Abstract
In this work, we report a systematic study on charge transport and thermo-electric properties of poly (3,4-ethylenedioxythiophene):poly(styrene sulfonate):poly(ethylene glycol) (PEDOT–PSS:PEG) organic thin films doped with silver nanoparticles (AgNPs). Transparent and flexible hybrid nanocomposite films were prepared by a simple strategy via bar coating technique. The effect of PEG treatment and AgNPs nanoparticles distribution in PEDOT–PSS films was examined through various characterization techniques such as scanning electron microscopy (SEM), atomic force microscopy (AFM), Fourier transform infra-red spectroscopy (FTIR), and thermo gravimetric analysis (TGA). The content of AgNPs in PEDOT–PSS:PEG was varied and optimized for 10 wt% as a percolation threshold. The addition of AgNPs and subsequent PEG treatment enhances the conductivity of PEDOT–PSS films from 2 to 420.33 S/cm due to the removal of non-complexed PSS and synergetic interaction between PEDOT–PSS and AgNPs segments via PEG. These highly conductive nanocomposite films were employed in an organic thermo-electric (TE) device to investigate the TE properties. These PEG treated PEDOT-PSS: AgNPs nanocomposite organic films exhibit a enhanced power factor from 6 μW/mK2 to 85 μW/mK2 which is nearly 15 times higher than that of pure PEDOT-PSS thin films. Due to ease of processing, flexibility, excellent charge transport, and thermo-electric properties, these PEG-treated PEDOT–PSS:AgNPs nanocomposite films can be potential thermo-electric materials for organic electronic devices operated at room temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Thermo-electric materials have gained potential applications involving the generation of power, as a solid-state heating and cooling devices, for direct conversion of thermal energy into electrical energy without altering the mechanical components [1]. The conventional inorganic-based semiconductor materials such as SiGe and PbTe have grabbed the attention as thermo-electric materials due to their high power factors, but these materials are generally of high cost and lack in scalability for fabrication. Hence, there is a great demand to build organic thermo-electric materials with low cost and easy process ability to be used for various thermo-electric applications [2]. In the recent past, a lot of attention has been paid towards the development of polymer-based thermo-electric materials in modern applications for being economical with low thermal conductivity [3,4,5]. Among various conducting polymers, poly (3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT–PSS) has been extensively studied as a p-type TE material for tuning the thermo-electric properties, but unfortunately the electrical conductivity and thermo power factors are very low for pure PEDOT–PSS films which affected its performance in thermo-electric devices [6,7,8,9,10]. These drawbacks in transport and thermo-electric properties of PEDOT–PSS could be improved by doping with polar solvents like DMSO, ethylene glycol, NMP, polyethylene glycol, and sorbitol [11, 12]. Several research groups have reported the improved thermo-electric performance with power factors between 10 and 21 µW/mK2 by incorporating these solvents into PEDOT–PSS [13,14,15,16]. Another strategy is to incorporate inorganic nano fillers into PEDOT–PSS matrix which yields the desired composite having the combination of low thermal conductivity of the polymer and the high power factor of these inorganic fillers [17,18,19,20]. Several researchers in the recent past have reported improved thermo-electric performances of PEDOT–PSS as composite with carbon-based materials such as CNTs and graphene, yet the thermo power factors of these materials need improvement for practical applications such as organic thermo-electric generators, OTEGs [21,22,23]. AgNPs are generally used to functionalize the materials for improved thermal and electrical properties. In case of poly-vinyl-pyrrolidone (PVP), the AgNPs are generally used as capping agents for material applications. Inclusion of AgNPs in polymer matrix results in increase of charge conjugation length, which in turn leads to increased electrical and thermo-electric properties of the polymer. Therefore, AgNPs were chosen as efficient fillers which can greatly influence to optimize the electrical conductivity, thermal power factor, and Seebeck coefficients. PEDOT–PSS has an excellent film forming property after the treatment [24,25,26,27,28,29] and polyethylene glycol (PEG) is one of the best binders that provides strong adhesion between PEDOT–PSS and AgNPs resulting in the formation of stable thin films. The previous studies on thermo-electric performances of PEDOT–PSS-based composites used large quantity of filler concentrations (> 30–40%) to improve the thermo-electric performances. Recently, Yoshida and Toshima [30] have reported the thermo-electric properties of hybrid thin films of PEDOT–PSS with silver nanowires that exhibit a power factor of 48 µW/mK2 and a Seebeck coefficient of 20 µV/K using upto 100 wt% of Ag nanowires. To obtain better thermo-electric performance, using small quantities of filler concentrations needs much attention. To the best of our knowledge, the TE performance of PEDOT–PSS:AgNPs treated with solvents such as PEG with excellent TE properties is not been reported so far.
