Abstract
The magnetic properties of Sr2Fe1−xNixMoO6 perovskites, with x ≤ 0.2, in a large temperature range, are reported. The saturation magnetizations decrease, while the Curie temperatures increase, as the nickel content is higher. These trends are correlated with the distribution of magnetic ions in B and B’ sites. The reciprocal susceptibilities follow non linear temperature dependences. The magnetic properties are analysed assuming a two sublattices model, in the mean field approximation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The crystal structure and magnetic properties of Sr2FeMoO6 double perovskite were reported near 60 years ago [1]. Relatively recent studies, showed that this material is half-metallic, having large low field magnetoresistance [2]. Taking into account their Curie point, Tc ≈ 400 °C, above room temperature, this perovskite was shown to be suitable for spin electronics applications and magnetoresistance based devices.
The ideal structure of Sr2FeMoO6 perovskite can be viewed as a regular arrangement of corner-sharing FeO6 and MoO6 octahedra, alternating along the three directions of the crystal. Although the structure falls within the cubic Fm3m space group, the crystal lattice of Sr2FeMoO6 is distorted. Since of large size of Sr2+ ions, occupying the voids in between the octahedra, these undergo cooperative tilting distortions, towards the most energetically favourable structure, which causes reduction of the cube cell to the tetragonal I4/m lattice.
The Fe and Mo atoms, in the ideal structure of Sr2FeMoO6, occupy the B and B’ sites, respectively, favouring a NaCl type ordered structure. In this ideal atomic arrangement, the perovskite is ferrimagnetically ordered. The array of parallel Fe3+ magnetic moments are antiparallelly coupled with the Mo5+ spins; the resulting moment per formula unit is expected to be 4µB [3]. The experimentally determined magnetic moments, smaller than the above-mentioned value, are due to: (1) antisite B cation disorder, when some Mo5+ cations occupy the position of the Fe3+ cations and vice versa; (2) a fraction of iron has Fe2+ valence state, with concomitant presence of Mo6+ ions; (3) the Mo5+ magnetic moment, at T = 4 K, is smaller than the expected value of 1 µB.
A colinear magnetic structure, with MFe = 3.9 ± 0.1 µB and MMo = − 0.37 ± 0.6 µB was determined for Sr2FeMoO6 by a neutron diffraction measurement, at T = 15 K [3], or with MFe = 3.97 ± 0.1 µB and MMo = − 0.19 ± 0.1 µB T = 2 K [4]. By X-ray magnetic circular dichroism (XMCD), on a polycrystalline Sr2FeMoO6 perovskite, mean magnetic moments MFe = 2.89 ± 0.03 µB and MMo = 0.32 ± 0.03 µB, were also reported [5]. By using X-ray photoelectron spectroscopy (XPS) and X-ray absorption spectroscopy (XAS) on highly ordered Sr2FeMoO6 sample, a mixed valence state of iron ions, involving 66% Fe2+—Mo6+ and 34% Fe3+—Mo5+ states has been shown [6]. Also, the presence of a nonuniform composition inside the grains, as well as the effect of grain boundary on the transport properties of ordered Sr2FeMoO6 perovskite, were analysed [7].
The temperature dependence of the magnetic susceptibility, χ, of Sr2FeMoO6, above the Curie point, revealed the presence of magnetic ordered impurities [8]. The analysis of the data, according to Honda-Arrot plot, evidenced a Curie–Weiss type behaviour, with a Curie constant, smaller than that of Fe2+ ions [9]. Since the perovskite is ferrimagnetically ordered, deviations from a linear temperature dependence of χ−1 values are expected, although not observed in the above report.
The previous studies performed on Sr2Fe1−xNixMoO6 perovskites reported an increase of the Curie temperatures, Tc when increasing the nickel content [10, 11]. Different trend for magnetizations, Ms, determined at T = 300 K, were evidenced, either decreasing [10] or increasing [11], as the iron is gradually substituted by nickel. In the above reports, the Ms values were obtained at different reduced temperatures, T/Tc, located between 0.74 (x = 0) and 0.69 (x = 0.2) and therefore cannot describe correctly their composition dependence at low temperatures, due to different thermal effects. The sintering method influence also the magnetic properties. The increase of magnetizations in Sr2Fe1−xNixMoO6, as iron was substituted by nickel, was correlated with the increase of atomic ordering [11], despite that nickel in B site has a smaller magnetic moment than those of ferrous or ferric ions.
