Abstract
Repercussion of Swift Heavy Ion (SHI) irradiation on nickel-based nanorods of Metal-Organic Framework (NRs-Ni3HHTP2 MOF) for enhancement in the properties of ChemFET-based gas sensor has been investigated. Nanorods of Ni3HHTP2-MOF were synthesized by chemical method and exposed to C12+ ions irradiation with fluence 1 × 1011 ion/cm2 and 1 × 1012 ion/cm2. The structural, spectroscopic, morphological, and optical characterizations were carried out using x-ray diffraction (XRD), fourier transfer infrared spectroscopy (FTIR), atomic force microscopy (AFM) with scanning electron microscopy (SEM), and UV-visible spectroscopy, respectively, whereas the bandgap was calculated from the Tauc’s plot. The synthesized nanorods of Ni3HHTP2 MOF were drop-casted on gold-coated microelectrodes on silicon/silicon dioxide (Si/SiO2) substrate, where silicon layer serves as a gate and gold microelectrodes on silicon/silicon dioxide (Si/SiO2) substrate as a source and drain. The transmutations in material properties due to SHI irradiations were serviceable for enhancing field-effect transistor (transfer and output) properties and sensing properties. After Swift Heavy Ion (SHI) irradiation (1 × 1011 ion/cm2), it shows excellent response and recovery time i.e., 20 and 23 s, respectively, for 1 ppm SO2 concentration at room temp (RT) with a lower detection limit of 0.625 ppm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In the last few years, the Metal-organic framework (MOF) is one of the focusing materials in the research world due to its tunable properties. MOFs are ultra-high porous, large surface area, highly crystalline, and high-stability material and importantly it can be tunable by altering central metal or organic ligands [1,2,3,4]. MOFs are helpful in various applications like gas storage, sensor, chemical separations, biomedical imaging, catalysis, and drug delivery as precursors for cooking graphite and metal oxides materials [1, 2, 5, 6].
The most critical problem in front of modern society is a continuous increment in pollutions in terms of air, water, sound etc [7,8,9]. Among these air pollution absorbed through the respiratory system. Above permissible exposure limit (PEL), hazardous gases are immediately life-threatening [10]. Sulfur dioxide is one of the responsible gases for increasing cardiorespiratory mortality and morbidity in human beings as well as the creation of corrosion in nonliving things [10, 11]. Therefore, researchers have been focusing on enhancing sensing properties of detectors for detection of various gases including SO2 [12,13,14,15]. Since last few years, MOF has been one of the mostly explored materials for detection of various gases including SO2. M. Tchalala [16] et al. reported fluorinated metal-organic frameworks (MOFs) used for the selective removal and sensing of SO2 analytes. Therefore, screening of new materials and their modification for enhancing sensing properties are continuous process in the research area.
Since the last few decenniums, sundry materials have been modified extensively by high energy particles (electron, proton) of heavy ions [17,18,19,20,21]. The irradiation of energetic ion beams engenders several types of defects in materials like chain scission, ionization, or excitation and ion track formation etc. The Swift Heavy Ion (SHI) irradiation is one of the promising implements for material modifications and workable for enhancing electrical properties [22,23,24,25]. Zhang et al. [23] studied the performance of SHI-irradiated MoSe2 material. The electrical changes were observed by using TMDC-channel field-effect transistors (FETs). Zeng et al. [26] explored the effects of electrical properties in graphene devices by exposing it to energetic ion beam irradiation where graphene was irradiated by 1.79 GeV Ta ions. It was observed that SHI-irradiated graphene at lower fluence exhibited optimized field-effect transistors performance, whereas at higher fluence, devices were significantly depreciated of electrical properties after the irradiation process. Also, Manikanthababu et al. [27] reported electrical characterization of vertical Schottky barrier diodes (SBDs) based on Ag ion-irradiated Ni/β-Ga2O3 materials with 120 MeV.