Therefore, these factors inspired our interest in PEDOT–PSS:PEG organic thin films doped with AgNPs for optimizing their thermo-electric performance. We have demonstrated through this work that small quantity of additives of AgNPs (~ 10 wt%) is sufficient to achieve excellent thermo-electric performance in PEDOT–PSS-based systems. In this work, a systematic study has been presented towards understanding the enhancement in conductivity and thermo-electric properties of these materials by investigating their structural and morphological changes after doping with PEG and AgNPs in PEDOT–PSS. The prepared thin films were characterized by SEM, AFM, FTIR, and TGA techniques for investigating their structure, surface morphology, and stability studies. The transport properties in terms of DC conductivity, impedance behavior, and dielectric attributes of the prepared thin films were studied in detail and the study leads to the conclusion that concentration of nano fillers and dopant in PEDOT–PSS has significant influence on the transport properties. Further, the prepared thin films were explored for thermo-electric applications based on the thermo-electric power factor and Seebeck coefficient values. The organic thin films of PEDOT–PSS:AgNPs composite treated with PEG exhibit a thermo power factor of 85 µW/mK2 (with an addition of only 10 wt% of AgNPs in PEDOT–PSS) which is highest in terms of PEDOT–PSS-based systems (without being chemically treated using strong acids) investigated so far.
2 Experimental
2.1 Materials and chemicals
PEDOT–PSS (0.5 wt% of PEDOT and 0.8 wt. of PSS dissolved in aqueous media), silver nanoparticles (AgNPs) (99.99% purity) of particle size 40–50 nm, sodium citrate (tribasic dihydrate), and polyethylene glycol (PEG, 99% purity) were procured from Sigma-Aldrich (India).
2.2 Preparation of PEDOT–PSS:PEG doped with AgNPs organic thin films
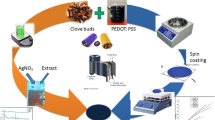
The AgNPs were mixed with tri sodium citrate solvent and sonicated for 6 h to get uniform colloidal dispersion of AgNPs in tri sodium citrate, and the resulting solution is filtered by using Whatman filter paper of pore size 0.65 µm to produce a pure AgNPs solution. The aqueous solutions of commercially procured PEDOT–PSS:PEG (10 vol%) and AgNPs colloidal dispersion in tri sodium acetate were mixed together with varying content of AgNPs (2, 4, 6, 8, 10, 12 and 14 wt% in the total solution of PEDOT–PSS). These mixed dispersions were further sonicated in an ice bath using a probe sonicator (QSonica-Q500) followed by bar coating onto pre-cleaned glass substrate (2.5 × 2.5 cm2) and dried in air at 75 °C for 2 h. These organic thin films were annealed at 100 °C for 3 h at ambient condition to eliminate the moisture content from thin films. Incorporation of AgNPs in PEDOT–PSS:PEG polymer matrix induces the conformational changes in polymer backbone from coiled structure to linear structure as depicted in Fig. 1. Contact electrodes were prepared through thermal vapor deposition using silver on pure PEDOT–PSS and PEDOT–PSS:PEG/AgNPs nanocomposite films to investigate the electrical and TE characteristics.
2.3 Characterization
The surface morphology of pure PEDOT–PSS and PEDOT–PSS:PEG doped with AgNPs organic thin films was recorded by using SEM (Zeiss Ultra-60-Pollland) and AFM (Bruker Dimension Icon-Japan). Chemical structure and different functional groups present in the prepared thin films were identified through FTIR spectrophotometer (Perkin Elmer Frontier-USA). Thermal stability of the thin films was investigated using a thermal analyzer (NETZSCH STA-409PC-Germany). Temperature-dependent conductivity of the films (2.5 × 2.5 cm2) was carried out by four probe technique using the Keithley source meter (2400) and multimeter (2700) in the temperature range 20–250 °C. The frequency-dependent dielectric and impedance measurement of the films were carried out by two probe method using a LCR impedance analyzer (Wayne Kerr-6500B London) in the frequency range of 60 Hz to 2 MHz. Thickness of the composite films were recorded using surface profiler (Alpha-Step-D500 Stylus profiler-KLA-Tencor). Thermo-electric properties of the prepared nanocomposite films (shown in Fig. 2) were performed by source meter (Keithley-2400), power supply (Keithley-2200), nanovoltmeter (Keithley-2182A), and switch system (Keithley-7001). During the measurement of Seebeck coefficient, a temperature drop (ΔT) of 5 ± 0.5 K was controlled by an Ohm resistive heater and was calculated by S = − ΔV/ΔT (where ΔV is the induced TE voltage at the ends of the sample).
