Abstract
Several off-stoichiometric compositions Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.12, 0.14) were deliberately synthesized to produce in-situ composites based on compositional engineering approach. The structural characterization of these materials employing XRD, SEM, and HR-TEM reveals the formation of in-situ-composites containing Bi0.5Sb1.5Te3 as matrix phase and minor phases of either Sb rich or Te rich in different compositions. Thermoelectric properties of these Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.12, 0.14) composites were studied in a wide range of temperatures extending from room temperature to 500 K. The electronic transport of these composites exhibits p-type semiconducting materials. The lowest thermal conductivity of ~ 0.69 W/m K @310 K was observed for Bi0.5Sb1.7Te3.12 composite, which was noted to be 14% reduced thermal conductivity when compared with that of the state-of-the-art Bi0.5Sb1.5Te3 (κ\(=\) 0.82 W/m K) material. In addition to this, an enhanced power factor was also observed in Bi0.5Sb1.7Te3.12 which is primarily due to increased electrical conductivity of these materials. This enhanced power factor of the composition of Bi0.5Sb1.7Te3.12 coupled with reduced thermal conductivity yields to high ZT ~ 1.13 at nearly room temperature, making these materials viable for large scale applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Thermoelectric (TE) technology enables solid-state cooling in refrigeration, air conditioning devices, and electronic devices by using small Peltier devices. For the use of TE devices at a large scale, improvement in the performance of thermoelectric materials is a prerequisite. TE performance of materials can be computed by a dimensionless quantity, called figure of merit (ZT). Figure of merit (ZT) = (α2σ/κ) T where σ, α, and κ are the electrical conductivity, Seebeck coefficient, and total thermal conductivity respectively at the absolute temperature (T) [1]. High ZT can be optimized by decoupling of α, σ and κ as they have close interdependences. Solid solution alloying [2, 3], band engineering [4,5,6,7], mass fluctuation [8,9,10,11], nanostructuring/nanocomposites [12,13,14], doping [15,16,17], phonon-glass electron-crystal (PGEC) [18, 19] and defect engineering [20,21,22], have been implemented in order to enhance the value of ZT up to a certain extent.
Among all thermoelectric materials, Bi2Te3 is known as potential state-of-the-art thermoelectric materials exhibiting a typical narrow-gap semiconductor which has been extensively studied in regard to the investigation of thermoelectric properties. It exhibits rhombohedral structure (R−3 m, 166) consisting of three 2D quintuple layers of Te(2)–Bi–Te(1)–Bi–Te(2) along c axis. Ternary Bi0.5Sb1.5Te3 alloy has been shown so far the best commercial p-type materials for low-temperature applications with peak of ZT 1.1 near 300 K [23].When it is prepared with conventional and different processing methods [24, 25] such as nanostructuring/nanocomposites results to a different value of ZT. The maximum value of ZT ~ 1.8 at 316 K was realized in composition Bi0.4Sb1.6Te3 [26], through rapid solidification by melt-spinning. Thus, composition fluctuation in this ternary composition of Bi0.5Sb1.5Te3 coupled with various powder metallurgical engineering has been found to increase ZT significantly [27,28,29]. Usually replacing Sb at Bi site in Bi0.5Sb1.5Te3, creates an atomic disorder of Bi-Sb which scatters heat-carrying high-frequency phonons to reduce the κL, thereby increased ZT has been observed [30]. Furthermore, recently nanostructuring/nanocomposites have been used as an efficient strategy to improve ZT via reducing thermal conductivity through grain boundary and interface boundary scattering of phonons in this family of compound [31, 32]. However, reasonably high ZT (≥ 2) in Bi0.5Sb1.5Te3 bulk materials could not be get for its thermoelectric applications at large scale. Recently a remarkable finding by Kim et al. [33] producing a record high ZT ~ 1.85 at 320 K in 25wt% excess Te in Bi0.5Sb1.5Te3 was observed, with the hope for the TE industry to develop TE module for cooling applications. However, it had further been noticed by other research group [34] that such high recorded ZT ~ 1.85 could not be reproduced. A synergetic optimization of power factor and a drastic reduction in thermal conductivity may lead to an expected result of achieving ZT ≥ 2. With this in mind, our critical observation of low σ ~ 400S/cm of Bi0.5Sb1.5Te3 at room temperature [35] provides us an impetus to reasonably increase the electrical conductivity while either maintaining or increasing the Seebeck coefficient and reducing the thermal conductivity for realizing high yield of ZT.
