Abstract
Ceria-based electrolyte powders consisting of Fe2O3 and Sm2O3 double-doped ceria (Ce0.8Sm0.2−xFexO2−δ) were synthesized by a sol–gel method. The structure and electrochemical characters of the electrolyte materials have been studied. The phase formation of precursor powders was studied by the X-ray diffraction analysis (XRD). Microstructural and sinterability measurements were carried out on the sintered electrolyte discs. Electrochemical impedance spectroscopy (EIS) was used for estimate the electrochemical properties. The results displayed that crystalline cubic fluorite structured is formed after calcined at 700 °C. The electrochemical analysis results showed that the electrical properties of Ce0.8Sm0.2O1.9 (SDC) electrolytes substituted with certain Fe2O3 were superior and a total conductivity of 0.0263 S cm−1 could be obtained in the x = 0.1 sample. Therefore, it was concluded that co-doped with Fe2O3 and Sm2O3 could enhance the electrical conductivities of CeO2-based solid electrolyte, indicating that it is a potential electrolyte for SOFCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the development of the society, energy affects all sectors of the global economy. The increasing industrialization requires sustainable, highly efficient energy production. Solid oxide fuel cell (SOFC) is a type of electrochemical device that can convert chemical energy into electric energy efficiently and environment-friendly [1,2,3]. In SOFC, several doped ceria materials were identified as attractive electrolytes because of the high ionic conductivity [4,5,6,7,8,9,10,11]. The substitution of trivalent ions for Ce4+ ions can enhance the vacancies number and result in the increasing of oxygen ion conductivities in ceria-based materials, so rare earth ions doped ceria exhibit high conductivity [12,13,14,15,16]. When CeO2-based electrolyte doped with a trivalent rare earth metal oxide or a divalent alkaline earth metal oxide, the oxygen vacancies formed in the oxygen sublattice due to the charge compensation, thereby increasing the ionic conductivity. The total conductivity (σtot) of a polycrystalline CeO2-based electrolyte is determined by the grain conductivity (σgi) and the grain boundary conductivity (σgb). σgi is usually 100–1000 times higher than σgb [17, 18]. During the sintering process, there are a certain amount of impurities exist at grain boundaries which increase the grain boundary resistance and resulting in the decrease of σgb.
In the study of ceria-based materials, double doped ceria is found to have superior electrical properties. Ceria-based electrolytes co-doped with alkaline earth (Ca2+, Sr2+) and rare earth ions (Gd3+, Sm3+, Y3+) have been extensively studied in the past decade years. In addition, to further improve the ionic conductivity, co-doped samples such as Ce0.8Ln0.2−xMxO1.9 (Ln = La, Gd, Sm, M = Y, Mg, Pr, etc.) have been studied [19, 20]. Usually, ceria-based materials co-doped with alkaline earth and rare earth ions were sintered at high temperature in order to obtain dense solid electrolyte with a single-phase of cubic fluorite structure for SOFC applications. In order to improve the densification, the sintering aids such as CuO and MnO2 have been added [21]. Duncan P. Fagg reported small (2 mol%) cobalt oxide additions to ceria-gadolinia materials considerably improve sinterability, making it possible to obtain ceramics with 95–99% density [22].
CeO2-based electrolytes doping with transition metal oxides such as Co2O3, Fe2O3 and Al2O3 have a certain effect on scavenging impurities in the electrolyte materials, which can improve the grain boundary conductivity and total conductivity [23,24,25]. Among them, Fe2O3 can be used not only as a impurities degreaser at grain boundaries, but also has the role of sintering aids for CeO2-based electrolyte [26, 27].
Ceria-based materials are often prepared by conventional solid-state reactions, lower temperature reactions including hydrothermal, precipitation, auto-combustion and other soft chemical processes [28,29,30,31]. Among these, the sol–gel method offers a new way to prepare electrolyte powders because of its advantages such as short reaction time, and it is simple more efficient than other methods. Moreover, high purity products with small particle sizes and narrow particle size distributions can be obtained from the sol–gel process [32].