3 Results and discussion
3.1 Scanning electron microscope
Figure 3a, b, c illustrate the SEM micrographs of pure PEDOT–PSS, AgNPs, and PEDOT–PSS:PEG doped with 10 wt% of AgNPs, respectively. The micrographs of pure PEDOT–PSS presented in Fig. 3a have a smoother surface with the formation of micrograins in the polymer matrix. The SEM micrograph of AgNPs represented in Fig. 3b reveals the formation of cluster of spherical-shaped AgNPs particles with an average size of ~ 45 nm. The elemental composition analysis through EDX spectra shows the presence of strongest peak which corresponds to Ag signals and weaker signals resulting from Cl atoms. This shows the pure crystalline nature solely composed of silver. The inclusion of AgNPs in PEDOT–PSS at the percolation threshold (10 wt% of AgNPs) results into a highly clustered morphology with AgNPs being covered with PEDOT–PSS:PEG as shown in Fig. 3c. The elemental analysis of composite sample through EDX shows the presence of signals resulting from C, S, and O confirming the presence of PEDOT–PSS, whereas the peaks resulting from Ag reveals the presence silver nanoparticles in the composite spectra. Hence, the EDX spectra of the nanocomposite film strongly supports the uniform distribution of both PEDOT–PSS and Ag phases. The presence of AgNPs in PEDOT–PSS:PEG induces a well-interconnected morphology in the composite film that is favorable for charge transport mechanism resulting in better conducting channels in the system.
3.2 Atomic force microscope
Three-dimensional (3D) AFM images of pure PEDOT–PSS and PEDOT–PSS:PEG thin films doped with 10 wt% of AgNPs were represented in Fig. 4a and b, respectively. It can be observed from AFM images that the rms roughness is low for pure PEDOT–PSS nearly of 1.4 nm. Smaller rms roughness in pure PEDOT–PSS film implies a decrease in the electrostatic interaction between PEDOT and PSS which hinders the charge transport in the polymer backbone. The AgNPs-doped PEDOT–PSS:PEG thin film represents (Fig. 4b) significant changes due to rearrangement of PEDOT clusters and PSS. The rms roughness in the nanocomposites have been improved significantly to 5.2 nm after the addition of 10 wt% of AgNPs in PEDOT–PSS:PEG thin film. The lighter zone in the AFM micrographs represents the PEDOT-rich phase and dark zone represents PSS-rich phase [31, 32]. Inclusion of AgNPs in PEDOT–PSS:PEG decreases the phase separation between conducting PEDOT and insulating PSS segments. The improved rms roughness in AgNPs-doped PEDOT–PSS:PEG film might be due to the thinning effect with the subsequent removal of PSS from PEDOT–PSS:PEG.
3.3 FTIR analysis
The FTIR spectra of pure PEDOT–PSS, AgNPs, and PEDOT–PSS:PEG thin film doped with 10 wt% of AgNPs are shown in Fig. 5. The inferences obtained from FTIR spectra were used to identify the different functional groups present in pure PEDOT–PSS, AgNPs, and PEDOT–PSS:PEG doped with AgNPs thin film. The prominent peaks in PEDOT–PSS at 3500 cm−1 is due to the O–H stretching, peak at 1630 cm−1 is due to the stretching of C–H functional group, peak at 1360 cm−1 is due to C=O stretching and the major stretching at 1120 cm−1, and 620 cm−1 are the bands linked with the sulfur bonds in PEDOT–PSS [33]. The main characteristic stretching of AgNPs appeared at 2830 cm−1, 1460 cm−1, 1030 cm−1 and 720 cm−1, arising from Ag–Ag metallic and Ag–O ionic bonds [34]. In case of PEDOT–PSS:PEG doped with AgNPs thin film, the main peak at 3400 cm−1 is due to the silver metal nanoparticle and corresponding peaks at 1640 cm−1, 1370 cm−1, 1120 cm−1, and 570 cm−1 are the prominent peaks of PEDOT–PSS. The FTIR studies confirm a strong dispersion of AgNPs in PEDOT–PSS:PEG. Due to AgNPs doping, the stretching vibrations of PEDOT–PSS shifts in the FTIR spectra indicates the formation of composite with strong interactions between two phases.