Initially, we have taken excess Sb; Bi0.5Sb1.7Te3 to increase hole concentration for high electrical conductivity. The increase in hole concentration can be realized because the difference in size and electronegativity between Sb–Te is much smaller than that of between Bi–Sb and Bi-Te, which makes Sb preferable to occupy the site as proposed by χ−r model [36]. Also, occupying Sb to Te site adds holes as Sb3+gets replaced with Te2−. Further, in order to optimize the Seebeck coefficient simultaneously with electrical conductivity in nominal composition Bi0.5Sb1.7Te3, off-stoichiometric compositions Bi0.5Sb1.7Te3+δ (δ = 0.12, 0.14) with an excess of Te were synthesized. Interestingly, the composition Bi0.5Sb1.7Te3 with an excess of Sb significantly increases electrical conductivity-parallel- i.e., σ// ~ 1200 S/cm at room temperature which is 200% larger than that of commercially reported Bi0.5Sb1.5Te3 composition by Hao et al. [35]. However, α was found to be ~ 51% decreased (α// ~ 110 μV/K) in comparison to that of Bi0.5Sb1.5Te3(α//\(=\) 225 μV/K at 310 K) [35] but excess Sb and Te together in this work give synergistic effect to control over σ & α. Hence, increase in power factor along with reduced κ yielding a high ZT ~ 1.13 at room temperature.
2 Experimental details
2.1 Materials synthesis
Several stoichiometric compositions Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.12, 0.14) were measured by taking high purity elements Sb powder (99%), Bi powder (99.9%), and Te powder (99%). The mixed powders were then sealed in a quartz tube in a vacuum at a pressure of 10−5 Torr. The sealed powders were further reacted in a furnace at 800 °C for 8 h and subsequently, water quenched at room temperature. The molten ingots were then grounded into fine powders for 15 min. These powder materials were then loaded into 12.7 mm diameter of graphite die and sintered by SPS at 673 K, pressure of 50 MPa with holding time of 3 min.
2.2 Phase and microstructure characterization
In order to investigate phase formation, powder X-ray diffractometer (PXRD, Model: Rigaku Mini Flex II) operated at 30 kV and 20 mA using Cu-Kα(λ\(=\) 1.54 Å) has been performed on all composite materials. For microstructure investigation, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used. Energy dispersive spectroscopy (EDS) was performed for composition and elemental analysis.
2.3 Thermoelectric property measurement
Laser Flash Analysis (Lineseis, LFA 1000) was performed on circular disc samples with a diameter of 12.7 mm and thickness of 1.5 mm for thermal diffusivity. For the estimation of specific heat, differential scanning calorimetry (DSC) (822e Mettler Toledo) is used. Further, thermal conductivity was estimated by using the relation κ\(=\)d × Cp × ρ, where d, Cp and ρ are diffusivity, specific heat, and density, respectively. The density (ρ) of all composites was found to be > 95% which was estimated using the Archimedes principle. The Seebeck coefficient and resistivity were measured by ULVAC, ZEM-3 from room temperature to 500 K.
3 Results and discussion
3.1 XRD analysis
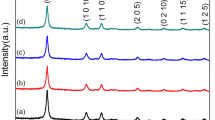
Figure 1a shows the X-ray diffraction for samples Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.12, 0.14). All the peaks are primarily indexed with Bi2Te3 type rhombohedral structure together with a trace amount of either Sb or Te. It is envisaged from XRD (Fig. 1a) that the peak positions corresponding to a rhombohedral structure are found to be slightly shifted towards a low angle side indicating an increase in a and c. The Rietveld analysis was performed on all the samples to identify the phases and accurate estimation of lattice parameters and the Rietveld refinement corresponding to best-optimized Bi0.5Sb1.7Te3.12 composition is presented in Fig. 1b. Variation of a and c parameters with increasing Te is presented in Fig. 1c. From Fig. 1c it is evident that the excess Te does not lead to the formation of a single solid solution phase as the trend does not follow the straights line of Vegard’s law for solid solution phase formation. Evidently, increasing Te concentration increases the c-parameter with a slight change in 2ϴ. It is important to point out that an increase in the c-parameter may be attributed to the Te occupied in the interstitial site between Te(1)–Te(1) Vander Waal gap.
In order to investigate the surface morphology and composition analysis, we have performed scanning electron microscopy (SEM), presented in the supporting information S1. SEM investigation reveals the formation of a composite phase containing a matrix phase of Bi0.5Sb1.5Te3 and the minor phases of Sb and Te which is evident in supporting information (S1) on SEM investigation.