In this study, Fe2O3 and Sm2O3 double-doped ceria-based electrolyte powders have been prepared by sol–gel process. The samples were characterized using thermogravimetric and differential scanning calorimetry (TG–DSC), X-ray diffraction (XRD), scanning electron microscope (SEM) and electrochemical impedance spectroscopy (EIS). The results of this work can contribute to the fabrication of novel electrolyte for the application to SOFC devices.
2 Experimental
2.1 Sample preparation
Ce0.8Sm0.2−xFexO2−δ (x = 0–0.2) ultrafine powders were prepared by the sol–gel method. In the process, analytical pure cerium nitrate (Ce(NO3)3·6H2O), samarium nitrate (Sm(NO3)3·6H2O) and Fe(NO3)3·9H2O were used as the raw materials.
During the preparation process, the aforementioned three nitrates were dissolved in deionized water and mixed together, citric acid was then added as complexing agents to form a homogenous solution, the molar ratio of citric acid to metal ion (Ce, Sm, Fe) is 1.5:1. The above mixture was continuously stirred using a magnetic agitator to form a homogenous solution at 80 °C, then converted to a viscous gel. The gel was dried in an oven at 120 °C for 24 h to remove the solvents, and then calcined at 700 °C for 2 h in a muffle furnace. The synthesized Ce0.8Sm0.2−xFexO2−δ powders were pressed into discs with size of ϕ11 × 1 mm, finally sintered at 1400 °C for 4 h in air atmosphere to obtain wafers used for testing purposes.
2.2 Materials characterisation
Ce0.8Sm0.2−xFexO2−δ xerogels were studied using TG–DSC simultaneous thermal analyzer (Netzsch STA 409PC, Germany) in a nitrogen atmosphere. The phase composition of the calcined Ce0.8Sm0.2−xFexO2−δ powders was analyzed by X-ray diffraction (XRD) method (Rigaku, Japan), the diffractograms were scanned in 2θ from 20° to 70° using Cu Kα line (λ = 0.154056 nm). The microstructure was analyzed with a scanning electron microscope (SEM, Model S4800). The electrochemical performance of the sintered discs was tested using an electrochemical analyzer (CHI660D, China). For electrochemical testing, silver paste was coated uniformly on both sides of each sintered disc and then fired at 700 °C for 10 min to form Ag electrodes. Measurement of the data were conducted in the frequency range 0.1 Hz–100 kHz from 400 to 800 °C in increments of 50 °C, the obtained impedance spectra were processed using ZSimpWin software.
3 Results and discussion
3.1 TG–DSC analysis
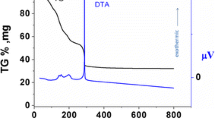
The thermal evolution of the xerogels were analyzed by recording TG–DSC curves. As shown in Fig. 1, the thermal decomposition and weightlessness of the xerogels can be divided into three stages. The first stage: from room temperature to 160 °C, the weight loss of the sample is about 7.6%, which corresponds to the loss of adsorbed water and citric acid in the sample. In the second stage(160–650 °C), there is a strong exothermic peak on the DSC curve and the weight loss of the sample is about 45%, corresponding to the decomposition process of some organic groups and ions in the sample. When the temperature is above 650 °C, the change of TG curve is not obvious, which indicates that the decomposition of organic matter in the sample has been completed. However, an obvious exothermic peak appears in the DSC curve at about 670 °C, which may be formed by the phase transition and crystallization process of the sample, indicating that a solid solution of Ce0.8Sm0.2−xFexO2−δ has been formed at this temperature. By analyzing the thermal behavior of Ce0.8Sm0.2−xFexO2−δ xerogels, precursor powders were calcined at 700 °C for 2 h in present study.
3.2 XRD analysis
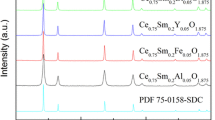
Figure 2 shows the XRD patterns of the Ce0.8Sm0.2−xFexO2−δ powders along with CeO2 standard patterns with cubic fluorite structure. Ce0.8Sm0.2−xFexO2−δ powders calcined at 700 °C crystallized well, and no second phase can be recognized. The powders showing all main reflections corresponding to the reference spectra (JCPDS No. 43-1002), indicative of the complete formation of CeO2-based solid solution with typical cubic fluorite structure.
