater binding energy results in the peaks at 285.7 eV, 287.5 eV and 289.2 eV, corresponding to the C–OH bond, C=O bond and O=C–OH bond, respectively. Figure 5c, d manifest the XPS spectrum of the Ti 2p orbital. Figure 5c displays the XPS spectrum of TiO2, there are two strong signal peaks at 458.6 eV and 464.5 eV, corresponding to Ti 2p 3/2 and Ti 2p 1/2 of Ti4+, respectively. There are two main signal peaks of TiOx at 458.6 eV and 463.5 eV can be clearly observed in Fig. 5d. They are derived from Ti 2p 3/2 and Ti 2p 1/2 of Ti4+, respectively. While two weak acromial peaks at 456.4 eV and 461.8 eV correspond to Ti 2p 3/2 and Ti 2p 1/2 of Ti3+, respectively, indicating the existence of Ti3+ in TiOx /rGO composite.
3.5 FESEM analysis
As shown in Fig. 6a, the average diameter and length of TiOx nanorods with smooth surface are about 50 nm and 1.5 μm, respectively. It can be seen in Fig. 6b that the rGO nanosheets are transparent and undulated like waves, indicating that the nanosheets are thin. This is because some folds are still retained after GO was reduced to rGO. In Fig. 6c, d, TiOx nanorods are evenly distributed in rGO nanosheets, and some TiOxNRs and rGO are interwoven together to form a network structure, which can reduce the agglomeration of rGO nanosheets.
3.6 Electrical performance analysis
The charge–discharge curves of TiOx/rGO composite with different mass fractions of TiOx (0 wt%, 10 wt%, 20 wt%, 30 wt% and 40 wt%) at a constant current of 1 A g−1 is displayed in Fig. 7a. The charging curve corresponds to the positive scan curve in the cyclic voltammetry curve while the discharge curve is consistent with the negative scan curve in the cyclic voltammetry curve. The two platforms represented the oxidation peak and reduction peak independently. As shown in Fig. 7a, the charge and discharge potential of TiOx/rGO composite changes non-linearly with time, indicating that the Faraday reaction exists in the charge storage process. In addition, the longest charge–discharge time is the TiOx/rGO composite with 20 wt% TiOx nanorods at the same charge–discharge current density, which makes clear that more charge can be stored in the TiOx/rGO composite with 20 wt% TiOx nanorods comparing to other TiOx content of the composite. It can be seen from Fig. 7b that as the current density increases, the specific capacitance decreases. This is because as the current density increases, the charge–discharge rate increases and the electrode reaction is insufficient. When the current density is 1 A/g and 10 A/g, the specific capacitance of the composite material is 149.5 F/g and 89.2 F/g, respectively, which shows that the composite material has excellent rate performance.
In order to measure the electrochemical properties of the TiOx/rGO composite, CV test is performed according to the electrochemical workstation in the laboratory. The specific capacitance of the TiOx/rGO composite can be calculated according to the CV curves. The calculation formula is as follows:
where Cs represents specific capacitance; I is the current; V displays the scanning rate; M expresses the weight of active substance; ΔV signifies the potential window. According to Eq. (1), at the same scanning rate, the larger the area enclosed by CV curve is, the larger the specific capacitance is.