3.2 Transmission electron microscopy investigation
High-resolution of the best performing composition Bi0.5Sb1.7Te3.12 was performed to examine more closely at the microstructure. A bright-field image obtained from the sample Bi0.5Sb1.7Te3.12 is shown in Fig. 2a with grains size ranging from 100 to 200 nm. The enlarged view of the area (marked red circle in Fig. 2a) is shown in Fig. 2b. The high density of dislocation at the grain boundaries is found in this region. The enlarged view of the white box in Fig. 2b is presented in Fig. 2c. The d spacings are measured in two similar oriented grains marked as region 1 and region 2 (dotted circle). The measured d spacing in these two regions is noticed to be 3.01 Å and 3.15 Å. The difference in d spacing might have caused due to dislocation at the interface of both grains. Selected area electron diffraction (SAED) was recorded from the grains shown in Fig. 2d along [− 1 1 − 11] zone axis corresponding to R − 3 m (166) structure. EDAX spectrum and quantification obtained from Bi0.5Sb1.7Te3.12 are shown in Fig. 2e indicates all the elements are present and the presence of Cu was also noted which is appearing from Cu-grid. Quantification of EDAX analysis (inset Fig. 2e) reveals the composition close to the nominal composition with a slight variation in Bi concentration, which is noticed within the instrumental error.
a Bright field electron micrograph of the Bi0.5Sb1.7Te3.12 composite displaying the dislocation embedded at the grain boundary (red circle); b enlarged view of circled region in (a); showing dislocation more clearly (red arrow) (c) HR-TEM image showing Misfit type of dislocation at interface; d HR-TEM image with inset of corresponding SAED confirming rhombohedral structure with a \(=\) 4.31 and c \(=\) 30.51 along zone axis [− 1 1 − 11] and e EDAX spectrum and quantification obtained from Bi0.5Sb1.7Te3.12 revealing the composition close to the nominal composition (Color figure online)
3.3 Hall measurement
Carrier concentration is an important parameter to be analyzed to understand the thermoelectric properties. Table 1 represents the room temperature hall coefficient measurement. The carrier concentration was estimated by using nH = \(\frac{1}{{R}_{H}e}\) and mobility by σ = neμ where μ = mobility, e = electron charge and σ = electrical conductivity and RH = hall coefficient. For composition with excess Sb; Bi0.5Sb1.7Te3, the carrier concentration nH\(=\) 3.2 × 1019/cm3 at room temperature, was observed which is about 52% larger than the commercially reported compound Bi0.5Sb1.5Te3 (nH\(=\) 2.1 × 1019/cm3) [35]. Further, on increasing the Te content; Bi0.5Sb1.7Te3.12 the carrier concentration decreases than the nominal composition Bi0.5Sb1.7Te3 due to increase in TeSb antisite defects as shown in Table 1. However, surprisingly, excess Te; the Bi05Sb1.7Te3.12 exhibits the much higher mobility (μ\(=\) 309.6 cm2 V−1 s−1) than the matrix Bi0.5Sb1.5Te3 (μ\(=\) 120 cm2 V−1 s−1) [35].
3.4 Electronic transport properties
Figure 3 depicts electrical conductivity (a, b) and Seebeck coefficient (c, d) measured parallel and perpendicular to pressing direction (α) of Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.12, 0.14) samples with increasing temperature. The results are also compared with that of commercially reported Bi0.5Sb1.5Te3 [35] alloy. All samples exhibit a positive value of the Seebeck coefficient in the entire temperature range suggesting holes to be the majority charge carrier. Interestingly, the composition with excess Sb; Bi0.5Sb1.7Te3, exhibits significantly increased electrical conductivity in both direction (σ// ~ 1200 S/cm and σ// ~ 2400 S/cm) at room temperature) which is 200% higher than that of commercially reported Bi0.5Sb1.5Te3 (σ// ~ 400 S/cm) [35] material in the parallel direction. This enhancement in electrical conductivity can be attributed to enhanced nH (3.2 × 1019 cm−3) due to increased SbTe antisite defects and also to increased mobility(μ\(=\) 456 cm2 V−1 s−1) due to reduced alloy scattering similar to other reports [37, 38]. It can be emphasized that the scattering and mobility of carriers may be co-related based on the Mathiessien rule [39]. In normal doped semiconductors, there are two contributions namely acoustic (μAC) and the impurities (μI) scattering and the total mobility can be given by Matthiessen’s rule,
where \({\mu }_{I}\) and \({\mu }_{AC}\) is the mobility for ionized impurity scattering and acoustic phonon scattering, respectively.