3.4 Thermo gravimetric analysis
Figure 6 represents the thermograms of pristine PEDOT–PSS and PEDOT–PSS:PEG doped with 10 wt% of AgNPs thin films. The thermograms of the prepared PEDOT–PSS-based organic thin films show a three-step weight loss [35, 36]. First step of weight loss was observed at 100 °C which is due to evaporation of moisture content in the thin film. Second step of weight loss corresponds to 450 °C which is due to loss of polyethylene glycol in the host PEDOT–PSS. The third step of weight loss at 580 °C is due to disruption of polymer backbone chain in PEDOT–PSS:PEG film. The nature of thermograms reveals that pure PEDOT–PSS thin film degrades at a faster rate with temperature and retains a steady weight of 25%, whereas PEDOT–PSS:PEG doped with AgNPs thin film exhibits a gradual weight loss and retains about 35% of the weight at higher temperatures. Hence, the PEDOT–PSS:PEG doped with AgNPs thin film is thermally more stable when compared to pure PEDOT–PSS film.
3.5 Temperature-dependent conductivity
Figure 7 represents the variation of temperature-dependent conductivity (σdc) for pure PEDOT–PSS and PEDOT–PSS:PEG doped with various concentrations of AgNPs in the temperature range from 20 °C to 250 °C. The pristine PEDOT–PSS thin film exhibits lowest conductivity of the order of 2 S/cm, whereas PEDOT–PSS:PEG doped with 10 wt% of AgNPs (percolation threshold) shows maximum conductivity of 420.3 S/cm. The conductivity in PEDOT–PSS:PEG thin film doped with 10 wt% of AgNPs was increased by three orders of magnitude compared to pure PEDOT–PSS film. The reason for improvement in the conductivity of doped PEDOT–PSS:PEG thin film can be attributed to the formation of more charge carriers (both polarons and bipolarons) in the polymer matrix at the percolation threshold and these charge carriers find electrical pathways to easily hop between conducting segments upon being doped with AgNPs in PEDOT–PSS:PEG thin film [24]. In the microscopic domain, coil conformations are expected to be present in PEDOT–PSS polymer backbone, whereas in doped PEDOT–PSS:PEG film, coil conformations change into linear conformations upon inclusion of AgNPs and PEG treatment in PEDOT–PSS (as illustrated in Fig. 2) leading to enhancement in the π–π conjugation length in the doped sample. The screening effect induced by AgNPs in PEDOT–PSS is one of the driving forces for improved conductivity in PEDOT–PSS:PEG thin films which further lead to a significant decrease in the activation energies as depicted in Table 1. The conductivity of the nanocomposites have been found to increase upon secondary doping of PEG. The presence of non-complexed PSS in large volume is found to reduce the conductivity, total carrier densities, and increase in tunneling distances, thereby drastically decreasing the carrier mobility of PEDOT–PSS films [21]. The secondary doping of PEG could remove the insulating PSS segment thereby effectively decreasing the distance between AgNPs and PEDOT that results into improved conductivity. For the AgNPs composition beyond 10 wt%, the increased density of AgNPs, in PEDOT–PSS:PEG, partially blocks the charge carrier hopping thereby reducing the conductivity of the composite films. Hence, better conductivity can be achieved in these composites at a percolation threshold of 10 wt% of AgNPs in PEDOT–PSS:PEG.
3.6 Dielectric studies
The dielectric response in terms of real part of dielectric constant and dielectric loss for PEDOT–PSS/AgNPs composite at 2 MHz with and without PEG are shown in Figs. 8 and 9. Both dielectric constant and dielectric loss strongly depends on AgNPs concentration as well as PEG in the PEDOT–PSS matrix. The values of ε′ and tan(δ) gradually decreases with increasing concentration of AgNPs in PEDOT–PSS due to increased grain boundaries as well as decrease in interfacial polarization. The enhancement in ε′ values for pure PEDOT–PSS can be attributed to the formation of large number of microcapacitors due to close packing of PEDOT–PSS leading to Maxwell–Wagner–Sillar (MWS) polarization at the grain boundaries [8, 37]. Addition of AgNPs in the PEDOT–PSS matrix creates a large number of micrograins that reduces the interfacial polarization at the grain boundaries. The PEG treatment in PEDOT–PSS:AgNPs, which acts as a binder, further hinders the dipolar orientation in the electric field resulting into least values for both ε′ and dielectric loss. PEDOT–PSS:AgNPs treated with PEG exhibits very small values of loss that can be attributed to DC conduction associated with interfacial polarization of barrier effects. For higher concentration of AgNPs (> 10 wt%) in PEDOT–PSS:PEG, the dielectric constant and dielectric loss shows increment, following the percolation-dependent behavior in agreement with conductivity measurements. These PEG-treated PEDOT–PSS:AgNPs composites with excellent dielectric constant and small dielectric losses could be a valuable material for electronic applications.