Figure 8a manifests the cyclic voltammograms of the TiOx/rGO composite at different scan rates, in which there is a pair of obvious Faraday redox peaks, indicating that the TiOx/rGO composite acts as a pseudo capacitance. Under the action of diffusion control and polarization, the Faraday redox peak moves to the direction of a more positive potential and a more negative potential with the increase of scan rate [38, 39]. When the scan rate was increased from 10 to 80 mV s−1, the oxidation peak and reduction peak shifted by 70 mV and 20 mV, respectively. The specific capacitance is 117.3 F g−1, 125.2 F g−1, 149.5 F g−1 and 122.4 F g−1, corresponding to the scan rate at 10 mV s−1, 20 mV s−1, 40 mV s−1 and 80 mV s−1, respectively. Figure 8b lists the comparison of cyclic voltammetry of TiOx/rGO, TiO2/rGO and rGO composites with a scan rate at 40 mV s−1. As can be seen in Fig. 8 that the curve area of the TiOx/rGO composite is larger than that of TiO2/rGO composite and rGO, revealing the capacity of charge storage of the TiOx/rGO composite is greater. The reasons are as follows: First, TiOx/rGO includes both the double-layer capacitance produced by graphene and the pseudo capacitance produced by TiOx The combination of the two materials improves the overall energy storage capacity. Secondly, the presence of TiOx nanorods is beneficial to reduce the agglomeration of graphene and increase the contact area between the material and the electrolyte, thereby improving the performance of the composite. By calculating the specific capacitances of TiOx/rGO, TiO2/rGO and rGO composites are 149.5 F g−1, 118.6 F g−1 and 76.4 F g−1, respectively at the scan rate 40 mV s−1. Thus, the TiOx nanorods reduction by TiO2 nanorods improve the capacitance performance of the TiOx/rGO composite.
Cyclic performance is an important index to characterize the sustainable use of materials. The change of capacity can be investigated by constant current charge–discharge test. Based on the above reasons, the stability of the TiOx/rGO composite was tested at a current density of 1 A g−1. As shown in Fig. 9, the capacitance retention rate still reaches 89.57% after 2 000 cycles, indicating that the TiOx/rGO composite has excellent cycle stability.
Typical impedance behavior of supercapacitors is analyzed by Nyquist graph. Figure 10 describes the dependence of real part Z′ and imaginary part Z″ impedance in the frequency range of 10 MHz ~ 100 kHz. In Fig. 10a, the observed regions are high frequency and medium frequency impedance regions, which basically represent the electrolyte internal resistance characteristics RS and the interface charge transfer resistance RCT of the supercapacitor system. A semicircle fitted from the impedance data of the Nyquist diagrams in the high and medium frequency regions is the result of the parallel interaction between the charge transfer resistance and the double-layer capacitor (CdI) at the electrode–electrolyte interface, as shown in the equivalent circuit in Fig. 10. Therefore, the internal resistance RS (RS = 0.9 Ω) can be calculated by the real part Z′ intercept after the circuit is closed. The IF intercept of the semicircle produces RS + RCT, resulting in RCT (RCT = 3.5 Ω), which is a relatively low resistance value. Besides, the higher the slope of the line in the low and middle frequency region, the smaller the resistance, which reveals the diffusion resistance of electrolyte ions at the electrode gap. The straight slope, in the right low frequency part of the Nyquist curve of the TiOxNRs/rGO composite electrode, is large. The result indicates that the diffusion rate of electrolyte ions in the TiOxNRs/rGO composite electrode is high. Figure 10b and c are comparison diagrams of the EIS diagrams of TiO2/rGO, rGO and TiOxNRs/rGO composites. From this, the impedance semicircle of TiOx/rGO composite is significantly smaller than that of TiO2/rGO and the slope of TiOx/rGO is greater than that of TiO2/rGO in the low frequency area, which shows that the TiOxNRs/rGO composite has better capacitance characteristics. In addition, there is almost no semicircle in rGO in the figure, indicating that the charge transfer resistance is low, impedance is low, and conductivity is good.