In this report, starting from experimental results, the magnetic properties of Sr2Fe1−xNixMoO6 were analysed assuming a two sublattices mean field model, corresponding to B and B’ sites magnetizations. The locations of constituent’s ions, their valence states as well as the exchange interactions inside and between magnetic sublattices were discussed in correlation with the sample’s compositions.
2 Experimental
The Sr2Fe1−xNixMoO6 perovskites, with x ≤ 0.2, were prepared by solid state reaction, as already described [12]. Stoichiometric amounts of high purity SrCO3, Fe2O3, MoO3 and NiO powders were mixed and calcinated in argon atmosphere, at T = 900 °C. The calcinated powders were pelletized and sintered at T = 1300 °C, during 8 h in a mixture of Ar/3%H2 atmosphere.
The phase purity, crystal structure and lattice parameters were determined by XRD, in Bragg–Brentano geometry, by using a Seifert-type equipment and CuKα radiation (λ = 1.54059 Å). The spectra were analysed by Rietveld method.
The antisite contents were estimated from the intensities of (101), (200) and (112) lines of XRD spectra, I(101)/[I(200) + I(112)] as already suggested [13] or starting from saturation magnetizations. The Sr2FeMoO6 sample has been already studied by XPS and XAS and was assumed to have a composition close to the nominal one [6]. Only the composition of the sample with x = 0.2 has been investigated. There are small differences between the grain’s compositions, the normalized ones to Sr2 content for two grains being Sr2Fe0.77–0.81Ni0.19–0.22O5.85–5.9. Analysing the magnetic properties, we assumed that Sr2Fe1−xNixMoO6 perovskites, have compositions close to the nominal ones. As will be shown latter, the errors introduced, as in case of sample with x = 0.2, are of the order of ≈ 3%.
Magnetic measurements were made at T = 4.2 K, in field up to 70 kOe, by extraction method with a Maglab 2000 type equipment from Oxford Instruments, working in the temperature range 4 K ≤ T ≤ 400 K. The Curie temperatures were determined analysing the temperature dependencies of magnetizations in low fields. At T ≥ Tc, the magnetic susceptibilities were determined also by using a Faraday—type balance up to T = 800 K. The magnetic susceptibilities were determined according to Honda-Arrot plot, from their field dependences, χ0 = χ + c \({M}_{s}^{^{\prime}}\) H−1, by extrapolating the measured values at H−1 → 0 [14]. By c is denoted a presumed magnetic ordered impurity content having \({M}_{s}^{^{\prime}}\) magnetization. In this way any possible alteration of the magnetic susceptibility, due to presence of small quantity of magnetic ordered phase, is avoided.
3 Experimental results
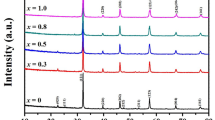
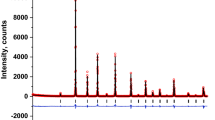
The XRD patterns of Sr2Fe1−xNixMoO6 perovskites, at ambient temperature, indicated the presence of a single-phase double perovskite in the composition range x ≤ 0.2. These crystallize in the tetragonal type structure, space group I4/mmm; only traces of impurities were identified. Both the a and c lattice parameters decrease when increasing the nickel content—Fig. 1. The tetragonal distortion parameters increase from 2.73 × 10−3 (x = 0) to 2.90 × 10−3 (x = 0.2), being only little smaller than those previously determined on the same system [11].
The presence of (101) spots, which reflect the ordering of Fe/Mo ions in B/B’ sites, have been evidenced in all samples. The antisite defects, AS, i.e. occupations of Fe(Mo) ions in the Mo(Fe) sites, were determined from the relative intensity of the ratio I101/(I122 + I200) [13]. The AS content increases from ≈ 4% (x = 0) up to 11% (x = 0.2). These values are little smaller than that already reported on the same system, [11] being associated with higher sintering temperature and time, than used in previous reports. The AS defects were estimated also from the composition dependence of magnetization, expected as Ni2+ ions are located in B sites and that experimentally determined. These are little higher than those obtained by XRD—Fig. 1 inset.