To date, researchers have explored SHI irradiation for modification of properties of various materials like Conducting Polymers (CPs), Single-Walled Carbon Nanotubes (SWNTs), Graphene (Gr), Metal Oxides (MOs) etc. by using various SHI and fluence rate [28,29,30,31]. Researchers have also explored SHI irradiation on various MOF materials. R. Dutta et al. [32] have reported SHI irradiation on NiBTC MOF using 100 MeV O7+, which exhibited enhancement in electrochemical sensing properties. Recently, P. Sayyad et al. [33] studied the effect of Au ion with 100 MeV at fluence 1 × 1011 ion/cm2 and 1 × 1012 ion/cm2 irradiation on FeBTC MOF. They observed drastic changes for higher ion fluence rate. Moreover, decrease in crystallite size, increase of energy bandgap, decrease in average surface roughness, and new functional group (C–H) were observed after SHI irradiation at a higher fluence 1 × 1012 ion/cm2.
Recently, we have explored nickel-based NRs-Ni3HHTP2 MOF for detection of sulfur dioxide (SO2) using ChemFET modality [34]. However, to the best of our knowledge, the influence of SHI irradiation for enhancing properties of materials for ChemFET sensing has not been explored so far. In the present work, SHI irradiation has been explored to enhance the ChemFET sensing properties of NRs-Ni3HHTP2 MOF using C12+ ion with fluence rate 1 × 1011 and 1 × 1012 ion/cm2 irradiations. The carbon ions exhibited a distinguishable energy distribution in depth, which is known as the “Bragg Peak”. Therefore, the majority of energy is released in the target [35], which will be very much useful to create defects in sensing materials. The influence of irradiation on the NRs-Ni3HHTP2 MOF was investigated by using structural analysis, surface morphological, electrical, and optical properties.
2 Experimental details
2.1 Fabrication of microelectrode
The device platform was prepared by using a typical photolithography process as reported earlier [36]. Highly boron (B)-doped silicon (Si) substrate having p-type nature with thickness 525 μm performed as a back gate terminal in FET measurement. A 100-nm-thick SiO2 layer was deposited on the Si substrate by low-pressure chemical vapor deposition. It is followed by deposition of Cr layer (20 nm) and Au layer (120 nm) by e-beam evaporator and standard lift-off technique. The width of Au microelectrodes was 200 μm and the gap between two microelectrodes was 3 μm. Later micropatterned substrates were immersed in piranha solution (70% concentration H2SO4/30%H2O2) followed by rinsing and drying under N2 flow before use.
2.2 Synthesis of NRs-Ni3HHTP2 MOF
Nickel (II) acetate (tetrahydrate) (99.99%, purchased from Sigma Aldrich) was used without further purification along with 2,3,6,7,10,11-Hexahydroxytripenylne Hydrate (HHTP) (98%, purchased from TCI) followed by earlier reported [34]. The chemical method followed for synthesis of NRs-Ni3HHTP2 MOF is as follows: 2.63 mmol nickel (II) acetate (tetrahydrate) was continuously stirred with 4ml deionized water using 5ml beaker. Further addition of 1.31 mmol HHTP in continuously stirred chemical with constant heating at 80 °C for 8 h was provided. After 8 h, solution become thicker than initial solution.
The chemically synthesized NRs-Ni3HHTP2 solution was drop-casted to create channel in between two gold microelectrodes which was dried in room atmospheric conditions. The gold microelectrodes work as a source and drain, whereas drop-casted material create channel between them and Si substrate performed as a back gate terminal in FET measurement. For experimental test, 6 sensor devices were prepared.
2.3 SHI irradiation
The SHI irradiation was carried out by using material science beamline, 15UD Pelletron tandem accelerators at the Inter-University Accelerator Center, New Delhi, India. The scanning area of ion irradiation was 1 × 1cm2 of devices riding on a ladder which was placed in the irradiated vacuum chamber under 10− 6 mbar pressure. The targeted material was irradiated with C12+ ion with 50 MeV at 1pnA for fluence 1 × 1011 ion/cm2 (16 s.) and 1 × 1012 ion/cm2(160 s.).
The values of electronic stopping, nuclear stopping, and range of ions in NRs-Ni3HHTP2 were calculated by using the SRIM simulator program. The calculated values were 1647 eV/Å, 10.84 eV/Å, and 10.25 μm, respectively.