The crystallite sizes were calculated using the Scherrer equation [33]:
where D is the crystallite size, λ is the X-ray radiation wavelength, 2θ represents the diffraction angle and β is the strongest Bragg’s peak corresponding to the reflection for the samples. \(\beta = \sqrt {\beta_{m}^{2} - \beta_{s}^{2} }\) is the corrected FWHM of the observed halfwidth: βm is that of the (111) reflection in samples and βs is that of the (111) reflection in a standard sample (Si from NIST). Table 1 shows the cell parameters of the samples calculated from the XRD patterns using Jade software.
Fe3+ co-doping decreases the lattice constant compared to SDC as expected from effective ionic radii considerations [34]. The SDC powder lattice parameter (a = 0.5433 nm) is slightly bigger than that of pure ceria (a = 0.5411 nm, in accordance with JCPDS card 43-1002), while co-doping with Fe3+ leads to a decrease in the lattice parameter, which attribute to the ionic radius of Fe3+ (0.065 nm) is smaller than that of Sm3+ (0.108 nm) and Ce4+ (0.097 nm). The lattice parameters depend on the ionic radius, amount, and solubility limit of the dopant ions, thus the lattice parameter of SDC decreases as the Fe content increasing [35,36,37].
3.3 Microstructure and sinterability analysis
Figure 3a–c display the SEM micrographs of the discs sintered at 1400 °C. The image show that the discs were dense and uniform. There are no obvious cracks and pores at the interface of the samples. It indicates good sintering of Ce0.8Sm0.2−xFexO2−δ samples. The mean grain sizes of the pellet was approximately 1–5 μm.
In order to estimate the influence of sintering temperature to the density of electrolyte ceramics, the Ce0.8Sm0.2−xFexO2−δ samples were sintered at different temperatures. Figure 3d–f display the surface topography of Ce0.8Sm0.1Fe0.1O2−δ sintered at 1100 °C, 1200 °C and 1300 °C, respectively. It can be found that the density of the sample increased with the increasing of sintering temperature. There are many pores on the surface of sintered sample at low temperatures (1100 °C and 1200 °C, Fig. 3d, e), while the pores on the surface of the sample are less and less with the increase of sintering temperature. There are no obvious pores at the interface of the samples which sintered at 1400 °C (Fig. 3b).Based above discussions, the samples should sintered at 1400 °C to form dense electrolyte ceramics.
The bulk density (ρ) and open porosity of Ce0.8Sm0.2−xFexO2−δ sintered discs was measured using Archimedes method with deionized water:
where WDry is the weight of sintered discs, ρwater is the density of water (0.997 g·cm−3), WWet is the wet weight (boil the discs in water for 30 min), and Wsus represents the suspended weight of sintered discs in water. The relative density of Ce0.8Sm0.2−xFexO2−δ discs sintering at 1400 °C reached above 95%, which confirmed that the sintered discs were dense with low porosity. The results of density and porosity testing showed that Ce0.8Sm0.2−xFexO2−δ powders prepared by sol–gel method have good sinterability.
Figure 4 illustrates the shrinkage curves of the Ce0.8Sm0.2−xFexO2−δ samples. The onset of Ce0.8Sm0.2−xFexO2−δ shrinkage occurred at lower temperature and rapid shrinkage occurred over a narrow temperature region (600–900 °C), and final shrinkage was approximately 12%. When the temperature is above 1400 °C, the change of shrinkage curve is not obvious, which indicates that the density of the samples were high. Thus, we selected 1400 °C as the sintering temperature, as it is sufficient to obtain the dense electrolyte ceramics.
3.4 Electrochemical analysis
Electrochemical impedance spectroscopy (EIS) is a powerful electrochemical technique with wide applications in SOFC development. It can characterize the information of ohmic losses, electrochemical kinetics, and mass transfer processes individually. It can obtain a symmetrical cell from a representative impedance spectrum, Rb and Rgb are the bulk and grain-boundary resistances of the electrolyte, Re is the electrode resistance. There are two semicircles of the electrolyte process in such circuit at high frequencies are represented by two resistances, Rb and Rgb [38].