The saturation magnetizations, determined at T = 4.2 K, decrease when increasing nickel content, while the Curie temperatures, Tc, follow an opposite trend—Figs. 2 and 3. Such a behaviour is rather interesting; commonly an increase of magnetizations taking place parallel with those of Tc values.
The Sr2Fe1-xNixMoO6 double perovskites are ferrimagnetically ordered, the magnetic moments of the ions located in B sites, being antiparallelly oriented to those situated at B’ sites. Such a magnetic structure can be analysed assuming a two sublattices model, in the mean field approximation [15]. Additional information on such a system can be obtained from their magnetic behaviour at T > Tc—Fig. 4.
The thermal variations of the magnetic susceptibilities follow non-linear dependences, characteristic for a ferrimagnetic system, as described by Nèel relation [15]:
By C is denoted the Curie constant and \({\chi }_{0}^{-1}\), \(\sigma \) and \(\theta \) parameters are related to the exchange interactions between and inside the magnetic sublattices as well as the contributions to the Curie constant of the two sublattices. The parameters involved in relation (1), obtained by fitting \({\chi }^{-1}\) versus T data with relation (1), are listed in Table 1.
The contributions to the Curie constants of the ions situated in B and B’ sites, CB and CB’ were evaluated starting from the additional law of magnetic susceptibilities [15] as well as the experimentally determined saturation magnetizations. An ionic model was assumed, where the effective moments of constituent elements are given by their free ion values [9].
According to additional law of magnetic susceptibilities, Curie constants, respectively [15, 16] we have:
Taking into account also the charge neutrality rule, the content and valences of constituting elements were determined assuming nominal compositions of perovskites. The Fe3+ fractions u = 0.39 (x = 0) and u = 0.41 (x = 0.2) were obtained. When the mean composition of sample with x = 0.2, as determined by SEM, is used, the determined Fe3+ content was 0.425 very close to above mentioned values. The content of ferric ions reported on the same Sr2FeMoO6 sample, by XPS and XAS, implies 66% Fe2+—Mo6+ and 34% Fe3+—Mo5+ states [6], little different from our estimation using magnetic measurements.
The composition dependences of the experimentally determined magnetizations are best described assuming: (1) the Mo5+ magnetic moment, at T = 4 K, is ≈ 0.4 µB/atom, as suggested by neutron diffraction [3, 4] and XMCD [5] studies on Sr2FeMoO6 and those of Fe3+, Fe2+ and Ni2+ of 5 µB, 4 µB and 2 µB, respectively; (2) the Ni2+ ions replace ferrous ions in B site; (3) in the B’ sites are located, in addition to molybdenum ions, ferric ions, according to antisites content. The magnetizations thus obtained, as shown in Fig. 3, describe rather well the experimental results, the differences being of the order of 3–4%
Starting from above distributions of ions in B and B’ sites, the contributions to the Curie constants of the B (CB) and B’ (CB’) sublattices were determined, C = CB + CB’. The ratio CB/CB’ is rather high, stressing the dominant contribution of B sublattice to the magnetic susceptibility. Consequently, there are relatively small deviations from a linear χ−1 versus T dependence, outside the temperature range Tc < T < Tc + 180 K. Thus, in some reports, the magnetic susceptibility of Sr2FeMoO6 was assumed to follow a Curie–Weiss type behaviour [8].
The parameters Jij (i, j = B, B’) describing the mean exchange interactions inside and between magnetic sublattices, were determined from paramagnetic data, according to Néel’s model [15, 16]—Table 2. The exchange interactions parameters JBB’ between the two sublattices are negative as expected for a ferrimagnetic system. These values, in absolute magnitude, increase as the iron is substituted by nickel. The JB’B’ values do not change, while a small increase of JBB is shown for sample with x = 0.2, as compared to that determined for the end series perovskite.
4 Conclusions
The Sr2Fe1−xNixMoO6 ordered solutions, crystallize in a tetragonal structure, in the composition range x ≤ 0.2. These perovskites are ferrimagnetically ordered. The Curie temperatures increase while the saturation magnetizations decrease, when increasing nickel content. An opposite trend for the composition dependence of the magnetization, at T = 300 K, was shown in disordered Sr2Fe1−xNixMoO6 [11]. This behaviour was attributed to the increase of the atomic ordering, followed by those of the magnetizations, although the nickel located in B site has a smaller magnetic moment that the substituted ferrous ions.
