Abstract
Effect of additional Zn2+ dopant on the ferromagnetism (FM) of Co doped CeO2 nanoparticles was studied. The Zn and Co co-doped CeO2 nanoparticles (Ce0.97−xZnxCo0.03O2: where x = 0, 0.01, 0.03, 0.05) were prepared by sol–gel technique. The crystal structure, morphology, and magnetic properties were analyzed by X-ray diffraction (XRD), scanning electron microscopy (SEM), Raman spectroscopy (Raman) and physical property measurement system (PPMS). XRD and Raman studied showed that certain amount of Zn2+ can readily be incorporated into the lattice of Co doped CeO2 with single-phase of CeO2 original cubic fluorite crystal structure, and no ferromagnetic secondary phase was observed. SEM images show Zn and Co co-doped CeO2 nanoparticles were spherical and uniform size. The PPMS studied indicate that the room-temperature FM of Co doped CeO2 nanoparticles increase with additional Zn2+ dopant. This result is helpful in understanding the origin of FM in diluted magnetic oxides (DMO) as well as improving the magnetic property of DMO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Physical properties and potential applications of semiconductor nanomaterials have been studied intensively [1,2,3]. Diluted magnetic semiconductors (DMS) have attracted the attention of the scientific community because of their potential for combining semiconductor and magnetic properties that are possible to use electronic charge and spin at the same time in a material. Room temperature ferromagnetism (FM) of the DMS by transition metal doped oxide semiconductor or insulator (TM-DMO) is the focus of today’s research. The TM-DMO material combines the spin and charge of carriers, thus the implementation of spintronic devices is possible by using TM-DMO materials [4]. The discovery of more suitable doped elements and the development of various TM-DMO materials are the main tasks of the present [5,6,7]. The origin of room temperature FM in TM-DMO materials is another focus of the investigation. The reports in the literature are different, some even contrary, but it is generally believed that the ferromagnetic exchange in TM-DMO is mainly due to defects, electron transfer and oxygen vacancy (Vo) [7,8,9]. Among many oxides, rare earth oxide of cerium oxide (CeO2) has attracted special attention. CeO2 is widely used in luminescence, polishing, UV absorption, automobile exhaust purification, solid oxide fuel cells, optical coatings and oxygen sensors. In addition, CeO2 is a typical wide band gap oxide semiconductor. The structure of CeO2 belongs to cubic fluorite type, with lattice constant matching well with Si. Therefore, it is considered as a potential replacement for Si semiconductor. Recent studies have found room temperature FM in undoped CeO2 and doped CeO2 samples. These findings stimulate interest in the study of CeO2 systems [8,9,10]. This is because the Ce ions in CeO2 based diluted magnetic oxides (DMO) can have a variety of ionic states (+ 2, + 3, and + 4), Therefore, defects in CeO2 based DMO are easy to manipulate. This is convenient to verify the mechanism of FM induced by defects. Second, the cubic structure of CeO2 can accommodate a large number of oxygen vacancies, thus Vo in CeO2 based DMO can be controlled. It is easy to verify the ferromagnetic coupling mechanism based on Vo by experiment. Third, CeO2 based DMO are easy to integrate with semiconductor materials, this makes preparation of dilute magnetic/semiconductor superlattice structures easy. Therefore, new type of spin functional device can be developed. For understanding the origin of room temperature FM, a systematic study on doped CeO2 is demanded.
In the present work, Zn2+ was chosen as additional dopants for Co doped CeO2 DMO systems. The Zn and Co co-doped CeO2 nanoparticles (Ce0.97−xZnxCo0.03O2: where x = 0, 0.01, 0.03, 0.05) were synthesized by sol–gel technique. The crystal structure, morphology, and magnetic properties were analyzed by X-ray diffraction (XRD), scanning electron microscopy (SEM), Raman spectroscopy and physical property measurement system (PPMS). Effect of additional Zn2+ dopant on the FM of Co doped CeO2 nanoparticles has been discussed.
2 Experiments
Preparation of Zn and Co co-doped CeO2 samples (Ce0.97−xZnxCo0.03O2: where x = 0, 0.01, 0.03, 0.05) was done by sol–gel technique. The precursor solutions were prepared as follows. Stoichiometric amount of Ce(III) nitrate hexahydrate (Ce(NO3)3·6H2O), cobaltous nitrate (Co(NO3)2·6H2O) and zinc acetate dihydrate (Zn(CH3COO)2·2H2O) was used to obtain desired compositions. These solutes were completely dissolved in 2-methoxyethanol with stirring for 1 h at 65 °C, and then 3 h at room temperature. Acetylacetone (CH3COCH2COCH3) was added to the solution as a stabilizer. According to previous reported results [5, 7,8,9,10,11,12], the optimal FM was obtained from Co-doped CeO2 DMO system with the nominal Co content of 3 at.%. So here we kept Co doping concentration as a constant of 3 at.% with the variation of Zn2+ doping content from 0 to 5 at.%. Zn doping was achieved by the introduction of appropriate amount of Zn(CH3COO)2·2H2O. These precursor solutions were put in a furnace for 24 h at 100 °C to eliminate excess water and form the xerogel. Then, the xerogel was calcined at 700 °C for 2 h to eliminate organic materials and form a powder.