The conductivities of Fe doped SDC electrolyte ceramics were measured in air atmosphere. Figure 5 shows the electrochemical impedance spectra of sintered discs (x = 0.1). As seen in Fig. 5, there are two incomplete semicircles in the spectrum at lower testing temperatures corresponded to grain boundary and electrode interface resistance, respectively [39]. As the increasing of operating temperature, the first arc reduced gradually, when working temperature reached 800 °C, there is only a single depressed arc can be observed in the impedance spectra. From the intercepts of the semicircular arcs at “z” axes, it can obtain the values of Rb and Rgb [29, 31, 40]. Based on the resistance values, using the following equation, it can calculate the conductivities of Fe doped SDC electrolytes.
where L is the thickness of sintered disc, R is total resistance, S represents the electrode area of the sample. Conductivities and activation energies of the samples tested at 800 °C are listed in Table 2.
From the conductivities calculated results, the conductivity of Ce0.8Sm0.2−xFexO2−δ ceramics increases with the increasing of Fe content and reaches a maximum at x = 0.1, then decreases. On one hand, the grain grows due to the doping of Fe2O3 (shown as Fig. 3), so the contact between the grains is tighter, the density is increased and reduced the grain resistance, thus increasing the grain conductivity. On the other hand, oxygen vacancies formed when SDC doped with the trivalent Fe3+ ion, and most of these oxygen vacancies are mobile in lower dopant content range, which result the increase in conductivity. But defect associations formed at the cost of oxygen vacancies as doped Fe content increasing, so the conductivity decreased [41, 42]. Furthermore, doping Fe2O3 in SDC, there will be a small amount of Fe3+ entering the lattice to replace Ce4+ to generate oxygen vacancies:
Because the attraction between \({\text{Fe}}_{\text{Ce}}^{\prime }\) and \({\text{V}}_{\text{O}}^{ \cdot \cdot }\) is greater than that of \({\text{Sm}}_{\text{Ce}}^{\prime }\) and \({\text{V}}_{\text{O}}^{ \cdot \cdot }\), so \({\text{Fe}}_{\text{Ce}}^{\prime }\) and \({\text{V}}_{\text{O}}^{ \cdot \cdot }\) are easier to combine. When the doping amount of Fe2O3 is higher than 0.1(x), part of Fe3+ enters SDC lattice to replace Ce4+ to produce \({\text{Fe}}_{\text{Ce}}^{\prime }\). \({\text{Fe}}_{\text{Ce}}^{\prime }\) combines with \({\text{V}}_{\text{O}}^{ \cdot \cdot }\), to form an association defect \(\left[ {{\text{Fe}}_{\text{Ce}}^{\prime } \cdot {\text{V}}_{\text{O}}^{ \cdot \cdot } } \right]\) [43, 44]. It can be seen from formula (4), two Fe3+ ions will generate one oxygen vacancy, however, a Fe3+ ion will associate one free oxygen vacancy (\(\left[ {{\text{Fe}}_{\text{Ce}}^{\prime } \cdot {\text{V}}_{\text{O}}^{ \cdot \cdot } } \right]\)), which reduces the concentration of oxygen vacancies, and decreases the conductivity. All in all, doping Fe2O3 may have two effects on the SDC system: one is that Fe2O3 scavenges the impurities exist at grain boundaries to increase the conductivity; the second is that \({\text{Fe}}_{\text{Ce}}^{\prime }\) and \({\text{V}}_{\text{O}}^{ \cdot \cdot }\) combine to form the association \(\left[ {{\text{Fe}}_{\text{Ce}}^{\prime } \cdot {\text{V}}_{\text{O}}^{ \cdot \cdot } } \right]\), which results the decreasing of oxygen vacancies concentration and conductivities. The effects of the above two aspects resulted in the fact that SDC shows the superior conductivity with certain Fe2O3 content (x = 0.1). The electrical behavior of Ce0.8Sm0.2−xFexO2−δ ceramics was similar to other rare earth ions doped ceria electrolytes [45,46,47,48]. It demonstrates that the electrical properties of the ceria-based electrolyte could be enhanced doped with appropriate Fe content in SDC could enhance for SOFCs applications.