2.4 Material characterizations
The X-ray diffraction (XRD) was carried out using Bruker D8 Advance having potential difference 40 kV and current 40 kA with source CuKα (wavelength 1.5406Å). The FTIR spectrum was recorded using Bruker Alpha ATR. For surface morphology, Scanning Electron Microscopy (SEM) and Atomic Force Microscopy (AFM) were carried out by Tescan MIRA 3 LMH and Park XE-7 instruments, respectively, and UV-Vis spectroscopy was done by using Jasco V-750. FET measurements were carried out using Keithley 4200 A semiconductor parameter analyzer (SPA).
Sensing measurements were performed using indigenously developed dynamic gas sensing setup which was attached with corrosive and non-corrosive mass flow controllers (MFCs) and data performance was recorded using Keithley 4200 A. Tedlar bags were used to get the desired concentration of gas analyte.
3 Results and discussion
3.1 Structural characterization
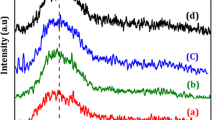
The structural analysis of pristine and SHI-irradiated NRs-Ni3HHTP2 MOF was carried out using X-ray diffraction (XRD) shown in Fig. 1a. In pristine NRs-Ni3HHTP2, (Fig. 1) (black) exhibits 2θ peaks at 4.5°, 9.4°, and 29° which exhibited good agreement in earlier reported data [37,38,39] which is consistent to (100), (020), and (311) Miller indices, respectively. The percentage of crystallinity for pristine NRs-Ni3HHTP2 is 48%. After C12+ ion irradiation, due to high energy, 2θ angle peak intensity decreases with increasing fluence rate at smaller angle (5° and 9.4°), whereas at larger angle (29°) peak intensity increases [37]. The resultant distinct 2θ peaks were observed after irradiation materials at 28°. The percentage of crystallinity after C12+ irradiation for fluence rate 1 × 1011 (Fig. 1a (red)) and 1 × 1012 ion/cm2 (Fig. 1, blue) was 23 and 20%, respectively. This result confirms the crystal structure collapse with increase in amorphous phase after SHI irradiation.
Besides giving crystalline structure information, the peaks of the diffraction pattern provide valuable information in other aspects of the material. The crystalline size of pristine and SHI-irradiated NRs-Ni3HHTP2 was calculated by using the Debye–Scherrer’s formula in equation (I) at 2θ angle 4.5150.
whereas D is crystallite size, λ is the wavelength of X-ray source radiation i.e., CuKα wavelength is 1.5406 Å, β is full width at half maxima (FWHM) calculated from Gauss fitting, and θ is the Braggs angle of diffraction. Also, the dislocation density () (equation II), distortion parameter (g) (equation III), and inter-chain separation (R) (equation IV) are shown in Table 1. The crystallite size of pristine NRs-Ni3HHTP2 was 170.1 Å increased with 180.4 Å and 197.1 Å with fluence rate 1 × 1011 and 1 × 1012 ion/cm2, respectively. Also, it is interesting to see that the micro-strain increases with increasing fluence rate.
3.2 Spectroscopy analysis
Fourier Transform Infrared spectroscopy (FTIR) spectrums were recorded for pristine and irradiated NRs-Ni3HHTP2 MOF materials in ZnSe window having range 4000−500 cm− 1 shown in the Fig. 1b. The bands 700–900 cm− 1 show continuous stretching containing CH3-metal group due to CH2 rocking vibration present in NRs-Ni3HHTP2 MOF. C=C stretching vibration bonds represent in between 1500 and 1580 cm− 1, whereas O-H stretching vibration was present in the 3200–3700 cm− 1 range. In the case of irradiated NRs-Ni3HHTP2 MOF materials, some of the bands become narrow and intensity decreases, these change are attributed to the scissoring and crosslinking of material by ion beam irradiation [40]. At higher fluence (1 × 1012 ion/cm2 (blue)), defects were created more than lower fluence (1 × 1011 ion/cm2 (red)). This fact might be responsible for the amorphous nature of NRs-Ni3HHTP2 MOF materials after irradiation. Therefore, at higher fluence (1 × 1012 ion/cm2 (blue)), low intensity represented lower absorption of light.