In Bi2Te3-based materials, though both \({\mu }_{I}\) and \({\mu }_{AC}\) contribute to overall mobility. However, Bi2Te3-based materials involve lots of defects, so ionized impurity scattering plays an important role in overall mobility. Therefore, we have estimated the \({\mu }_{I}\) of ionized scattering as suggested by Li et al. [35]
Herein, NI is the ionized impurity concentration, \({m}_{s}^{*}\) is a single valley effective mass given by m*\(={N}_{v}^{2/3}{m}_{s}^{*}\) where Nv is valence band degeneracy, ɛ is the dielectric constant. Dimensionless f (LD) parameter given above can be expressed as
where LD, ħ is Debye screen length and reduced Planck constant respectively and LD is defined as [35]:
The estimated value of LD by using the above relation for all our compositions is shown in Fig. 4. It is worth mentioning that short LD corresponds to low coulomb potential around ionized impurity as suggested by Li et al. [35] which in turn gives a weak effect on carrier transport. Based on this fact, we can notice the lowest value of LD in our nominal composition Bi0.5Sb1.7Te3 which results to maximum mobility and this could be due to smaller coulomb potential similar to the earlier reports [35].
Further with increasing Te concentration, the σ decreases in the entire measured temperature as compared to Bi0.5Sb1.7Te3 in both directions. The composition with excess Te; Bi0.5Sb1.7Te3.12 exhibits the electrical conductivity (σ//) measured parallel to the pressing direction to be of ~ 658 S/cm which is 64% larger than the commercially reported Bi0.5Sb1.5Te3 compound. On increasing the Te concentration further; for instance in the case of Bi0.5Sb1.7Te3.14the electrical conductivity was noted to be almost the same as in the case of Bi0.5Sb1.7Te3.12 for both directions. The Seebeck coefficient measured in parallel and perpendicular to the pressing direction of Bi0.5Sb1.7Te3 in the entire temperature range is presented in Fig. 3c, d. On increasing the Te concentration; for Bi0.5Sb1.7Te3.12 and Bi0.5Sb1.7Te3.14, the Seebeck coefficient is observed to be increased in parallel to pressing direction as well as in perpendicular to pressing direction [Fig. 3c, d] in comparison to that of nominal composition Bi0.5Sb1.7Te3 [35]. For instance, the Seebeck coefficient of best-optimized composition Bi0.5Sb1.7Te3.12 exhibits α// ~ 195 μV/K at room temperature which is much larger than α// ~ 110 μV/K for nominal composition Bi0.5Sb1.7Te3.
The rationale behind the increasing α on increasing the Te concentration in Bi0.5Sb1.7Te3.12 and Bi0.5Sb1.7Te3.14 as compared to the nominal composition Bi0.5Sb1.7Te3 of present work can be explained through a model which was proposed by Nolas et al. [40] for the Seebeck coefficient. This model relates the scattering factor with reduced Fermi energy which can be given as
where, KB, ξ, r is the Boltzmann constant, reduced Fermi energy and scattering factor, respectively. The carrier concentration is related to reduced Fermi energy and for samples Bi0.5Sb1.7Te3.12 and Bi0.5Sb1.7Te3.14, the low value of carrier concentration corresponds to reduced Fermi energy resulting in a high value of Seebeck coefficient as compared to the nominal composition Bi0.5Sb1.7Te3 in the present study.
In order to further understand the transport behavior of the present composites, we have plotted the Seebeck coefficient with carrier density, i.e., Pisarenko plot, obtained from a simple electron transport model, given below:
The Fig. 5 shows the Pisarenko plot for compositions Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.12, 0.14). The Seebeck coefficient for a given temperature is explained using a single parabolic band (SPB) based on effective mass (m*) due to single charge carriers. The density of states effective mass (m*) was estimated from the Seebeck coefficient and carrier concentration by using the above equation. The estimated effective mass for all samples is given in Table 1. We observed from the Pisarenko plot that, the value of m* for composites does not lie on the single parabolic curve indicating that the composites exhibit different effective mass and hence they do not follow the SPB. Thus, excess Sb and Te significantly alter the electronic band structure close in proximity of the Fermi level and hence the transport properties are significantly modified.
Figure 6 shows the power factor measured parallel to pressing direction and perpendicular to pressing direction (PF = α2σ) of Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.2, 0.4) samples with increasing temperature. The parallel power factor (PF//) of present derived compositions Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.2, 0.4) is observed to be significantly increased than that of commercially reported Bi0.5Sb1.5Te3 [35]. The enhancement in power factor in the entire temperature range is primarily due to increased electrical conductivity. Interestingly, at room temperature, the PF// for best-optimized composition Bi0.5Sb1.7Te3.12 is 25.5 × 10–4 W/m K2 which is 16% larger than that of the commercially reported Bi0.5Sb1.5Te3 compound (PF// ~ 22 × 10–4 W/m K2) [35].