4 Conclusion
In summary, the TiOxNRs/rGO composite was prepared with a three-step method. Firstly, TiO2 nanorods were synthesized with a one-step hydrothermal method under alkaline condition. Then, TiO2 nanorods were reduced into TiOx nanorods through solid phase reduction. Finally, TiOxNRs/rGO composite was prepared in liquid phase. rGO nanosheets are relatively thin. The average diameter and length of the TiOx/NRs composite is about 50 nm and 1.5 μm, respectively, which are evenly dispersed among rGO nanosheets. In addition, the mass ratio effect of TiOxNRs and rGO on the capacitance performance of TiOxNRs/rGO composite is studied. The results show that the specific capacitance of TiOxNRs/rGO composite is improved compared with that of TiO2/rGO composite and rGO nanosheets. When the mass ratio the TiOxNRs composite was 20 wt% in the TiOx/rGO composite, the specific capacitance of TiOx/rGO composite reaches the best (149.5 F g−1) at the scan rates of 40 mV s−1. In addition, at the current density of 1 A g−1, the specific capacitance of TiOxNRs/rGO composite remains 89.57% after 2000 cycles. Therefore, as the electrode of supercapacitor, the TiOxNRs/rGO composite exhibits excellent cycling stability and good electrochemical performances. In a word, it is a kind of material with great potential and application prospect in the future.
Data availability
Written informed consent for publication of this paper was obtained from the Zhongyuan University of Technology and all authors.
References
N.L. Wu, Nanocrystalline oxide supercapacitors. Mater. Chem. Phys. 75(1–3), 6–11 (2002)
G. Zhang, T. Wang, X. Yu, H. Zhang, H. Duan, B. Lu, Nanoforest of hierarchical Co3O4@NiCo2O4 nanowire arrays for high-performance supercapacitors. Nano Energy 2(5), 586–594 (2013)
R. Wei, Y. Gu, L. Zou, B. Xi, Q. Xu, Nanoribbon superstructures of graphene nanocages for efficient electrocatalytic hydrogen evolution. Nano Lett. 20(10), 7342–7349 (2020)
W. Hailiang, L. Yongye, D. Hongjie, S.C. Hernan, Ni(OH)2 nanoplates grown on graphene as advanced electrochemical pseudocapacitor materials. J. Am. Chem. Soc. 132(21), 7472–7477 (2010)
L. Wang, B. Wen, X. Bai, C. Liu, H. Yang, NiCo Alloy/carbon nanorods decorated with carbon nanotubes for microwave absorption. ACS Appl. Nano Mater. 2(12), 7827–7838 (2019)
X. Zhang, X. Xu, Y. Hu, G. Xu, W. He, J. Zhu, C60/Na4FeO3/Li3V2(PO4)3/soft carbon quaternary hybrid superstructure for high-performance battery-supercapacitor hybrid devices. NPG Asia Mater. 12(1), 8 (2020)
X. Yi, W. He, X. Zhang, Y. Yue, G. Yang, Z. Wang, M. Zhou, L. Wang, Graphene-like carbon sheet/Fe3O4 nanocomposites derived from soda papermaking black liquor for high performance lithium ion batteries. Electrochim. Acta 232, 550–560 (2017)
X. Yi, Y. Zhang, W. He, X. Zhang, G. Yang, Z. Wang, Y. Wang, Q. Cheng, Low-temperature synthesis of graphene/SiC nanocomposite anodes with super-long cycling stability. ChemElectroChem 4(6), 1320–1326 (2017)
Y. Wang, X. Gao, X. Wu, W. Zhang, Q. Wang, C. Luo, Hierarchical ZnFe2O4@RGO@CuS composite: Strong absorption and wide-frequency absorption properties. Ceram. Int. 44(8), 9816–9822 (2018)
L. Liu, L. Wang, Q. Li, X. Yu, X. Shi, J. Ding, W. You, L. Yang, Y. Zhang, R. Che, High-performance microwave absorption of MOF-derived core-shell Co@N-doped carbon anchored on reduced graphene oxide. ChemNanoMat 5(4), 558–565 (2019)