3.3 Morphological studies
Scanning electron microscopy (SEM) high-magnification images were recorded before and after C+ 12 ion with fluence rate of 1 × 1011 and 1 × 1012 ion/cm2 irradiation on NRs-Ni3HHTP2 MOF shown in Fig. 2a, b, and c, respectively. This confirms the presence of nanorods in synthesized Ni3HHTP2 MOF [34]. The average size of nanorods (pristine Ni3HHTP2, 38 nm) decreases with fluence rate i.e., C12+ with fluence rate 1 × 1011 and 1 × 1012 ion/cm2, 35nm, and 28nm, respectively. This was done due to the high energetic ion passed through materials which loses electronic energy and creates defects [23]. An increased influence rate shows more clusters which are attributed to scissoring and crosslinking of material and is in good agreement with XRD and FTIR results.
Atomic force microscopy (AFM) was carried out (shown in Fig. 2d, e and f) for determination of roughness (shown in Fig. 2g, h and i) of pristine and SHI-irradiated C12+ with fluence rate 1 × 1011 and 1 × 1012 ion/cm2 on NRs-Ni3HHTP2 MOF, respectively. The roughness was calculated by XEI image-processing software. The roughness of pristine NRs-Ni3HHTP2 MOF was 32.518nm and roughness after C12+ ion-irradiated sample was 20.053 nm and 19.475 nm with fluence rate 1 × 1011 and 1 × 1012 ion/cm2, respectively. The decrease in surface roughness is due to discontinuous tracks and the creation of defects after irradiation of high energetic ion. Higher fluence creates more clusters in material which reduce the surface roughness as compared to lower fluence.
3.4 Optical studies
The optical absorbance spectra of pristine (black) and C12+-irradiated with fluence rate 1 × 1011 ion/cm2 (red) and 1 × 1012 ion/cm2 (blue) on NRs-Ni3HHTP2 MOF shown in Fig. 3. It was observed that the pristine NR-Ni3HHTP2 and irradiated materials absorbance peaks are in the 400–550 nm visible wavelength range. The intensity reduction was observed in irradiated materials. The bands at 450 − 500 nm have been frequently associated with defect absorption [41]. This was due to the creation of free radicals and ions formed by irradiation on NRs-Ni3HHTP2 materials. It has affected the bandgap of materials. Moreover, there is another fundamental gap present i.e., HOMO-LOMO gap. Due to defect creation minimum energy was formed in separated, uncorrelated free electron and hole, and associated with the transport of single particles in the solid, corresponding to the onset of optical absorption and formation of abound electron-hole pair or exciton. The bandgap of pristine NRs-Ni3HHTP2 was 3.54 eV; after C+ 12 ion irradiation band gap decreases. It was 3.41 eV and 3.44 eV for fluence rate of 1 × 1011 ion/cm2 and 1 × 1012 ion/cm2, respectively, shown in Fig. 3b-1, b-2 and b-3. This was due to the creation of pronounced coalescence phenomena in higher fluence (1 × 1012 ion/cm2)-irradiated material [42]. It shows complementary results with structural and morphological characteristics. Due to high energy, a little bit more damage was created in the material.
The bandgap was calculated by using Tauc’s equation I.
where Eg is an energy bandgap of the material, α is the coefficient of absorbance calculated from equation (II), A is absorbance, and d is the thickness of deposited material.