The variation in the real (Z′) and imaginary (Z″) part of impedance with frequency for pure PEDOT–PSS and PEDOT–PSS:PEG doped with AgNPs thin films recorded through impedance analyzer are illustrated as Nyquist curves in Fig. 10. The values of impedance are found to depend on the overall resistance of the system which is the sum of grain resistance and bulk resistance. The Nyquist plots for all the samples exhibit semicircular arc in lower frequency region indicating single relaxation resulting from Debye behavior. It can be noted that, as the AgNPs concentration increases, the area under the curve reduces indicating a decrease in electrical resistance of the PEDOT–PSS:PEG composite films. From the Nyquist plots, it has been observed that thin film of PEDOT–PSS:PEG doped with 10 wt% AgNPs exhibits a small area under the semicircular arc implying a small resistance value compared to other samples. For the higher concentration of AgNPs (> 10 wt%) in PEDOT–PSS:PEG, the trend will be reversed indicating the percolation-dependent behavior in these composites. The analysis of Nyquist plots also indicates a low relaxation time of the order of 0.212 µs for the composite sample at the percolation threshold. The response of the LCR equivalent circuit corresponds to a resistor and a capacitor in parallel as depicted in the inset of the Fig. 10. Decrease in the arc area implies a drop in resistance value indicating that the inclusion of dopant at the percolation threshold leads to the formation of high conduction paths at the grain boundaries contributing to enhanced conductivity.
3.7 Thermo-electric study
The variation of thermo-electric power as a function of temperature difference between the cold and hot junctions for pure PEDOT–PSS and PEDOT–PSS:PEG doped with various concentrations of AgNPs are illustrated in Fig. 11. From the plot, it can be inferred that the thermo-electric power gradually increases with increase in dopant concentration in comparison to pure PEDOT–PSS. Further, it can be analyzed as a two-step variation wherein the change in the thermo-electric power value is very low in the initial region (ΔT) with a sudden increase towards the higher temperature difference (ΔT). Among all the prepared thin films, the sample of PEDOT–PSS:PEG doped with 10 wt% AgNPs thin film exhibits higher thermo-electric power and is attributed to the change in morphology and generation of charge carriers (both polarons/bipolarons) in the polymer backbone at the percolation threshold. The decrease in thermo-electric power beyond 10 wt% of AgNPs in PEDOT–PSS:PEG further confirms the percolation-dependent thermo-electric characteristics of these composites. Molecular alignment of the polymer is one of the most important factors for improvement of thermo-electric performance of the percolated structure, thereby suggesting that the inclusion of metallic nanoparticles such as AgNPs lead to greater alteration of properties. Increase in charge carrier concentration and easy diffusion of these charge carriers from the hot end to the cold junction has resulted in the improved thermo-electric power of 44 mV/K in PEDOT–PSS:PEG doped with 10 wt% AgNPs thin film compared to pure PEDOT–PSS thin film. The enhanced electrical conductivity in the percolated structure is the main factor responsible for the increased power factor. Although the power factors of the nanocomposite films have been increased significantly by the addition of AgNPs and post treatment of PEG, further improvement in the power factor could be needed.
Figure 12 represents the Seebeck coefficient for different weight percentage of AgNPs in PEDOT–PSS:PEG thin films. It was observed that the Seebeck coefficient increases with concentration of AgNPs in PEDOT–PSS upto 10 wt%. Further increase in the AgNPs concentration (> 10 wt%),shows reduction in Seebeck coefficient values which confirms the percolation-dependent Seebeck effect in these composites. The major reason for improved Seebeck coefficient in PEDOT–PSS doped with 10 wt% AgNPs thin film is interpreted as mainly due to morphological changes in terms of improved alignment of polymer molecules or clusters and creation of more charge carriers with increased carrier mobility upon inclusion of AgNPs in PEDOT–PSS. The π–π stacking interaction between PEDOT chains with AgNPs induces an enhanced Seebeck coefficient and thermo power factor in doped PEDOT–PSS thin films. Among all the prepared thin films, the PEDOT–PSS thin film doped with 10 wt% of AgNPs displays higher Seebeck coefficient of the order of 25.2 μV/K. The conductivity (σ) can be related to carrier concentration, carrier charge, and carrier mobility respectively using σ = nqµ. The increased conductivity of PEG-treated PEDOT–PSS:AgNPs is expected to exhibit low Seebeck coefficient as they inversely depend on carrier concentration. The decreased values of Seebeck coefficient in PEG-treated nanocomposite film further confirms the increase in charge carriers of the system that supports enhanced conductivity.