The crystal structure of Ce0.97−xZnxCo0.03O2 powders were characterized by using RIGAKU D-MAX 2200 VPC X-ray diffractometer (XRD) equipped with a Cu-Kα (λ = 1.54 Å) source. The Raman spectroscopy of these powders was analyzed by a Confocal Micro-Raman Spectrometer (inVia Reflex, Renishaw) with 514 nm excitation source under air ambient condition. The morphology of the samples was observed by a thermal field SEM (Quanta 400F, FEI/OXFORD/HKL). Energy dispersive spectroscopy (EDS) was used to determine the amount of doping in the powder. Magnetic properties were investigated by a commercial physical property measurement system (PPMS, QUANTUM DESIGN, MOOEL 6000). In Table 1, some properties of Ce0.97−xZnxCo0.03O2 powders, such as Zn content determined by EDS (xEDS), particle size (D), saturation moment (Ms), and coercivity (HC) are shown.
3 Results and discussion
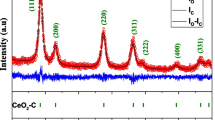
The XRD patterns of the Ce0.97−xZnxCo0.03O2 (where x = 0, 0.01, 0.03, 0.05) powders are depicted in Fig. 1. The obtained XRD patterns matched the powder diffraction data for CeO2 obtained using JCPDF 34-0394, which suggests a cubic symmetry belonging to the Fm-3m space group. Within the XRD detection limit, no extra diffraction peak related to the second phases of Co or Zn, which confirms the complete solubility of Zn and Co ions in the ceria crystal structure. This indicates that Zn and Co substituted for Ce in CeO2 host without changing the fluorite crystal structure. From Fig. 1, it is seen that all diffraction peaks were broadened, which indicated the fine nature of the small particles. By using Debye Scherrer formalism \(D=0.89\lambda /\beta \cos \theta ,\) where β is the full-width at half-maximum in radians, θ is the Bragg’s angle in degrees, and λ is the wavelength of X-rays (1.54 Å for Cu-Kα), the average particle size of Ce0.97−xZnxCo0.03O2 powders has been determined from the peak (111) broadening in the XRD patterns. The calculated particle size was 16.7, 14.6, 14.2 and 11.9 nm for x values of 0, 0.01, 0.03, and 0.05, respectively. The particle size and the peak intensity decrease with the increase of the amount of Zn2+ (see Table 1).
Figure 2 shows the SEM images of Ce0.97−xZnxCo0.03O2 powders with x = 0, 0.03. The particles were spherical, uniform size, and good dispersion. The particle size was about 20 nm for Ce0.97−xZnxCo0.03O2 powders with x = 0, 0.03, which agree well with the results calculated from Scherrer equation. The EDS spectrum (data not shown), which was collected from different parts of the samples, indicted that the Ce0.97−xZnxCo0.03O2 powders consist of Ce, Co, Zn and O only. A semi-quantitative analysis confirmed that the Co and Zn content in the samples were consistent with the design of our experiments (see Table 1).
The structure of the Ce0.97−xZnxCo0.03O2 nanoparticles was further studied by Raman spectroscopy. Figure 3 shows the Raman spectrum of the Ce0.97−xZnxCo0.03O2 (where x = 0, 0.01, 0.03, 0.05) nanoparticles, measured in the 200–700 cm−1 range. A strong band at 462.5 cm−1 was observed for all nanoparticles. This band belongs to the lattice mode (F2g) of the cubic fluorite metal dioxides. It related to a first-order symmetrical stretching mode of the Ce–O8 vibrational unit. This band (462.5 cm−1) is sensitive to any disorder in the oxygen sublattice [13]. The F2g mode shifts to the lower frequencies with additional Zn2+ dopant. This shift indicates the increase in the tensile stress of the crystal lattice caused by defects, such as vacancies generated when Co and Zn substitutes for Ce in the CeO2 matrix [14]. A weak band at 545 cm−1 was also observed for Zn and Co co-doped CeO2 nanoparticles. This band is attributed to the oxygen hole absorption peak [15], which indicates Vo was generated due to charge compensation in the Zn and Co co-doped CeO2 nanoparticles. The peak intensity of the Raman modes was found to decrease by increasing the Zn doping concentrations. We think that the increased Zn concentrations lead to shrinkage in unit cell volume. This increases the optical absorption. Thus the intensity of the Raman peaks decreases. Therefore, the observed decrease in the intensity of Raman active mode is the result of additional Zn doping induced structural modification in Co-doping CeO2 nanoparticles. Furthermore, we have not observed any Raman active modes of Zn- or Co-related oxide in this study. Hence, the Raman studies strengthened the XRD data and help us to conclude that: (i) all the samples are in nanocrystalline form; (ii) the Vo maybe present in the samples, and Vo concentration is varied with additional Zn doping; and (iii) no secondary phases could be detected, indicative of substitutional doping of Zn and Co.