3.5 Field effect transistor (FET) measurements
The FET measurements were carried out by measuring the output and transfer characteristics of pristine and irradiated MOFs. Output characteristics were performed by modulating drain to source voltage (Vds) in the window 0 to 10 V at gate voltage (Vgs) 1 to 4 V for NRs-Ni3HHTP2 MOF shown in Fig. 4a, whereas for C+ 12 ion-irradiated MOF materials output characteristics were performed by keeping same Vds window at constant gate voltage (Vgs) 1 V as shown in Fig. 4b. Moreover, transfer characteristics (as shown in Fig. 4c) were measured by modulating gate to source voltage in window − 30 to 30 Vgs at Vds = 0.5 V. Excellent ON/OFF behavior of FET was observed. The significant changes in output and transfer characteristics in pristine and SHI-irradiated device were observed. Significant enhancement in drain current (Ids) was observed in SHI-irradiated MOF materials. This is due to SHI-induced defects and the creation of free radicals and ions in irradiated MOF. The SHI enhances charge transportation in irradiated MOF materials. Moreover, at higher fluence, saturation level was observed. Irradiation of higher energy creates more clusters in material which reduces the surface roughness as compared to lower fluence.
4 ChemFET sensing
The NRs-Ni3HHTP2 and C+ 12 ion irradiated with fluence 1 × 1011 ion/cm2 and 1 × 1012 ion/cm2 devices were tested in ChemFET modality for detection of SO2 gas analytes from 1 to 0.625 ppm levels by keeping Vgs= 1 V and Vds = 0.5 V constant as shown in Fig. 4d. The SHI irradiation was used as a tool for maximizing the surface area of the sensor. It was viable approach for increasing the efficacy of sensing. This means that surfaces with higher porosity offer a greater number of sites for the adsorption of toxic gas molecules, increasing the number of reactions with sensor molecules. Accordingly, the porosity of the sensor surface has a direct relation with the sensor performance, sensor sensitivity, and sensor response. Reversible ChemFET sensing was observed for SO2 gas analytes. It was showing remarkable dynamic sensing response against SO2 analytes. The significant improvement in the sensing performance was observed in case of SHI-irradiated MOF. The enhancement in sensing performance may be attributed to the increased active sites for SO2 adsorption on MOF after SHI irradiation. The statistical approach for calculating regression coefficient for SO2 analytes was adopted by using linear regression equations and normalized current responses are plotted against gas concentrations for pristine and SHI-irradiated MOF as shown in Fig. 5a. For NRs-Ni3HHTP2 MOF linear regression equation was y = 0.0018x -1.009 with R square 0.785. Moreover, for SHI-irradiated MOF ( C+ 12 ion-irradiated MOF with fluence 1 × 1011 ion/cm2 and 1 × 1012 ion/cm2 ) linear regression equation was y = 0.0018x − 0.675 with R square 0.931 and y = 0.0014x – 0.588 with R square 0.9142, respectively. The C+ 12 ion-irradiated MOF with fluence 1 × 1011 ion/cm2 shows excellent (0.931) regression coefficient. On that line, repeatability was tested for 1 ppm using C+ 12 ion-irradiated MOF with fluence 1 × 1011 ion/cm2 as shown in Fig. 5-b and it shows excellent repeatability.
The standard error bar is one of the crucial points in sensor properties. The calibration plot is shown in Fig. 5-c. It exhibited lower deviation at higher concentration of SO2 analytes, whereas higher deviations at lower concentrations. The cross-selectivity performance of SHI-irradiated MOF for various gas analytes i.e., SO2, NO2, NH3, CO, and CH4 were also investigated. It shows good selectivity towards SO2 analytes as shown in Fig. 5-d. The SHI-irradiated MOF (C+ 12 ion irradiated with fluence 1 × 1011 ion/cm2 sensor ) exhibits improved selectivity due to increased stacking coefficient for SO2 adsorption in sensing material. The long-term stability was also investigated continuously for 60 days on pristine as well as irradiated sensors as shown in supporting information (SI) figure S1-1. The SHI-irradiated MOF exhibited degradation after 25 days. It might be because of creation of more number of defects due to SHI irradiation. The low-energy SHI-irradiated MOF showed better results than higher-energy SHI-irradiated MOF in terms of long-term stability. The most important parameters in the sensor are response and recovery time, lower detection limit, and operating temperature. Table 2 shows comparison of abovementioned sensing parameters of earlier reported work and present work. It can be concluded that present work shows better results as compared to earlier reported work. It shows excellent response and recovery time i.e., 20 and 23 s for 1 ppm SO2 concentration at room temp (RT) with a lower detection limit of 0.625 ppm.