3.5 Thermal transport properties
The thermal conductivity (κ) is another important property to be studied for the estimation of ZT. Figure 7a, b represents the temperature-dependent thermal conductivity of Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.2, 0.4) alloys measured parallel as well as perpendicular to pressing direction. We have also compared the thermal conductivity measured in the parallel direction (κ//) with the reported values measured in the parallel direction of commercially Bi0.5Sb1.5Te3 [35] material. The total thermal conductivity for all samples increases with increasing temperature in both directions which is consistent with temperature dependence electrical conductivity and Seebeck coefficient. Interestingly, the parallel thermal conductivity of optimized composition Bi0.5Sb1.7Te3.12 exhibits much reduced thermal conductivity compared to that of commercially reported Bi0.5Sb1.5Te3 [35] and lowest value of κ// found to be 0.7 W/m K at 311 K for best performing composition Bi0.5Sb1.7Te3.12 which is 14% smaller than that of commercial composition Bi0.5Sb1.5Te3(κ// ~ 0.8 W/m K) [35]. The total thermal conductivity has three contributions i.e., lattice thermal conductivity (κL), electronic thermal conductivity (κe) and bipolar thermal conductivity (κbipolar). For Bi2Te3-based TE materials, an inverse relationship between κL and T(\(\kappa_{L} { = }aT^{ - 1} + b\)) above the Debye temperature (160 K) follows the Umklapp scattering mechanism [41]. Here we have estimated the lattice thermal conductivity κL (by subtracting the electronic thermal conductivity κe\(=\)LσT where L is the Lorentz number, here L\(=\) 1.6 × 10–8/V2K2 [33] is considered in the present study) from total thermal conductivity over the temperature in which intrinsic excitation is neglected. Then, we calculated the κL for higher temperature according to the relation \(\kappa_{L} { = }aT^{ - 1} + b\) and κbipolar using equation κtotal = κL + κe+κBipolar which are shown in Fig. 7c–f. Interestingly, with increasing the Te concentration the value of lattice thermal conductivity decreases and reached the lowest parallel lattice thermal conductivity ~ 0.40 W/ m K for Bi0.5Sb1.7Te3.12, at room temperature which is about 33% lower than that of commercially reported compound Bi0.5Sb1.5Te3 (κ//\(=\) 0.6 W/m K at 300 K). The reduced lattice thermal conductivity in this alloy is attributed to the scattering of phonons from grain boundaries, defects, dislocations and interfaces produced by excess Te.
3.6 Thermoelectric figure of merit (ZT)
The estimated value of parallel and perpendicular ZT are displayed in Fig. 8a, b. As shown in Fig. 8a, the highest ZT// ~ 1.13 near room temperature was achieved for Bi0.5Sb1.7Te3.12 which is about 60% larger compared to the commercially reported Bi0.5Sb1.5Te3 (ZT//\(=\) 0.71 at 310 K) [35]. Such high ZT ~ 1.13 at room temperature makes it feasible to develop thermoelectric devices because maintaining the room temperature gradient between hot and cold end is easier than maintaining the temperature gradient more than 100 °C in the commercially available thermoelectric materials.
This favorable situation might have occurred due to unusual electronic transport due to excess Te. Further, unusual increase of m* and unusual decrease in μ in the present work derives a new measuring parameter, ie. quality factor (B) [42] which governs the thermoelectric figure of merit. The quality factor basically originates from the parameters associated with the electronic and lattice structure, namely, m*, μ, and κL and defined by
where μκL and m* is the carrier mobility, lattice thermal conductivity, and effective mass, respectively. The estimated quality factor for all compositions is plotted with their corresponding ZT. Evidently, the increasing quality factor results in increasing the ZT (Fig. 9) which is consistent with the model [42].
3.7 Thermoelectric compatibility factor
Thermoelectric compatibility has been computed by Snyder et al. [43] using a relation \(s= \frac{\sqrt{\left(1+ZT\right)}-1}{\alpha T}\) to find the applicability of a segment of thermoelectric materials with other thermoelectric segment for better efficiency. Herein, we have also estimated the compatibility factor(s) for Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.12, 0.14) (Fig. 10) to have an understanding for the suitability of these materials to be deployed with another counterpart in making a thermoelectric couple for maximum performance of the device. It can be noticed that the compatibility factor of Bi0.5Sb1.7Te3.12 is 7.61 V−1 at 310 K which is favorable as suggested by Snyder et al. [43] for maximum efficiency [44].