I. Abdalla, A. Elhassan, J. Yu, Z. Li, B. Ding, A hybrid comprised of porous carbon nanofibers and rGO for efficient electromagnetic wave absorption. Carbon 157, 703–713 (2020)
S. Wang, Y. Xu, R. Fu, H. Zhu, Y. Zhao, Rational construction of hierarchically porous Fe–Co/N-doped carbon/rGO composites for broadband microwave absorption. Nano-Micro Lett. 11(4), 1–16 (2019)
B. Hu, C. Guo, C. Xu, Y. Cen, J. Hu, Y. Li, S. Yang, Y. Liu, D. Yu, C. Chen, Rational construction of V2O5@rGO with enhanced pseudocapacitive storage for high-performance flexible energy storage device. ChemElectroChem 6(23), 5845–5855 (2019)
Y. Wang, X. Gao, W. Zhang, C. Luo, L. Zhang, P. Xue, Synthesis of hierarchical CuS/RGO/PANI/Fe3O4 quaternary composite and enhanced microwave absorption performance. J. Alloy. Compd. 757, 372–381 (2018)
H. Zhang, C. Lu, C. Chen, L. Xie, P. Zhou, Q. Kong, 2D layered α-Fe2O3/rGO flexible electrode prepared through colloidal electrostatic self-assembly. ChemElectroChem 4(8), 1990–1996 (2017)
Q. Zeng, X. Xiong, P. Chen, Q. Yu, Q. Wang, R. Wang, H. Chu, Air@rGO€Fe3O4 microspheres with spongy shells: self-assembly and microwave absorption performance. J. Mater. Chem. C 4(44), 10518–10528 (2016)
J.S. Sanchez, A. Pendashteh, J. Palma, M. Anderson, R. Marcilla, Anchored Fe3O4 nanoparticles on rGO nanosheets as high-power negative electrodes for aqueous batteries. ChemElectroChem 4(6), 1295–1305 (2017)
L. Liu, J. Lang, P. Zhang, B. Hu, X. Yan, Facile synthesis of Fe2O3 nano-dots@nitrogen-doped graphene for supercapacitor electrode with ultralong cycle life in KOH electrolyte. ACS Appl. Mater. Interfaces 8(14), 9335–9344 (2016)
M.D. Stoller, S. Park, Y. Zhu, J. An, R.S. Ruoff, Graphene-based ultracapacitors. Nano Lett. 8(10), 3498–3502 (2008)
Z. Xu, Z. Zhang, H. Yin, S. Hou, H. Lin, J. Zhou, S. Zhuo, Investigation on the role of different conductive polymers in supercapacitors based on a zinc sulfide/reduced graphene oxide/conductive polymer ternary composite electrode. RSC Adv. 10(6), 3122–3129 (2020)
Y. Li, M.V. Zijll, S. Chiang, P. Ning, KOH modified graphene nanosheets for supercapacitor electrodes. J. Power Sources 196(14), 6003–6006 (2011)
D. Wang, X. Li, J. Chen, X. Tao, Enhanced photoelectrocatalytic activity of reduced graphene oxide/TiO2 composite films for dye degradation. Chem. Eng. J. 198, 547–554 (2012)
X. Lu, G. Wang, T. Zhai, M. Yu, Y. Li, Hydrogenated TiO2 nanotube arrays for supercapacitors. Nano Lett. 12(3), 1690–1696 (2012)
M.M. Abutalib, A. Rajeh, Influence of MWCNTs/Li-doped TiO2 nanoparticles on the structural, thermal, electrical and mechanical properties of poly (ethylene oxide)/poly (methylmethacrylate) composite. J. Organomet. Chem. 918, 121309 (2020)
M. Kazazi, Z.A. Zafar, M. Delshad, J. Cervenka, C. Chen, TiO2/CNT nanocomposite as an improved anode material for aqueous rechargeable aluminum batteries. Solid State Ion. 320, 64–69 (2018)
S. Muthusamy, J. Charles, Metal–organic framework of nanostructured polypyrrole incorporated with TiO2 nanoparticles for supercapacitor electrode. J. Mater. Sci. 32(6), 7349–7365 (2021)
L.Y. Zhu, Zemin, Z. Zhou, M. Zhu, Preparation of TiO2 nanowires-reduced graphene oxide composites by one-step hydrothermal method for supercapacitor. Mod. Chem. Indust. 39, 146–150 (2019).