4.1 Sensing mechanism
The sensing mechanism is the key factor to understand the sensing behavior of materials. To understand the sensing mechanism, we have calculated bandgap using Tauc’s plot as shown in Fig. 3-b (1,2 and 3). It shows decrease in banggap after SHI irradiation. After SHI irradiation, defects, free radicals, and ions were created on sites of materials. These defects act as an electron trapper with adsorption of the oxygen species from gas analytes. Moreover, these defects are responsible for adsorbing oxygen species and creating oxygen ions thereby preventing electron-hole recombination rate [46]. This was responsible for decreasing drain current after exposing to gas analyte as shown in Fig. 6.
5 Conclusions
The NRs-Ni3HHTP2 MOFs were successfully synthesized by the chemical method. The SHI irradiation C12+ with fluence 1 × 1011 ion/cm2 and 1 × 1012 ion/cm2 have induced changes in structural, spectroscopic, morphological, optical, and FET properties of NRs-Ni3HHTP2 MOFs. The NRs-Ni3HHTP2 MOF was amorphized after SHI irradiation which was confirmed by XRD. The XRD pattern exhibits the creation of defects in irradiated materials. The size of NRs-Ni3HHTP2 MOF decreases with decreasing the surface roughness and forms a cluster in irradiated materials which was confirmed from surface morphology by SEM and AFM. The decrease in surface roughness was attributed to discontinuous tracks, which lead to amorphization. The drain current of NRs-Ni3HHTP2 MOFs-based FET was enhanced due to the trapping of free mobile carriers after the SHI irradiations. The SHI-induced surface defects in NRs-Ni3HHTP2 MOFs were responsible for enhancing sensing properties. It shows excellent response and recovery time i.e., 20 and 23 s for 1 ppm SO2 concentration at room temp (RT) with a lower detection limit of 0.625 ppm. Therefore, it can be concluded that SHI-irradiated NRs-Ni3HHTP2 MOFs showed improved material properties which were responsible for enhancing sensing properties.
References
G.A. Bodkhe et al., Field effect transistor based on proton conductive metal organic framework (CuBTC). J. Phys. D: Appl. Phys. 52(33), 335105 (2019)
D. Wang, D. Jana, Y. Zhao, Metal–Organic Framework Derived Nanozymes in Biomedicine. Acc. Chem. Res. 53(7), 1389–1400 (2020)
G.A. Bodkhe et al., Selective and sensitive detection of lead Pb(II) ions: Au/SWNT nanocomposite-embedded MOF-199. J. Mater. Sci. 56(1), 474–487 (2020)
A. Rashti et al., Tuning MOF-derived Co3O4/NiCo2O4 nanostructures for high-performance energy storage. ACS Appl. Energy Mater. 4(2), 1537–1547 (2021)
N. Ingle et al., Sulfur dioxide (SO2) detection using composite of Nickel benzene carboxylic (Ni3BTC2) and OH-functionalized single walled carbon nanotubes (OH-SWNTs). Front. Mater. 7, 93 (2020)
M. Dinari, F. Jamshidian, Preparation of MIL-101-NH2 MOF/triazine based covalent organic framework hybrid and its application in acid blue 9 removals. Polymer 215, 123383 (2021)
M. Mahadik et al., EDTA modified PANI/GO composite based detection of Hg(II) ions. Front. Mater. 7, 81 (2020)
H. Nazemi et al., Advanced micro-and nano-gas sensor technology: a review. Sensors 19(6), 1285 (2019)
A. Nowoświat, L. Dulak, Impact of cement dust pollution on the surface of sound-absorbing panels on their acoustic properties. Materials 13(6), 1422 (2020)
P.W. Sayyad et al., Chemiresistive SO2 sensor: graphene oxide (GO) anchored poly (3,4-ethylenedioxythiophene): poly (4styrenesulfonate)(PEDOT: PSS). Appl. Phys. A 126(11), 1–8 (2020)