4 Conclusion
Novel in-situ composites have been synthesized by varying stoichiometric Bi0.5Sb1.5+xTe3+δ (x = 0.2; δ = 0, 0.12, 0.14) compositions based on compositional engineering approach. The addition of an excess amount of Sb in a nominal composition Bi0.5Sb1.7Te3 primarily brings the system of significantly increased electrical conductivity. Further, excess Te incorporation in such derived Bi0.5Sb1.7Te3 composition leads to synergistically modify the Seebeck coefficient and thermal conductivity. The optimum combination of Sb and Te in present compositions Bi0.5Sb1.5 +xTe3+δ (x = 0.2; δ = 0, 0.12, 0.14) leads to modify the materials into composites with unusual electronic transport behavior favorable to high ZT. A remarkably high ZT ~ 1.13 at room temperature was optimized in the best performing composition Bi0.5Sb1.7Te3.12. The enhanced ZT is attributed due to synergistically optimized large power factor (25.5 × 10–4 W/m K2) and reduced thermal conductivity (0.69 W/m K at 311 K). This approach of compositional modification to synthesize in-situ composites provides excellent means of twinning the thermoelectric parameters. Further, it may be thought that controlled compositional engineering in Bi2Te3-based thermoelectric materials can lead to a result even higher ZT than ZT ~ 1.13 at room temperature. We strongly believe that such ZT materials at room temperature will enable them to be deployed for thermoelectric devices for power generation/cooling on a large scale.
References
G.J. Snyder, E.S. Toberer, Complex thermoelectric materials. Nat. Mater. 7, 105–114 (2008). https://doi.org/10.1038/nmat2090
M.E. Anderson, S.S.N. Bharadwaya, R.E. Schaak, Modified polyol synthesis of bulk-scale nanostructured bismuth antimony telluride. J. Mater. Chem. 20, 8362–8367 (2010). https://doi.org/10.1039/C0JM01424A
S. Dutta, V. Shubha, T.G. Ramesh, Reduction in thermal conductivity of Bi-Te alloys through grain refinement method. Bull. Mater. Sci. 36, 853–857 (2013). https://doi.org/10.1007/s12034-013-0532-6
Y. Zheng, Q. Zhang, X. Su, H. Xie, S. Shu, T. Chen, G. Tan, Y. Yan, X. Tang, C. Uher, Mechanically robust BiSbTe alloys with superior thermoelectric performance: a case study of stable hierarchical nanostructured thermoelectric materials. Adv. Energy Mater. 5, 1401391 (2015)
Y. Zhang, X. Jia, H. Sun, B. Sun, B. Liu, H. Liu, L. Kong, H. Ma, Enhanced thermoelectric performance of nanostructured CNTs/BiSbTe bulk composite from rapid pressure-quenching induced multi-scale microstructure. J. Mater. 2, 316–323 (2016)
Y. Pei, X. Shi, A. LaLonde, H. Wang, L. Chen, G.J. Snyder, Convergence of electronic bands for high performance bulk thermoelectrics. Nature 473, 66–69 (2011). https://doi.org/10.1038/nature09996s
M. Cho, Two-dimensional optical spectroscopy (CRC Press, Boca Raton, 2009). https://doi.org/10.1201/9781420084306
C. Fu, S. Bai, Y. Liu, Y. Tang, L. Chen, X. Zhao, T. Zhu, Realizing high figure of merit in heavy-band p-type half-Heusler thermoelectric materials. Nat. Commun. 6, 8144 (2015)
Q. Zhang, J. He, T.J. Zhu, S.N. Zhang, X.B. Zhao, T.M. Tritt, High figures of merit and natural nanostructures in Mg 2 Si 0.4 Sn 0.6 based thermoelectric materials. Appl. Phys. Lett. 93, 102109 (2008)
M. Guan, K. Zhao, P. Qiu, D. Ren, X. Shi, L. Chen, Enhanced thermoelectric performance of quaternary Cu2–2XAg2XSe1–XSX Liquid-like Chalcogenides. ACS Appl. Mater. Interfaces 11, 13433–13440 (2019)
N.S. Chauhan, S. Bathula, B. Gahtori, Y.V. Kolen’ko, A. Dhar, Enhanced thermoelectric performance in Hf-Free p-type (Ti, Zr) CoSb half-heusler alloys. J. Electron. Mater. 48, 6700–6709 (2019)