J. Macak, M. Zlamal, J. Krysa, P. Schmuki, Self-organized TiO2 nanotube layers as highly efficient photocatalysts. Small 3(2), 300–304 (2010)
J.M. Macak, S. Aldabergerova, A. Ghicov, P. Schmuki, Smooth anodic TiO2 nanotubes: annealing and structure. Phys. Status Solidi 203(10), R67–R69 (2010)
X. He, C.P. Yang, G.L. Zhang, D.W. Shi, Q.A. Huang, H.B. Xiao, Y. Liu, R. Xiong, Supercapacitor of TiO2 nanofibers by electrospinning and KOH treatment. Mater. Des. 106, 74–80 (2016)
M. Selvakumar, D.K. Bhat, Microwave synthesized nanostructured TiO2-activated carbon composite electrodes for supercapacitor. Appl. Surf. Sci. 263, 236–241 (2012)
J. Ding, L. Cheng, X. Zhang, Q. Liu, Synthesis of multilayered micro flower NiCo2O4/GN/Fe3O4 composite for enhanced electromagnetic microwave (EM) absorption performance. J. Mater. Sci. 30(9), 8864–8875 (2019)
J. Liu, S. Shao, B. Meng, G. Fang, Z. Xie, L. Wang, X. Li, Enhancement of inverted polymer solar cells with solution-processed ZnO-TiOX composite as cathode buffer layer. Appl. Phys. Lett. 100(21), 213906 (2012)
X. Bao, L. Sun, W. Shen, C. Yang, W. Chen, R. Yang, Facile preparation of TiOX film as an interface material for efficient inverted polymer solar cells. J. Mater. Chem. 2(6), 1732–1737 (2014)
M. Nakamura, S. Kato, T. Aoki, L. Sirghi, Y. Hatanaka, Formation mechanism for TiOx thin film obtained by remote plasma enhanced chemical vapor deposition in H2–O2 mixture gas plasma. Thin Solid Films 401(1), 138–144 (2001)
L. Cheng, Q. Wang, J. Ding, One-step hydrothermal synthesis of the FeNi3/rGO composite for electrochemical supercapacitor. J Mater Sci: Mater Electron 32(6), 7226–7236 (2021)
H. Yue, E. Guan, X. Gao, F. Yao, Q. Wang, T. Zhang, Z. Wang, S. Song, H. Zhang, One-step hydrothermal synthesis of TiO2 nanowires-reduced graphene oxide nanocomposite for supercapacitor. Ionics 25, 1–8 (2018)
L. Mei, T. Yang, C. Xu, M. Zhang, L. Chen, Q. Li, T. Wang, Hierarchical mushroom-like CoNi2S4 arrays as a novel electrode material for supercapacitors. Nano Energy 3, 36–45 (2014)
X.F. Lu, D.J. Wu, R.Z. Li, Q. Li, S.H. Ye, Y.X. Tong, G.R. Li, Hierarchical NiCo2O4 nanosheets@hollow microrod arrays for high-performance asymmetric supercapacitors. J. Mater. Chem. A 2(13), 4706 (2014)
Funding
The authors acknowledge the financial support from the Henan Province Science and Technology Project (Grant No. 212102210584), Cultivation Project of “Young Key Teachers in Universities of Henan Province, Funded by Young Key Teachers of Zhongyuan Institute of Technology” in 2019 (Jiaogao [2019] No. 350, Zhong Gongong [2018] No. 60).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by JD and LC. The first draft of the manuscript was written by JD and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict and competing of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ding, J., Cheng, L. Preparation of Ti3+ self-doped TiOxNRs/rGO composite: application in supercapacitors. J Mater Sci: Mater Electron 32, 19947–19957 (2021). https://doi.org/10.1007/s10854-021-06519-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06519-9