Y. Wu et al., The high-resolution estimation of sulfur dioxide (SO2) concentration, health effect and monetary costs in Beijing. Chemosphere 241, 125031 (2020)
A. Yang et al., Single ultrathin WO3 nanowire as a superior gas sensor for SO2 and H2S: Selective adsorption and distinct IV response. Mater. Chem. Phys. 240, 122165 (2020)
Q. Zhou et al., High sensitive and low-concentration sulfur dioxide (SO2) gas sensor application of heterostructure NiO-ZnO nanodisks. Sens. Actuators B 298, 126870 (2019)
V. Kumar, D.R. Roy, Single-layer stanane as potential gas sensor for NO2, SO2, CO2 and NH3 under DFT investigation. Physica E 110, 100–106 (2019)
N. Ingle et al., A chemiresistive gas sensor for sensitive detection of SO 2 employing Ni-MOF modified–OH-SWNTs and–OH-MWNTs. Appl. Phys. A 127(2), 1–10 (2021)
M. Tchalala et al., Fluorinated MOF platform for selective removal and sensing of SO2 from flue gas and air. Nat. Commun. 10(1), 1–10 (2019)
A.S. El-Said et al., Tuning tailored single-walled carbon nanotubes by highly energetic heavy ions. Phys. Rev. Appl. 13(4), 044073 (2020)
H. Gupta et al., Defect-induced photoluminescence from gallium-doped zinc oxide thin films: influence of doping and energetic ion irradiation. Phys. Chem. Chem. Phys. 21(27), 15019–15029 (2019)
P. Esquinazi et al., Induced magnetic ordering by proton irradiation in graphite. Phys. Rev. Lett. 91(22), 227201 (2003)
A. Krasheninnikov, K. Nordlund, Ion and electron irradiation-induced effects in nanostructured materials. J. Appl. Phys. 107(7), 3 (2010)
I.I.I. Wood et al., Perspectives on the relationship between materials chemistry and roll-to-roll electrode manufacturing for high-energy lithium-ion batteries. Energy Storage Mater. 29, 254–265 (2020)
S. Raghuvanshi et al., Dual control on structure and magnetic properties of Mg ferrite: role of swift heavy ion irradiation. J. Magn. Magn. Mater. 471, 521–528 (2019)
A. Ratan et al., Enhanced electrical properties of few layers MoS2-PVA nanocomposite film via homogeneous dispersion and annealing effect induced by 80 MeV Carbon6 + swift heavy ion irradiation. Mater. Sci. Semicond. Process. 108, 104877 (2020)
M. Karlušić et al., Nanopatterning surfaces by grazing incidence swift heavy ion irradiation. Appl. Surf. Sci. 541, 148467 (2021)
R. Singh et al., Tuning of defects induced visible photoluminescence by swift heavy ion irradiation and thermal annealing in zinc oxide films. Radiat. Phys. Chem. 183, 109400 (2021)
J. Zeng et al., Graphene electrical properties modulated by swift heavy ion irradiation. Carbon 154, 244–253 (2019)
N. Manikanthababu et al., Swift heavy ion irradiation-induced modifications in the electrical and surface properties of β-Ga2O3. Appl. Phys. Lett. 117(14), 142105 (2020)
H.K. Patil et al., Reinforcement of polyaniline and poly-(o-toluidine) with SWNTs and tuning of their physicochemical properties by heavy ion beams. Appl. Phys. A 124(7), 491 (2018)
H.K. Patil, et al., Spectroscopic investigations upon 100 MeV oxygen ions irradiation on polyaniline and poly-o-toluidine. In: AIP Conference Proceedings. 2018. AIP Publishing LLC
O. Ochedowski et al., Radiation hardness of graphene and MoS2 field effect devices against swift heavy ion irradiation. J. Appl. Phys. 113(21), 214306 (2013)
T. Bolse, H. Paulus, W. Bolse, Swift heavy ion induced dewetting of metal oxide thin films on silicon. Nucl. Instrum. Methods Phys. Res. Sect. B 245(1), 264–268 (2006)