The variation of thermo-electric power factor as a function of temperature-dependent part of the conductivity is shown in Fig. 13. It is observed that the thermo-electric power factor increases with the increasing content of AgNPs in PEDOT–PSS:PEG and this linear rise can be attributed to thermally assisted hopping of charge carriers. The value of thermo-electric power is recorded to be lowest for pure PEDOT–PSS thin film of 6 µW/mK2 and for PEDOT–PSS:PEG doped with 10 wt% AgNPs, the thermo-electric power factor was found to be 85 µW/mK2 which is found to be highest reported so far in the literature using a filler concentration of just 10 wt% without chemical treatment of organic thin films using strong acids. Superior thermo-electric properties of PEDOT–PSS:PEG thin film doped with AgNPs is attributed to the existence of strong π–π conjugation between AgNPs and PEDOT–PSS:PEG. The π–π conjugation interactions lead to ordering of the chain arrangement in PEDOT–PSS:PEG molecules with the surface of AgNPs, thereby reducing the defects in PEDOT–PSS:PEG molecular chain. However, these strong π–π interactions function as a bridge that facilitates charge carrier motion between PEDOT–PSS:PEG and AgNPs. These aspects aid to increase in charge carrier mobility resulting in enhanced conductivity and improved thermo-electric properties in PEDOT–PSS:PEG system.
Figure 14 indicates the variation of thermo power factor as a function of inverse hot junction temperature. In these plots, it has been noticed that the thermo power factor not only decreases gradually with temperature of hot junction, but also with the concentration of AgNPs in PEDOT–PSS:PEG thin films. Also, it can be observed that the oxidation level in PEDOT–PSS:PEG decreases at lower inverse hot junction temperature [38]. It has been observed that, upon doping AgNPs followed by PEG treatment in PEDOT–PSS, the thin film thickness decreases upto 10 wt% of AgNPs (as shown in Fig. 15) which may be due to the removal of excess of non-complexed PSS segments in PEDOT–PSS. For the AgNPs inclusion beyond 10 wt% (observed to be as threshold limit), the film thickness shows small increments, due to excessive loading of AgNPs in PEDOT–PSS:PEG, which needs further in-depth understanding.
4 Conclusions
In this work, we present a simple strategy to prepare PEDOT–PSS:AgNPs nanocomposites treated with PEG by bar coating technique, as an excellent material for thermo-electric applications. These organic thin film nanocomposites were characterized using SEM, AFM, FTIR, and TGA techniques. SEM and AFM images of the nanocomposite films reveals a modified surface morphology due to the presence of AgNPs. FTIR spectra of the nanocomposite films shows a strong dispersion of AgNPs in PEDOT–PSS:PEG matrix. TGA thermograms of the nanocomposite films confirm that AgNPs-doped PEDOT–PSS:PEG are thermally more stable compared to bare PEDOT–PSS thin films. Doping of AgNPs in PEDOT–PSS:PEG remarkably enhances the conductivity and dielectric attributes of the bare PEDOT–PSS by several orders. The increased charge transport behavior is mainly due to the conformational changes in the polymer backbone due to the addition of AgNPs. Addition of AgNPs in various concentrations and PEG treatment significantly enhances the thermo-electric performance of PEDOT–PSS organic thin films. These PEG-treated PEDOT–PSS:AgNPs nanocomposites exhibit thermo-electric power factor of 85 µW/mK2 with a Seebeck coefficient of 25.2 μV/K for the optimal concentration of PEDOT–PSS:PEG doped with 10 wt% of AgNPs. Due to low cost, ease of fabrication, enhanced conductivity, and excellent thermo-electric performance, these AgNPs-doped flexible PEDOT–PSS:PEG nanocomposites could be promising thermo-electric materials for designing flexible electronic devices operated at room temperature.