Y. Du, K.F. Cai, S. Chen, P. Cizek, T. Lin, Facile preparation and thermoelectric properties of Bi2Te3 based alloy nanosheet/PEDOT: PSS composite films. ACS Appl. Mater. Interfaces 6, 5735–5743 (2014)
X.B. Zhao, X.H. Ji, Y.H. Zhang, T.J. Zhu, J.P. Tu, X.B. Zhang, Bismuth telluride nanotubes and the effects on the thermoelectric properties of nanotube-containing nanocomposites. Appl. Phys. Lett. 86, 62111 (2005)
W. Xie, J. He, H.J. Kang, X. Tang, S. Zhu, M. Laver, S. Wang, J.R.D. Copley, C.M. Brown, Q. Zhang, Identifying the specific nanostructures responsible for the high thermoelectric performance of (Bi, Sb)2Te3 nanocomposites. Nano Lett. 10, 3283–3289 (2010)
B. Chen, J. Li, M. Wu, L. Hu, F. Liu, W. Ao, Y. Li, H. Xie, C. Zhang, Simultaneous enhancement of the thermoelectric and mechanical performance in one-step sintered n-type Bi2Te3-based alloys via a facile MgB2 doping strategy. ACS Appl. Mater. Interfaces 11, 45746–45754 (2019)
M.U. Muzaffar, B. Zhu, Q. Yang, Y. Zhou, S. Zhang, Z. Zhang, J. He, Suppressing bipolar effect to broadening the optimum range of thermoelectric performance for p-type bismuth telluride–based alloys via calcium doping. Mater. Today Phys. 9, 100130 (2019)
Z. Wang, Y. Yokoyama, T. Onda, Y. Adachi, Z. Chen, Improved thermoelectric properties of hot-extruded Bi–Te–Se bulk materials with Cu doping and property predictions via machine learning. Adv. Electron. Mater. 5, 1900079 (2019)
P.-C. Gharleghi, F.-H. Hung, C.-J. Lin, Liu, Enhanced ZT of In x Co4Sb12–InSb nanocomposites fabricated by hydrothermal synthesis combined with solid-vapor reaction: a signature of phonon-glass and electron-crystal materials. ACS Appl. Mater. Interfaces 8, 35123–35131 (2016)
T. Zhu, Y. Liu, C. Fu, J.P. Heremans, J.G. Snyder, X. Zhao, Compromise and synergy in high-efficiency thermoelectric materials. Adv. Mater. 29, 1605884 (2017)
Q. Zhang, B. Gu, Y. Wu, T. Zhu, T. Fang, Y. Yang, J. Liu, B. Ye, X. Zhao, Evolution of the intrinsic point defects in bismuth telluride-based thermoelectric materials. ACS Appl. Mater. Interfaces 11, 41424–41431 (2019)
K.T. Kim, T.S. Min, S.-D. Kim, E.-A. Choi, D.W. Kim, S.-Y. Choi, Strain-mediated point defects in thermoelectric p-type bismuth telluride polycrystalline. Nano Energy 55, 486–493 (2019)
J. Li, C. Zhang, Y. Feng, C. Zhang, Y. Li, L. Hu, W. Ao, F. Liu, Effects on phase transition and thermoelectric properties in the Pb-doped GeTe-Bi2Te3 alloys with thermal annealing. J. Alloys Compd. 808, 151747 (2019)
W.M. Yim, E.V. Fitzke, F.D. Rosi, Thermoelectric properties of Bi2Te3-Sb2Te3-Sb2Se3 pseudo-ternary alloys in the temperature range 77 to 300°K. J. Mater. Sci. 1, 52–65 (1966). https://doi.org/10.1007/BF00549720
D.M. Rowe, CRC handbook of thermoelectrics (CRC Press, Boca Raton, 2018)
B. Poudel, Q. Hao, Y. Ma, Y. Lan, A. Minnich, B. Yu, X. Yan, D. Wang, A. Muto, D. Vashaee, X. Chen, J. Liu, M.S. Dresselhaus, G. Chen, Z. Ren, High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 320, 634–638 (2008). https://doi.org/10.1126/science.1156446
S. Fan, J. Zhao, J. Guo, Q. Yan, J. Ma, H.H. Hng, p-type Bi 0.4 Sb 1.6 Te 3 nanocomposites with enhanced figure of merit. Appl. Phys. Lett. 96, 182104 (2010)
S.V. Ovsyannikov, N.V. Morozova, I.V. Korobeinikov, L.N. Lukyanova, A.Y. Manakov, A.Y. Likhacheva, A.I. Ancharov, A.P. Vokhmyanin, I.F. Berger, O.A. Usov, V.A. Kutasov, V.A. Kulbachinskii, T. Okada, V.V. Shchennikov, Enhanced power factor and high-pressure effects in (Bi, Sb)2(Te, Se)3 thermoelectrics. Appl. Phys. Lett. 106, 3–8 (2015). https://doi.org/10.1063/1.4916947
Y.Q. Cao, X.B. Zhao, T.J. Zhu, X.B. Zhang, J.P. Tu, Syntheses and thermoelectric properties of Bi2 Te3 Sb2 Te3 bulk nanocomposites with laminated nanostructure. Appl. Phys. Lett. (2008). https://doi.org/10.1063/1.2900960