R. Dutta, A. Kumar, 100 MeV O7+ ion irradiation induced electrochemical enhancement in NiBTC metal-organic framework based composite polymer electrolytes incorporated with ionic liquid. Mater. Res. Exp. 6(8), 085305 (2019)
P.W. Sayyad et al., Tuning the properties of Fe-BTC metal-organic frameworks (MOFs) by swift heavy ion (SHI) irradiation. Radiat Effects Defects Solids (2020). https://doi.org/10.1080/10420150.2020.1825958
N. Ingle et al., ChemFET Sensor: nanorods of nickel-substituted metal–organic framework for detection of SO2. Appl. Phys. A 126(9), 1–9 (2020)
T.D. Malouff et al., Carbon ion therapy: a modern review of an emerging technology. Front. Oncol. 10, 82 (2020)
K. Datta et al., Organic field-effect transistors: predictive control on performance parameters. J. Phys. D: Appl. Phys. 46(49), 495110 (2013)
M. Ko et al., Employing conductive metal–organic frameworks for voltammetric detection of neurochemicals. J. Am. Chem. Soc. 142(27), 11717–11733 (2020)
M.K. Smith et al., Direct self-assembly of conductive nanorods of metal–organic frameworks into chemiresistive devices on shrinkable polymer films. Chem. Mater. 28(15), 5264–5268 (2016)
M.K. Smith, K.A. Mirica, Self-organized frameworks on textiles (SOFT): conductive fabrics for simultaneous sensing, capture, and filtration of gases. J. Am. Chem. Soc. 139(46), 16759–16767 (2017)
J. Ram et al., Ion beam engineering in WO3-PEDOT: PSS hybrid nanocomposite thin films for gas sensing measurement at room temperature. Inorg. Chem. Commun. 119, 108000 (2020)
H. Xu et al., Ag/Ag2S nanoparticle-induced sensitization of recovered sulfur-doped SnO2 nanoparticles for SO2 detection. ACS Appl. Nano Mater. 3(8), 8075–8087 (2020)
R.U. Mene, M.P. Mahabole, R.S. Khairnar, Surface modified hydroxyapatite thick films for CO2 gas sensing application: effect of swift heavy ion irradiation. Radiat. Phys. Chem. 80(6), 682–687 (2011)
H. Xu et al., Ag/Ag2S nanoparticle-induced sensitization of recovered sulfur-doped SnO2 nanoparticles for SO2 detection. ACS Appl. Nano Mater. 8, 8075–8087 (2020)
Y. Liu et al., An integrated micro-chip with Ru/Al2O3/ZnO as sensing material for SO2 detection. Sens. Actuators B 262, 26–34 (2018)
L. Liu, S. Liu, Oxygen vacancies as an efficient strategy for promotion of low concentration SO2 gas sensing: the case of Au-modified SnO2. ACS Sustain. Chem. Eng. 6(10), 13427–13434 (2018)
S.K. Lim et al., Preparation of ZnO nanorods by microemulsion synthesis and their application as a CO gas sensor. Sens. Actuators B 160(1), 94–98 (2011)
Acknowledgements
The authors are thankful to the Inter-University Accelerator Center (IUAC), New Delhi (UFR-62320 & UFR-62321) for material science beamline with SEM facilities and financial support and DST-SERB (Sanction No. EEQ/2017/000645), UGC-DAE CSR (RRCAT), Indore (Project No. CSR-IC-BL66/CSR-183/2016-17/847), UGC-SAP programme (F.530/16/DRS-1/2016 (SAP-II), dt. 16-04-2016), DST-FIST (Project No. No. SR/FST/PSI-210/2016(C) dtd. 16/12/2016), Rashtria Uchachatar Shiksha Abhiyan (RUSA), Government of Maharashtra for providing characterization facilities. The authors also thank Dr. Saif A. Khan, IUAC, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ingle, N.N., Shirsat, S., Sayyad, P. et al. Influence of swift heavy ion irradiation on sensing properties of nickel-(NRs-Ni3HHTP2) metal-organic framework. J Mater Sci: Mater Electron 32, 18657–18668 (2021). https://doi.org/10.1007/s10854-021-06353-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-021-06353-z