Change history
21 February 2023
This article has been retracted. Please see the Retraction Notice for more detail: https://doi.org/10.1007/s10854-023-10077-7
References
G.J. Snyder, E.S. Toberer, Complex thermoelectric materials. Nat. Mater. 7, 105 (2008)
C. Yu, Y.S. Kim, D. Kim, J.C. Grunlan, Thermoelectric behavior of segregated-network polymer nanocomposites. Nano Lett. 8, 4428 (2008)
Y. Chen, J. Xu, Y. Yang, S. Li, PEDOT–PSS/graphene/PEDOT ternary film for high performance electrochemical electrode. J. Mater. Sci. Mater. Electron (2015). https://doi.org/10.1007/s10854-015-3494-1
A. Feng, G. Wu, C. Pan, Y. Wang, The behavior of acid treating carbon fiber and the mechanical properties and the thermal conductivity of phenolic resin matrix composites. J. Nanosci. Nanotechnol. 17, 3786–3791 (2017)
D. Yoo, J. Kim, J.H. Kim, Direct synthesis of highly conductive poly(3,4-ethylenedioxythiophene):poly(4-styrenesulfonate) (PEDOT:PSS)/graphene composites and their applications in energy harvesting systems. Nano Res. 7(5), 717–730 (2014)
D. Lee, S. Sayed, S. Lee, C.A. Kuryak, J. Zhou, G. Chen, Y. Shao-Horn, Quantitative analyses of enhanced thermoelectric properties of modulation-doped PEDOT:PSS/undoped Si nano scale hetero structures. Nanoscale 8, 19754 (2016)
T.-C. Tsai, H.-C. Chang, C.-H. Chen, W.-T. Whang, Widely variable Seebeck coefficient and enhanced thermoelectric power of PEDOT–PSS films by blending thermal decomposable ammonium formate. Org. Electron. 12, 2159–2164 (2011)
S. Khasim, Y.K. Misra, N. Badi, A. Pasha, M Lakshmi Fabrication of high performance flexible supercapacitors using secondary doped PEDOT–PSS:graphene nanocomposite films for large area solid state devices. RSC Adv. 10, 10526–10539 (2020)
N. Toshima, S. Ichikawa, Conducting polymers and their hybrids as organic thermoelectric materials. J. Electron. Mater. 44(1), 384–390 (2015)
Y. Kalachyova, O. Guselnikova, P. Postnikov, P. Fitl, L. Lapcak, V. Svorcik, O. Lyutakov, Reversible switching of PEDOT:PSS conductivity in the dielectric-conductive range through the redistribution of light-governing polymer. RSC Adv. 8, 11198–11206 (2018)
D. Alemu, H.-Y. Wei, K.-C. Ho, C.-W. Chu, Highly conductive PEDOT:PSS electrode by simple film treatment with methanol for ITO-free polymer solar cells. Energy Environ. Sci. 5, 9662–9671 (2012)
D. Valtakari, J. Liu, V. Kumar, C. Xu, M. Toivakka, J.J. Saarinen, Conductivity of PEDOT–PSS on spin-coated and drop cast nano-fibrillar cellulose thin films. Nanoscale Res. Lett. 10, 386 (2015)
E. Liu, C. Liu, Z. Zhu, Preparation of poly(3,4 ethylenedioxythiophene):poly(4-styrenesulfonate)/silicon dioxide nanoparticles composite films with large thermoelectric power factor. J. Compos. Mater. 52(5), 621–627 (2018)
S. Liu, H. Deng, Y. Zhao, S. Ren, Q. Fu, The optimization of thermoelectric properties in a PEDOT:PSS thin film through post-treatment. RSC Adv. 5, 1910–1917 (2015)
J. Luo, D. Billep, T. Blaudeck, E. Sheremet, R.D. Rodriguez, Zahn DRT, Toader M, Hietschold M, Otto T, Gessner T, Chemical post-treatment and thermoelectric properties of poly (3,4-ethylenedioxythiophene):poly (styrenesulfonate) thin films. J. Appl. Phys. 115, 054908 (2014)
Y. Du, J. Xu, Y. Wang, T. Lin, Thermoelectric properties of graphite–PEDOT:PSS coated flexible polyester fabrics. J. Mater. Sci. Mater. Electron. 28, 5796–5801 (2017)
Y. Du, S.Z. Shen, K.F. Cai, P.S. Casey, Research progress on polymer–inorganic thermoelectric nanocomposite materials. Prog. Polym. Sci. 37, 820–841 (2012)
N. Saxena, J. Keilhofer, A.K. Maurya, P. Müller-Buschbaum, Facile optimization of thermoelectric properties in PEDOT:PSS thin films through acid–base and redox de doping using readily available salts. ACS Appl. Energy Mater. 1(2), 336–342 (2018)
Z. Fan, J. Ouyang, Thermoelectric properties of PEDOT–PSS. Adv. Electron. Mater. 12, 1234–1239 (2019)
J. Luo, D. Billep, T. Waechtler, T. Otto, M. Toader, O. Gordan, E. Sheremet, J. Martin, M. Hietschold, D.R.T. Zahn, Enhancement of the thermoelectric properties of PEDOT:PSS thin films by post-treatment. J. Mater. Chem. A 1, 7576 (2013)