The increase of the Curie temperatures when increasing nickel content can be corelated with the exchange interactions between B and B’ sublattices, as the number of the antisite content increases, B’ magnetization, respectively. Similar behaviour was already reported in Sr2FeMoO6 perovskite and correlated with changes in the fraction of antisites [17]. A theoretical model, also attributed to antisite defects, a role in stabilizing the magnetic ordered phase in perovskite [18].
The Curie temperatures, calculated with the exchange interactions parameters, determined mainly from paramagnetic data, according to mean field model [15], describe well the experimentally determined values—Fig. 3. This fact stresses the reliability of the data obtained both below and above Curie temperatures, in describing the magnetic properties of perovskites as already showed in case of Ca2Fe1−xNixMoO6 perovskites [19].
References
E. Burzo, Perovskites, Landolt Bornstein Handbuck, Vol. III27f1 (Springer Verlag, New York, 1996).
K.I. Kobayashi, T. Kimura, H. Sawada, K. Terakura, I. Tokura, Nature 395, 677 (1998)
D. Sánchez, J.A. Alonso, M. García-Hernández, M.J. Martínez-Lope, J.L. Martínez, A. Mellergård, Phys. Rev. B 65, 104426 (2002)
C. Ritter, M.R. Ibarra, L. Morellon, J. Blasco, J. Garcia, J.M. De Teresa, J. Phys. 12, 8295 (2000)
T. Koide, T. Sekine, H. Miyauchi, H. Manaka, D. Asakura, A. Fujimori, K.I. Kobayashi, Y. Tomioka, T. Kimura, Y. Tokura, J. Phys. 502, 012003 (2014)
K. Kuepper, I. Balasz, H. Hesse, A. Winiarski, K.C. Prince, M. Matteucci, D. Wett, R. Szargan, E. Burzo, M. Neumann, Phys. Stat. Solidi (A) 201, 3252 (2004)
E. Burzo, I. Balasz, S. Constantinescu, I.G. Deac, J. Magn and Magn. Mater. 316, e741 (2007)
J. Navarro, L.I. Balcells, B. Martınez, J. Fontcuberta, J. Appl. Phys. 89, 7684 (2001)
C. Kittel, Introduction to Solid State Physics (Wiley and Sons, New York, 1971).
A. Gaur, G.D. Varma, H.K. Singh, J. Alloys Compd. 460, 581 (2008)
L. Chen, C. Yuan, J. Xue, J. Wang, J. Phys. D. 38 (2005) 4003; J. Am. Ceram. Soc. 89 (2006) 671
E. Burzo, I. Balasz, M. Valeanu, I.G. Pop, J. Alloys Compd. 509, 105 (2011)
Ll. Balcells, J. Navarro, M. Bibes, A. Roig, B. Martınez, and J. Fontcuberta, Appl. Phys. Lett., 78 (2001) 781
L.F. Bates, Modern Magnetism (Cambridge University Press, Cambridge, 1951), p. 189
L. Nèel, J. Phys. (Paris) 3, 137 (1948)
E. Burzo, I. Balasz, M. Valeanu, D.P. Kozlenko, S.E. Kichanov, A.V. Rutkauskas, B.N. Savenko, J. Alloys Compd. 621, 71 (2015)
J. Navarro, J. Nogues, J.S. Munoz, J. Fontcuberta, Phys. Rev. B 67, 174416 (2003)
J.B. Solovyev, Phys. Rev. B 65, 144446 (2012)
E. Burzo, I. Balasz-Muresan, Rom. J. Phys. 62, 601 (2017)
Acknowledgements
We would like to acknowledge support from the Romanian UEFISCDI Project Number PN-III-P4-ID-PCCF-2016-0112 Nr. 6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Burzo, E., Souca, G. Magnetic properties of Sr2Fe1−xNixMoO6 perovskites. J Mater Sci: Mater Electron 32, 2200–2204 (2021). https://doi.org/10.1007/s10854-020-04985-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-04985-1