Representative Arrhenius plots of the reciprocal temperature dependence of log(σT) of Ce0.8Sm0.2−xFexO2−δ electrolytes are shown in Fig. 6 which follows the Arrhenius equation:
The activation energies calculated from Fig. 5 reach a minimum at x = 0.1(0.77 eV).
In the study of electrochemical properties of ceria-based materials, the interaction between dopant \(M_{Ce}^{\prime }\) or \(M_{Ce}^{\prime \prime }\) and oxygen vacancies \(V_{O}^{ \cdot \cdot }\), resulting the formation of associates \(2{\text{M}}_{\text{Ce}}^{\prime } - {\text{V}}_{\text{O}}^{ \cdot \cdot }\) or \({\text{M}}_{\text{Ce}}^{\prime \prime } - {\text{V}}_{\text{O}}^{ \cdot \cdot }\). It needs an additional energy to free the vacancy from the association, so Arrhenius plot of conductivity often shows a kink towards low-temperature regime to reflect the increasing activation energy. Moreover, there is a minimization of the lattice elastic strain, so the maximum conductivity can be observed for a specific dopant with the closest match in ionic radius of the dopant cation and Ce4+ in ceria based electrolytes. In reducing atmospheres, the excess electronic conduction can considerably elevates due to the presence of a variable Ce valence which named Ce3+ (\({\text{Ce}}_{\text{Ce}}^{\prime }\)) and Ce4+ (\({\text{Ce}}_{\text{Ce}}^{ \times }\)) by the following defect reaction.
Based on the electrochemical measurements, we suggested that Sm and Fe co-doped CeO2 composite material is a prospective electrolyte because of its superior electrochemical properties for future use in IT-SOFCs, and Ce0.8Sm0.2−xFexO2−δ directs a new strategy to design novel electrolyte materials for SOFCs applications.
4 Conclusion
Ce0.8Sm0.2−xFexO2−δ solid electrolyte powders were prepared by the sol–gel method. XRD studies confirmed the formation of ceria based electrolytes with cubic fluorite structure after doped with Sm3+ and Fe3+ ions. Microstructure measurements displayed that the Ce0.8Sm0.2−xFexO2−δ electrolyte ceramics are quite dense and uniform with good sinterability after sintered at 1400 °C for 4 h, and the relative densities reached above 95%. Electrochemical analysis indicated the electrical conductivities of Ce0.8Sm0.2−xFexO2−δ electrolytes were much higher than that of SDC, and the dopant content at x = 0.1, the electrolyte showed the best ionic conductivity of 0.0263 S cm−1 at 800 °C. In summary, CeO2-based solid electrolyte ceramics which doped with Fe and Sm had a potential application for solid oxide fuel cells in middle or low level temperatures range.
References
S. Prakash, B. Parthasarathi, S. Kumar, S.T. Aruna, Microstructure and electrical properties of plasma sprayed Gd 0.15 Ce 0.85 O 2 − δ coatings from solution combustion synthesized flowable powders. J. Eur. Ceram. Soc. 37, 271–279 (2017)
C. Yao, J. Meng, X. Liu, X. Zhang, X. Liu, F. Meng, X. Wu, J. Meng, Enhanced ionic conductivity in Gd-doped ceria and (Li/Na)2SO4 composite electrolytes for solid oxide fuel cells. Solid State Sci. 49, 90–96 (2015)
K.C. Anjaneya, G.P. Nayaka, J. Manjanna, V.M. Ashwin Kumar, G. Govindaraj, K.N. Ganesha, Investigation on the Sr-doped ceria Ce1 − xSrxO2−δ (x = 0.05–0.2) as an electrolyte for intermediate temperature SOFC. J. Alloys Compd. 598, 33–40 (2014)
