Abstract
In this paper, the effect of La3+ doping on the structure, charge ordering and magnetic properties of Sm0.5Ca0.5MnO3 manganite has been studied. A series of LaxSm0.5-xCa0.5MnO3 (x = 0, 0.25, 0.375, 0.5) samples are prepared by traditional solid state reaction. The crystal structures are studied by X-ray diffraction technology. The results show that they are single-phase and orthorhombic structures. The change of La3+ ion concentration does not change the crystal structure of the samples. The surface morphology and particle size of the samples are studied by scanning electron microscope. The average particle size of the samples is several micrometers. Energy-dispersive spectrometer is used to detect the composition of the samples. The results show that the actual element composition of the samples is consistent with that of the modulated element. Magnetic properties are analyzed by magnetization versus temperature (M–T) curves and magnetic hysteresis (M-H) loops. Below 50 K, the zero-field-cooling (ZFC) and field-cooling (FC) M–T curves diverge obviously, which is a typical feature of spin glass transition. The ZFC and FC M–T curves show a significant protuberance peak between 215 and 274 K, which represents the existence of charge-ordered (CO) anti-ferromagnetic (AFM) state. With increasing La3+ ion doping concentration, the CO temperature gradually decreases. Moreover, the butterfly-type M-H loops also confirm the existence of CO-AFM phase in samples. In a few words, the competition between FM and AFM phases determines the charge ordering behavior and magnetic properties of the samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Perovskite manganese oxides RE1-xAExMnO3 (RE are trivalent rare-earth elements; AE are bivalent alkaline-earth elements) have colossal magnetoresistance (CMR) effect, which is a hot topic in the field of magnetic materials. In doped manganese oxides, there are strong and complex interactions among charge, orbit, spin, and lattice [1,2,3]. In 1950, Jonker and Van Santen have studied the magnetism and conductivity of doped LaMnO3, and made some breakthrough progress [4]. After the 1990s, de Gennes has promoted the development of this field [5]. In perovskite manganites, ferromagnetic (FM) phase can be produced by double exchange of manganese ions, and antiferromagnetic (AFM) phase can be produced by super-exchange. The competition between AFM and FM phases can lead to disorderly freezing of some spins in CMR materials at low temperature, which is called spin glass (SG) state [6]. In 2002, SG transition is observed by suryanarayanan et al. in Sm0.85Ca0.15MnO3 manganite [7]. The doped perovskite Manganese oxides have a broad developmental prospect, especially in the field of magnetic recording. The RMnO3 with 50% of trivalent rare-earth ions of R3+ replaced by divalent alkaline-earth metal ions of A2+, R0.5A0.5MnO3 (R = La, Pr, Nd, etc.; A=Sr, Ca, Ba, etc.) are the interesting class of materials which exhibit real space ordering of 1:1 Mn3+/Mn4+ species accompanying a lattice structural change [8]. Meanwhile, charge ordering appears in R0.5A0.5MnO3, and at near charge-ordering temperature (TCO), these materials show anomalies in resistivity, magnetization, and lattice parameters as a function of temperature, magnetic field, and isotope mass [9]. These manganites have been extensively studied and found to show interesting electronic and magnetic properties, such as metal-to-insulator transition due to the double-exchange interaction, charge-ordering transitions due to the long-range Coulomb interaction among the carriers, and AFM transition due to the super-exchange interaction, depending on the combination of rare-earth ions (R3+) and alkaline-earth ions (A2+) [10]. The doping concentration is one of the key factors affecting their physical properties [11, 12]. It is generally believed that when the doping concentration of SmxCa1-xMnO3 reaches x = 0.5, there will be charge ordering, which corresponds to the optimal doping concentration for charge localization [13]. La1-xCaxMnO3 (x = 0.5) shows charge-ordered (CO) insulation behavior, and La0.5Ca0.5MnO3 is in the boundary of two different states (FM and AFM regions) [14]. Because both Sm0.5Ca0.5MnO3 and La0.5Ca0.5MnO3 exhibit charge ordering at different temperatures in the previous research [15, 16] and the electronic structure of Sm3+ is the similar to that of La3+, La3+ is selected to partially replace Sm3+ in SmxCa1-xMnO3 (x = 0.5) in this work. The single-phase polycrystalline LaxSm0.5-xCa0.5MnO3 (x = 0, 0.25, 0.375, 0.5) samples are prepared by high-temperature solid state reaction. The effects of La3+ doping on the charge ordering and magnetic properties of Sm0.5Ca0.5MnO3 are investigated.