J.J. Shen, T.J. Zhu, X.B. Zhao, S.N. Zhang, S.H. Yang, Z.Z. Yin, Recrystallization induced in situ nanostructures in bulk bismuth antimony tellurides: a simple top down route and improved thermoelectric properties. Energy Environ. Sci. (2010). https://doi.org/10.1039/c0ee00012d
H.J. Goldsmid, Electronic refrigeration (Pion, London, 1986), pp. 57–87
B.H. Poudel, Q. Ma, Y. Lan, Y. Minnich, A. Yu, B. Yan, X. Wang, D. Muto, A. Vashaee, D. Chen, X. Liu, J. Dresselhaus, M.S. Chen, and Ren Z, High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 320, 634–638 (2008)
Y. Ma, Q. Hao, B. Poudel, Y. Lan, B. Yu, D. Wang, G. Chen, Z. Ren, Enhanced thermoelectric figure-of-merit in p-type nanostructured bismuth antimony tellurium alloys made from elemental chunks. Nano Lett. 8, 2580–2584 (2008)
S. Il Kim, K.H. Lee, H.A. Mun, H.S. Kim, S.W. Hwang, J.W. Roh, D.J. Yang, W.H. Shin, X.S. Li, Y.H. Lee, G.J. Snyder, S.W. Kim, Dense dislocation arrays embedded in grain boundaries for high-performance bulk thermoelectrics. Science 348, 109–114 (2015). https://doi.org/10.1126/science.aaa4166
R. Deng, X. Su, Z. Zheng, W. Liu, Y. Yan, Q. Zhang, V.P. Dravid, C. Uher, M.G. Kanatzidis, X. Tang, Dense dislocation arrays at grain boundaries. Sci. Adv. 4, eaar5606 (2018)
P. Lu, L. Chen, H. Chen, F. Hao, D. Ren, Q. Song, P. Qiu, X. Shi, Roles of Cu in the enhanced thermoelectric properties in Bi0.5Sb1.5Te3. Materials (Basel). 10, 251 (2017). https://doi.org/10.3390/ma10030251
Z. Xu, H. Wu, T. Zhu, C. Fu, X. Liu, L. Hu, J. He, J. He, X. Zhao, Attaining high mid-temperature performance in (Bi, Sb)2Te3 thermoelectric materials via synergistic optimization. NPG Asia Mater. 8, e302 (2016)
W.M. Yim, F.D. Rosi, Compound tellurides and their alloys for peltier cooling—a review. Solid State Electron. 15, 1121–1140 (1972)
W.M. Yim, E.V. Fitzke, F.D. Rosi, Thermoelectric properties of Bi2Te3-Sb2Te3-Sb2Se3 pseudo-ternary alloys in the temperature range 77 to 300 K. J. Mater. Sci. 1, 52–65 (1966)
E.S. Zevalkink, W.G. Toberer, E. Zeier, G.J. Flage-Larsen, Snyder, Ca3AlSb3: an inexpensive, non-toxic thermoelectric material for waste heat recovery. Energy Environ. Sci. 4, 510–518 (2011). https://doi.org/10.1039/C0EE00517G
G.S. Nolas, J. Sharp, J. Goldsmid, Thermoelectrics: basic principles and new materials developments (Springer, New York, 2013)
F. Hao, P. Qiu, Y. Tang, S. Bai, T. Xing, H.-S. Chu, Q. Zhang, P. Lu, T. Zhang, D. Ren, High efficiency Bi2 Te3-based materials and devices for thermoelectric power generation between 100 and 300 C. Energy Environ. Sci. 9, 3120–3127 (2016)
G.J. Snyder, T.S. Ursell, Thermoelectric efficiency and compatibility. Phys. Rev. Lett. 91, 148301 (2003)
G.J. Snyder, Application of the compatibility factor to the design of segmented and cascaded thermoelectric generators. Appl. Phys. Lett. 84, 2436–2438 (2004)
E. Maciá, Compatibility factor of segmented thermoelectric generators based on quasicrystalline alloys. Phys. Rev. B 70, 100201 (2004)
Acknowledgements
Authors highly acknowledge Director, NPL, and Head of Department, Division of Indian Reference Materials, NPL, New Delhi for their constant encouragement and support. One of the authors, SB, greatly acknowledges the financial support from UGC. The authors acknowledge Dr. J.J. Pulikkotil for useful discussion.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bano, S., Kumar, A., Govind, B. et al. Room temperature Bi2Te3-based thermoelectric materials with high performance. J Mater Sci: Mater Electron 31, 8607–8617 (2020). https://doi.org/10.1007/s10854-020-03396-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-020-03396-6