A. Maheshwari, H.-D. Wiemhöfer, Sr2+–Gd3+ co-doped CeO2: a cost-effective variant for IT-SOFC electrolytes. Ceram. Int. 41, 9122–9130 (2015)
M. Dudek, M. Mosiałek, Utility of Ce0.8M0.2O1.9, Ce0.8M0.15Y0.05O1.9, M = Gd, Sm powders synthesized by aerosol decomposition method in solid oxide fuel cell technology. Electrochim. Acta 104, 339–347 (2013)
T. Somekawa, Y. Matsuzaki, Y. Tachikawa, S. Taniguchi, K. Sasaki, Study of the solid-state reaction at the interface between lanthanoid-doped ceria and yttria-stabilized zirconia for solid-oxide fuel cell applications. Solid State Ion. 282, 1–6 (2015)
N.C. Martins, S. Rajesh, F.M. Marques, Synthesis and electrochemical assessment of Ce0.5Yb0.5O1.75 ceramics and derived composite electrolytes. Mater. Res. Bull. 70, 449–455 (2015)
L. Spiridigliozzi, M. Biesuz, G. Dell’Agli, E. Di Bartolomeo, F. Zurlo, V.M. Sglavo, Microstructural and electrical investigation of flash-sintered Gd/Sm-doped ceria. J. Mater. Sci. 52, 7479–7488 (2017)
C. Rockenhäuser, B. Butz, N. Schichtel, J. Janek, R. Oberacker, M.J. Hoffmann, D. Gerthsen, Microstructure evolution and cation interdiffusion in thin Gd2O3 films on CeO2 substrates. J. Eur. Ceram. Soc. 34, 1235–1242 (2014)
H. Liu, J. Huang, D. Zhao, H. Yang, T. Zhang, Improving the electrical property of CeO2-containing sealing glass–ceramics for solid oxide fuel cell applications: effect of HfO2. J. Eur. Ceram. Soc. 36, 917–923 (2016)
E.O. Oh, C.M. Whang, Y.R. Lee, S.Y. Park, D.H. Prasad, K.J. Yoon, J.W. Son, J.H. Lee, H.W. Lee, Extremely thin bilayer electrolyte for solid oxide fuel cells (SOFCs) fabricated by chemical solution deposition (CSD). Adv. Mater. 24, 3373–3377 (2012)
S. Dutta, A. Nandy, A. Dutta, S.K. Pradhan, Structure and microstructure dependent ionic conductivity in 10 mol% Dy2O3 doped CeO2 nanoparticles synthesized by mechanical alloying. Mater. Res. Bull. 73, 446–451 (2016)
J.-E. Hong, S. Ida, T. Ishihara, Decreased sintering temperature of anode-supported solid oxide fuel cells with La-doped CeO2 and Sr- and Mg-doped LaGaO3 films by Co addition. J. Power Sources 259, 282–288 (2014)
H. Tinwala, P. Shah, K. Siddhapara, D. Shah, J. Menghani, Investigation of ionic conductivity of lanthanum cerium oxide nano crystalline powder synthesized by co precipitation method. J. Cryst. Growth 452, 54–56 (2016)
L. Guan, S. Le, X. Zhu, S. He, K. Sun, Densification and grain growth behavior study of trivalent MO1.5 (M = Gd, Bi) doped ceria systems. J. Eur. Ceram. Soc. 35, 2815–2821 (2015)
K.C. Anjaneya, G.P. Nayaka, J. Manjanna, G. Govindaraj, K.N. Ganesha, Preparation and characterization of Ce1 − xGdxO2 − δ (x = 0.1–0.3) as solid electrolyte for intermediate temperature SOFC. J. Alloys Compd. 578, 53–59 (2013)
B.C.H. Steele, Appraisal of Ce1 − yGdyO2 − y/2 electrolytes for IT-SOFC operation at 500°C. Solid State Ion. 129, 95–110 (2000)
J.L.M. Rupp, A. Infortuna, L.J. Gauckler, Microstrain and self-limited grain growth in nanocrystalline ceria ceramics. Acta Mater. 54, 1721–1730 (2006)
S.A. Muhammed Ali, M. Anwar, A.M. Abdalla, M.R. Somalu, A. Muchtar, Ce0.80Sm0.10Ba0.05Er0.05O2 − δ multi-doped ceria electrolyte for intermediate temperature solid oxide fuel cells. Ceram. Int. 43, 1265–1271 (2017)