2 Experimental details
Polycrystalline LaxSm0.5-xCa0.5MnO3 (x = 0, 0.25, 0.375, 0.5) manganites were synthesized by high-temperature solid state reaction. Stoichiometric raw powders (with 99.99% purity) of Sm2O3, CaCO3, MnO2, and La2O3 for the LaxSm0.5-xCa0.5MnO3 (x = 0, 0.25, 0.375, 0.5) were intimately mixed and thoroughly ground and then were heated at 1100 °C for 24 h. The obtained powders were then pressed into the disk-shaped pellets. These pellets were sintered at 1200 °C for 24 h. Finally, these pellets were slowly cooled down to room temperature. The crystal structure of the samples was measured by X-ray diffraction (XRD) with the X-ray wavelength of 1.5405 Å. The surface structure of the samples was observed by scanning electron microscope (SEM). The composition of the samples is detected by energy-dispersive spectrometer (EDS). The magnetization versus temperature (M–T) curves and magnetic hysteresis (M-H) loops of the samples were measured by physical properties measurement systems (PPMS) with vibrating sample magnetometer (VSM). In the process of magnetic measurement, the mass of the samples was kept as 1 g.
3 Results and discussion
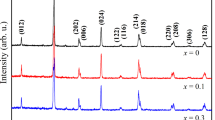
Figure 1a shows the XRD spectra of LaxSm0.5-xCa0.5MnO3 (x = 0, 0.25, 0.375, 0.5) manganites. The samples are single phase without phase impurity (no extra impurity peak). The XRD spectra can be indexed in an orthorhombic structure with Pbnm space group. By observing the diffraction patterns shown in Fig. 1b, it can be found that with the increase of doping concentration x, the position of (121) diffraction peaks (the corresponding 2θ angle value) shifts to the left, from 33.365° to 33.225°, 33.149°, and 33.090°, i.e., the diffraction peak gradually moves to the small angle direction, indicating that the average lattice constant increases. The lattice parameters of LaxSm0.5-xCa0.5MnO3 (x = 0, 0.25, 0.375, 0.5) samples can be obtained by the Retiveld fitting calculation, as listed in Table 1. With increasing La3+ concentration, lattice volume (V) increases, which is related to the relatively large ionic radius of La3+ ion (compared with Sm3+ ion).
Figure 2 shows the SEM images of LaxSm0.5-xCa0.5MnO3 (x = 0, 0.25, 0.375, 0.5) samples. The average particle size of the four samples is of several micrometers. In the sample Sm0.5Ca0.5MnO3 without La3+ ion doping, as shown in Fig. 2a, it can be found that the crystal particles are relatively small (about 3.5 μm), densely distributed, agglomerated, with irregular grain orientation, and more rough surface with average roughness of about 156.7 nm. In Fig. 2b and c, for the LaxSm0.5-xCa0.5MnO3 (x = 0.25 and 0.375), the particle size of two samples is about 4 μm and the surface roughness is 126.5 nm for La0.25Sm0.25Ca0.5MnO3 and 88.4 nm for La0.375Sm0.125Ca0.5MnO3. In Fig. 2d, for the LaxSm0.5-xCa0.5MnO3 (x = 0.5), the particle size is about 4.5 μm and the surface roughness is 86.5 nm for La0.5Ca0.5MnO3. With the increase of La3+ doping concentration, as shown in Fig. 2b–d, the particle size and smoothness of the sample increase, and the overall arrangement begins to become orderly, and the proportion of irregular-shaped particles decreases. When La3+ doping concentration reaches x = 0.25 (La3+/Sm3+ ratio reaches 1:1), as shown in Fig. 2b, the crystal distribution is the most dispersed.