W. Lee, Y.H. Kang, J.Y. Lee, K.-S. Jang, S.Y. Cho, Improving the thermoelectric power factor of CNT/PEDOT:PSS nanocomposite films by ethylene glycol treatment. RSC Adv. 6, 53339 (2016)
F.-P. Du, N.-N. Cao, Y.-F. Zhang, P. Fu, Y.-G. Wu, Z.-D. Lin, R. Shi, A. Amini, C. Cheng, PEDOT:PSS/graphene quantum dots films with enhanced thermoelectric properties via strong interfacial interaction and phase separation. Sci. Rep. 8, 6441 (2018)
X. Sun, Y. Wei, J. Li, J. Zhao, L. Zhao, Q. Li, Ultralight conducting PEDOT:PSS/carbon nanotube aerogels doped with silver for thermoelectric materials. Sci. China Mater. 60(2), 159–166 (2017)
A. Pasha, S. Khasim, O.A. Al-Hartomy, M. Lakshmi, K.G. Manjunatha, Highly sensitive ethylene glycol-doped PEDOT–PSS organic thin films for LPG sensing. RSC Adv. 8, 18074–18083 (2018)
F.X. Jiang, J.K. Xu, B.Y. Lu, Y. Xie, R.J. Huang, L.F. Li, Thermoelectric performance of poly (3,4-ethylenedioxythiophene):poly(styrenesulfonate). Chin. Phys. Lett. 25, 2202–2205 (2008)
B. Zhang, J. Sun, H.E. Katz, Fang F, Opila RL, Promising thermoelectric properties of commercial PEDOT–PSS materials and their Bi2Te3 powder composites. ACS Appl. Mater. Interfaces 2, 3170–3178 (2010)
I. Nuramdhani, A.T. Gokceoren, S.A. Odhiambo, G. De Mey, C. Hertleer, L. Van Langenhove, Electrochemical impedance analysis of a PEDOT:PSS-based textile energy storage device. Materials (Basel) 11(1), 48 (2018)
N. Massonnet, A. Carella, O. Jaudouin, P. Rannou, G. Laval, J.P. Simonato, Improvement of the Seebeck coefficient of PEDOT–PSS by chemical reduction combined with a novel method for its transfer using free-standing thin films. J. Mater. Chem. C 2, 12781283 (2014)
Q. Meng, K. Cai, Y. Du, Preparation and thermoelectric properties of SWCNT/PEDOT–PSS coated tellurium nano-rod composite films. J. Alloys Compds. 778, 163–169 (2019)
A. Yoshida, N. Toshima, Thermoelectric properties of hybrid thin films of PEDOT–PSS and silver nanowires. J. Electron. Mater. 45(6), 2914–2919 (2016)
S. Khasim, A. Pasha, A.S. Roy, A. Parveen, N. Badi, Effect of secondary doping using sorbitol on structure and transport properties of PEDOT–PSS thin films. J. Electron. Mater. 46(7), 4439–4447 (2017)
U. Lang, E. Muller, N. Naujoks, J. Dual, Microscopical investigations of PEDOT–PSS thin films. Adv. Funct. Mater. 19(8), 1215 (2009)
D.A. Mengistie, M.A. Ibrahem, P.C. Wang, Chu CW, Highly conductive PEDOT–PSS treated with formic acid for ITO-free polymer solar cells. ACS Appl. Mater. Interfaces 6, 2292 (2014)
M. Reyes-Reyes, I. Cruz-Cruz, R. Lopez-Sandoval, Enhancement of the electrical conductivity in PEDOT–PSS films by the addition of dimethyl sulfate. J. Phys. Chem. C 114, 20220–20224 (2010)
K. Jyoti, M. Baunthiyal, A. Singh, Characterization of silver nanoparticles synthesized using Urtica dioica Linn leaves and their synergistic effects with antibiotics. J. Radiat. Res. Appl. Sci. 9, 217–227 (2016)
D.A. Mengistie, M.A. Ibrahem, P.-C. Wang, C.W. Chu, Highly conductive PEDOT–PSS treated with formic acid for ITO-free polymer solar cells. ACS Appl. Mater. Interfaces 6(4), 1193–1199 (2014)
M.S. Mahajan, D.M. Marathe, S.S. Ghosh, V. Ganesan, J.V. Sali, Changes in in-plane electrical conductivity of PEDOT–PSS thin films due to electric field induced dipolar reorientation. RSC Adv. 5, 86393–86401 (2015)
O. Bubnova, Z.U. Khan, A. Malti, S. Braun, M. Fahlman, M. Berggren, X. Crispin, Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 10, 429433 (2011)
Acknowledgements
The authors would like to acknowledge financial support for this work, from the Deanship of Scientific Research (DSR), University of Tabuk, Tabuk, Saudi Arabia, under Grant No. S-1440-0267.
Author information
Authors and Affiliations
Contributions
Apsar Pasha and Syed Khasim contributed equally towards carrying out this research and designing the manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s10854-023-10077-7
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pasha, A., Khasim, S. RETRACTED ARTICLE: Highly conductive organic thin films of PEDOT–PSS:silver nanocomposite treated with PEG as a promising thermo-electric material. J Mater Sci: Mater Electron 31, 9185–9195 (2020). https://doi.org/10.1007/s10854-020-03448-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03448-x