E.Y. Pikalova, A.A. Murashkina, V.I. Maragou, A.K. Demin, V.N. Strekalovsky, P.E. Tsiakaras, CeO2 based materials doped with lanthanides for applications in intermediate temperature electrochemical devices. Int. J. Hydrog. Energy 36, 6175–6183 (2011)
X. Zhang, C. Decès-Petit, S. Yick, M. Robertson, O. Kesler, R. Maric, D. Ghosh, A study on sintering aids for Sm0.2Ce0.8O1.9 electrolyte. J. Power Sources 162, 480–485 (2006)
D.P. Fagg, V.V. Kharton, J.R. Frade, Transport in ceria electrolytes modified with sintering aids: effects on oxygen reduction kinetics. J. Solid State Electrochem. 8, 618–625 (2004)
W. Zając, L. Suescun, K. Świerczek, J. Molenda, Structural and electrical properties of grain boundaries in Ce0.85Gd0.15O1.925 solid electrolyte modified by addition of transition metal ions. J. Power Sources 194, 2–9 (2009)
T.S. Zhang, J. Ma, L.B. Kong, P. Hing, Y.J. Leng, S.H. Chan, J.A. Kilner, Sinterability and ionic conductivity of coprecipitated Ce0.8Gd0.2O2 − δ powders treated via a high-energy ball-milling process. J. Power Sources 124, 26–33 (2003)
H.J. Park, G.M. Choi, The electrical conductivity and oxygen permeation of ceria with alumina addition at high temperature. Solid State Ion. 178, 1746–1755 (2008)
D. Xu, X. Liu, S. Xu, D. Yan, L. Pei, C. Zhu, D. Wang, W. Su, Fabrication and performance of Ce0.85Sm0.15O1.925–Fe2O3 electrolytes in IT-SOFCs. Solid State Ion. 192, 510–514 (2011)
T.S. Zhang, J. Ma, S.H. Chan, J.A. Kilner, Grain boundary conduction of Ce0.9Gd0.1O2 − δ ceramics derived from oxalate coprecipitation: effects of Fe loading and sintering temperature. Solid State Ion. 176, 377–384 (2005)
Y.-C. Wu, Y.-Y. Liao, Effect of Ca2+ and Sr2+ doping on the microstructure and cell performance of samaria-doped ceria electrolytes used in solid oxide fuel cells. Int. J. Hydrog. Energy 41, 13591–13602 (2016)
K.C. Anjaneya, J. Manjanna, G.P. Nayaka, V.M. Ashwin Kumar, G. Govindaraj, K.N. Ganesha, Citrate-complexation synthesized Ce0.85Gd0.15O2 − δ (GDC15) as solid electrolyte for intermediate temperature SOFC. Phys. B 447, 51–55 (2014)
G. Chen, H. Kishimoto, K. Yamaji, K. Kuramoto, M. Gong, X. Liu, G. Hackett, K. Gerdes, T. Horita, Chemical reaction mechanisms between Y2O3 stabilized ZrO2 and Gd doped CeO2 with PH3 in coal syngas. J. Power Sources 268, 904–910 (2014)
C.G.M. Lima, T.H. Santos, J.P.F. Grilo, R.P.S. Dutra, R.M. Nascimento, S. Rajesh, F.C. Fonseca, D.A. Macedo, Synthesis and properties of CuO-doped Ce0.9Gd0.1O2 − δ electrolytes for SOFCs. Ceram. Int. 41, 4161–4168 (2015)
J. Cheng, W. Bao, C. Han, W. Cao, A novel electrolyte for intermediate solid oxide fuel cells. J. Power Sources 195, 1849–1853 (2010)
B. Matović, M. Stojmenović, J. Pantić, A. Varela, M. Žunić, N. Jiraborvornpongsa, T. Yano, Electrical and microstructural properties of Yb-doped CeO2. J. Asian Ceram. Soc. 2, 117–122 (2014)
L. Guan, S. Le, S. He, X. Zhu, T. Liu, K. Sun, Densification behavior and space charge blocking effect of Bi2O3 and Gd2O3 Co-doped CeO2 as electrolyte for solid oxide fuel cells. Electrochim. Acta 161, 129–136 (2015)