The EDS spectra of LaxSm0.5-xCa0.5MnO3 (x = 0, 0.25, 0.375, 0.5) samples have been measured and the components of four samples are analyzed in Table 2: For LaxSm0.5-xCa0.5MnO3 (x = 0), the nominally Sm:Mn = 1:2 and the actually Sm:Mn = 10.59:23.99; For LaxSm0.5-xCa0.5MnO3 (x = 0.25), the nominally La:Mn = 1:4 and the actually La:Mn = 4.14: 18.73; For LaxSm0.5-xCa0.5MnO3 (x = 0.375), the nominally La:Sm = 3:1 and the actually La:Sm = 7.00: 2.36; For LaxSm0.5-xCa0.5MnO3 (x = 0.5), the nominally La:Mn = 1:2, the actually La:Mn = 8.03:18.93. Therefore, the difference of the actual and nominal values of element percentage is small and can be ignored, confirming the high quality of samples prepared by solid state reaction. The investigation of X-ray photoelectron spectroscopy (XPS) spectra is helpful for the understanding of element valence [17]. In LaxSm0.5-xCa0.5MnO3 samples, the valence and element percentage can be obtained by the XRD and EDS. Therefore, XPS spectra are not provided.
Figure 3a shows the zero-field-cooling (ZFC) and field-cooling (FC) M–T curves of LaxSm0.5-xCa0.5MnO3 (x = 0) samples under an external magnetic field of 500 Oe. Figure 3b, c shows the M-H loops at different temperatures of LaxSm0.5-xCa0.5MnO3 (x = 0) samples. It has been reported by Wang et al. that Sm0.5Ca0.5MnO3 has CO-AFM phase transition at CO temperatures (TCO) of 270 K and SG state occurs in Sm0.5Ca0.5MnO3 at low temperature [15]. As seen from Fig. 3a, there is a significant protuberance peak at 274 K, which represents the existence of CO state in LaxSm0.5-xCa0.5MnO3 (x = 0). With the appearance of CO state, CE-type AFM phase appears [15, 16, 18, 19]. Below TCO, the AFM characteristic appears. With the decrease of temperature (T < 50 K), the ZFC and FC M–T curves bifurcate, which is caused by magnetic inhomogeneous clusters in the samples [20, 21]. The obvious bifurcation of FC and ZFC M–T curves is also a typical feature of spin glass transition [6, 7, 15]. When the M-H curve inclines to a straight line, this phenomenon indicates that the magnetic order is destroyed. In Fig. 3b, c, the magnetic order is destroyed within 100 K, which is consistent with the M–T curves in Fig. 3a.
Figures 4, 5, and 6 show the ZFC and FC M–T curves under 500 Oe field and the M-H loops at different temperatures of LaxSm0.5-xCa0.5MnO3 (x = 0.25, 0.375, 0.5) samples, respectively. It can be seen from Figs. 3a, 4a, 5a, and 6a that with the gradual increase of doping concentration x, the TCO is 274 K, 230 K, 220 K, and 215 K, respectively. TCO decreases with the increase of La3+ doping concentration. This demonstrates that La3+ doping can suppress the CO-AFM ordering. In Fig. 3b, c, for LaxSm0.5-xCa0.5MnO3 (x = 0), the magnetic order is destroyed within 100 K due to a straight line in the M-H curve. In Fig. 4b, c, for LaxSm0.5-xCa0.5MnO3 (x = 0.25), the magnetic order is destroyed between 100 and 150 K. In Fig. 5b, c, for LaxSm0.5-xCa0.5MnO3 (x = 0.375), the magnetic order is destroyed between 150 and 200 K. In Fig. 6b, c, for LaxSm0.5-xCa0.5MnO3 (x = 0.5), the magnetic order is destroyed around 250 K. Moreover, the butterfly-type magnetic hysteresis (M-H) loops can be found in all samples, which also indicates the existence of charge ordering in samples [14]. With decreasing temperature from room temperature, CO phase appears at TCO and then the samples are in a state of multi-phase coexistence including CO-AFM and FM phases, which can be observed in M–T curves and M-H loops in Figs. 3, 4, 5, 6. La3+ doping can destroy the long-range AFM ordering, leading to the coexistence of FM and AFM states. The competition between FM and AFM phases is the internal reason for the change of charge ordering and magnetic properties.