Y.-C. Wu, Y.-Y. Liao, Effect of Ca2 + and Sr2 + doping on the microstructure and cell performance of samaria-doped ceria electrolytes used in solid oxide fuel cells. Int. J. Hydrog. Energy 41, 13591–13602 (2016)
N.K. Singh, P. Singh, M.K. Singh, D. Kumar, O. Parkash, Auto-combustion synthesis and properties of Ce0.85Gd0.15O1.925 for intermediate temperature solid oxide fuel cells electrolyte. Solid State Ion. 192, 431–434 (2011)
S. Kumar, S. Gautam, T.K. Song, K.H. Chae, K.W. Jang, S.S. Kim, Electronic structure study of Co doped CeO2 nanoparticles using X-ray absorption fine structure spectroscopy. J. Alloys Compd. 611, 329–334 (2014)
K. Huang, J.B. Goodenough, Performance Characterization Techniques for a Solid Oxide Fuel Cell (SOFC) and its Components[M] (Elsevier, Amsterdam, 2009)
E. Drożdż, J. Wyrwa, K. Schneider, M. Rękas, Electrical properties of silica-doped 3 mol% yttria-stabilized tetragonal zirconia. J. Mater. Sci. 52, 674–685 (2016)
R.K. Lenka, T. Mahata, A.K. Tyagi, P.K. Sinha, Influence of grain size on the bulk and grain boundary ion conduction behavior in gadolinia-doped ceria. Solid State Ion. 181, 262–267 (2010)
D. Zhou, Y. Xia, J. Zhu, W. Guo, J. Meng, Preparation and electrical properties of new oxide ion conductors Ce6 − xGdxMoO15 − δ (0.0 ≤ x≤1.8). J. Am. Ceram. Soc. 92, 1042–1046 (2009)
B. Ji, C. Tian, C. Wang, T. Wu, J. Xie, M. Li, Preparation and characterization of Ce0.8Y0.2 − xCuxO2 − δ as electrolyte for intermediate temperature solid oxide fuel cells. J. Power Sources 278, 420–429 (2015)
Y. Zheng, M. Zhou, L. Ge, S. Li, H. Chen, L. Guo, Effect of Fe2O3 on Sm-doped ceria system solid electrolyte for IT-SOFCs. J. Alloys Compd. 509, 546–550 (2011)
T.S. Zhang, J. Ma, Y.J. Leng, S.H. Chan, P. Hing, J.A. Kilner, Effect of transition metal oxides on densification and electrical properties of Si-containing Ce0.8Gd0.2O2 − δ ceramics. Solid State Ion. 168, 187–195 (2004)
W. Sun, Z. Shi, J. Qian, Z. Wang, W. Liu, In-situ formed Ce0.8Sm0.2O2 − δ@Ba(Ce, Zr)1 − x(Sm, Y)xO3 − δ core/shell electron-blocking layer towards Ce0.8Sm0.2O2 − δ-based solid oxide fuel cells with high open circuit voltages. Nano Energy 8, 305–311 (2014)
M. Yashima, Invited review: some recent developments in the atomic-scale characterization of structural and transport properties of ceria-based catalysts and ionic conductors. Catal. Today 253, 3–19 (2015)
L. Zhang, F. Liu, K. Brinkman, K.L. Reifsnider, A.V. Virkar, A study of gadolinia-doped ceria electrolyte by electrochemical impedance spectroscopy. J. Power Sources 247, 947–960 (2014)
A. Sawka, A. Kwatera, Deposition of Sm2O3-doped CeO2 layers using the MOCVD method. Ceram. Int. 42, 1446–1452 (2016)
Acknowledgements
The work was kindly supported by the Natural Science Foundation of Colleges and Universities in Anhui Province (Grant Nos. KJ2016A591, KJ2018A0549) and the Nature Science Foundation of Anhui Province of China (No. 1708085ME112).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cheng, J., Tian, C. & Yang, J. Effects of Fe2O3 addition on the electrical properties of SDC solid electrolyte ceramics. J Mater Sci: Mater Electron 30, 16613–16620 (2019). https://doi.org/10.1007/s10854-019-02040-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-019-02040-2