4 Conclusion
Polycrystalline LaxSm0.5-xCa0.5MnO3 (x = 0, 0.25, 0.375, 0.5) samples are synthesized by solid state reaction method. These samples belong to single-phase orthorhombic structure. With La3+ doping, the CO temperature of LaxSm0.5-xCa0.5MnO3 samples decreases gradually and the long-range AFM ordering of samples is destroyed. The AFM and FM phases coexist in the samples, and the competition between them is the internal reason for the change of charge ordering and magnetic properties.
References
A.J. Millis, P.B. Littlewood, B.I. Shraiman, Phys. Rev. Lett. 74, 5144 (1995)
E. Dagotto, Science 309, 257 (2005)
H. Wang, W. Tan, AIP Adv. 10, 095113 (2020)
G.H. Jonker, J.H. Van Santen, Physica 16, 337 (1950)
P.-G. de Gennes, Phys. Rev. 118, 141 (1960)
J.M. De Teresa, M.R. Ibarra, J. García, J. Blasco, C. Ritter, P.A. Algarabel, C. Marquina, A. del Moral, Phys. Rev. Lett. 76, 3392 (1996)
R. Suryanarayanan, A. Vasil Év, T. Voloshok, J. Magnet. Magnet. Mater. 242–245, 695 (2002)
A. Srivastava, R. Thakur, N.K. Gaur, AIP Conf Proc. 1536, 913–914 (2013)
J. Lo´pez, O.F. de Lima, P.N. Lisboa-Filho, F.M. Araujo-Moreira, Phys. Rev. B. 66, 214402 (2002)
T. Terai, T. Sasaki, T. Kakeshita, T. Fukuda, T. Saburi et al., Phys. Rev. B 61, 3488–93 (2000)
Liu, Z.Z. Qiu, D.Z. Hou, J. Barker, K. Yamamoto, O. Gomonay, E. Sai Toh, Nat. Mater. 17, 577 (2018)
R.W. Li, Z.H. Wang, X. Chen, B.G. Shen, Acta. Phys. Sin. 48, S105 (1999). ((in Chinese))
H. Wang, H. Zhang, Su. Kunpeng, S. Huang, W. Tan, D. Huo, J. Mater. Sci.: Mater. Electron. 31, 14421 (2020)
H.O. Wang, K.P. Su, S. Huang, J. Ge, W.S. Tan, D.X. Huo, J. Superconduct. Novel Magnet. 32, 3887 (2019)
S.P. Wang, J.C. Zhang, G.X. Cao, J. Yu, C. Jing, S.X. Cao, Chin. Phys. Soc. 55, 370 (2006). ((in Chinese))
P.G. Radaelli, D.E. Cox, M. Marezio, S.W. Cheong, Phys. Rev. B 55, 3015 (1997)
Z. Ma, Hu. Ming Yue, Z.Y. Liu, K. Wei, H. Guan, H. Lin, M. Shen, S. An, Wu. Qiong, S. Sun, J. Am. Chem. Soc. 142, 8440–8446 (2020)
A.P. Ramirez, P. Schiffer, S.W. Cheong, C.H. Chen, W. Bao, T.T.M. Palstra, B. Zegarski, P.L. Gammel, D.J. Bishop, Phys. Rev. Lett. 76, 3188 (1996)
Y. Tomioka, T. Okuda, Y. Okimoto, A. Asamitsu, H. Kuwahara, Y. Tokura, J. Alloy. Compd. 326, 27 (2001)
J.A. De Toro, Phys. Rev. B 60, 12918 (1999)
H. Maniya, I. Nakatani, T. Furnbayashi, Phys. Rev. Lett. 80, 177 (1998)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (11604067, U1832143, 51702289, 51601049, 11704091). The authors would like to thank colleagues from Beijing Synchrotron Radiation Facility (BSRF) and Shanghai Synchrotron Radiation Facility (SSRF) for their help in XRD experiments.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, H., Wang, H. et al. Charge ordering and magnetic properties of LaxSm0.5-xCa0.5MnO3 manganite. J Mater Sci: Mater Electron 32, 18721–18727 (2021). https://doi.org/10.1007/s10854-021-06391-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06391-7