Magnetic properties of the Ce0.97−xZnxCo0.03O2 (where x = 0, 0.01, 0.03, 0.05) nanoparticles were studied by using the commercial physical property measurement system (PPMS, QUANTUM DESIGN, MOOEL 6000). Figure 4 dislpays magnetization (M) versus field (H) curves of Ce0.97−xZnxCo0.03O2 nanoparticles measured at 300 K. It can be seen from the M (H) curves that Ce0.97−xZnxCo0.03O2 nanoparticles show the magnetic hysteresis loop, which gives the indication of ferromagnetic ordering at 300 K. The coercive field (HC) values calculated from hysteresis loops were in the range from 300 to 550 Oe for different Zn2+ concentration (shown in the Table 1). The saturation magnetization (Ms) for x = 0, 0.01, 0.03, and 0.05 are 0.0018, 0.0022, 0.0031, and 0.004 emu/g, respectively. It is found that the Ms increases as the additional Zn2+ dopant content increases (see Table 1).
Combining with the analysis of XRD and Raman spectra, it could be concluded that the room temperature FM observed in the Ce0.97−xZnxCo0.03O2 nanoparticles may be intrinsic because it is very easy to rule out any impurity for the magnetic signal in Ce0.97−xZnxCo0.03O2 nanoparticles. Effect of additional Zn2+ dopant on the room temperature FM of Co doped CeO2 nanoparticles possibly results from Vo or defects. In fact, the Vo appear to increase with increases in the Zn2+ content according to the Raman investigation before. The enhancement of room temperature FM in Ce0.97−xZnxCo0.03O2 nanoparticles could be attributed to interactions between the Zn2+ and uncompensated acceptor defects that are incorporated inside the CeO2 crystal lattice during the calcination of the samples. As well known that, Zn2+ substitution of Ce4+ will produce more Vo due to charge compensation, and the formation of VO increases with the increase of the amount of Zn2+. The formation of VO is confirmed by our Raman spectra before. On the basis of F-center mediated ferromagnetic coupling mechanism used in insulated DMO [16,17,18,19], more Vo will provide more coupling centers thus induce larger Ms. The increase in Zn doping concentration favored ferromagnetic interactions because most of the VO are formed. Therefore, the Ms is enhanced with additional Zn2+ dopant. Defects have also been reported as one of the possible reason for the FM origination [20]. Owing to the quite close ionic radius, doped Zn2+ would preferably substitute the crystal Ce4+ rather than locate at interstice. The substitution of Ce4+ by Zn2+ will also produce the lattice distortion or form some other defects, because the ionic radius of Zn2+ (0.09 nm) is smaller than that of Ce4+ (0.097 nm). Basing on the FM mechanism of the defects/VO through the F-center exchange interaction, it is readily to expect the Ms enhancement by additional Zn2+ dopant.
4 Conclusions
In summary, the Zn and Co co-doped CeO2 nanoparticles (Ce0.97−xZnxCo0.03O2: where x = 0, 0.01, 0.03, 0.05) were synthesized by sol–gel technique. The XRD and Raman results indicated that all the samples had a face-centered cubic structure and that no secondary phase was detected. The influence of additional Zn2+ dopant on the FM of Co-doped CeO2 nanoparticles was studied. Results indicate that the FM of Co-doped CeO2 nanoparticles was enhanced with additional Zn2+ dopant. The FM behavior can be attributed to the presence of dopant ions mediated by Vo. We conclude that Zn2+ is a good candidate to be incorporated into the lattice of CeO2 with the substitution of Ce4+, which would enhance the FM of Co-doped CeO2 nanoparticles.
References
A. Sobhani-Nasab, A. Ziarati, M. Rahimi-Nasrabadi et al., Res. Chem. Intermed. 43(11), 6155–6165 (2017)
A. Ziarati, A. Sobhani-Nasab, M. Rahimi-Nasrabadi et al., J. Rare Earths 35(4), 374–381 (2017)
A. Sobhani-Nasab, Z. Zahraei, M. Akbari et al., J. Mol. Struct. 1139, 430–435 (2017)
T. Diet, H. Ohno, F. Matsukura et al., Science 287, 1019–1022 (2000)
R.K. Singhal, P. Kumari et al., J. Phys. D: Appl. Phys. 44, 165002 (2011)
N. Doğan, A. Bingölbali, L. Arda, J. Magn. Magn. Mater. 373, 226–230 (2015)
Y.Q. Song, H.W. Zhang et al., J. Appl. Phys. 102, 043912 (2007)
A. Tiwari, V.M. Bhosle, S. Ramachandran et al., Appl. Phys. Lett. 88, 142511 (2006)
B. Vodungbo, Y. Zheng, F. Vidal et al., Appl. Phys. Lett. 90(1–3), 062510 (2007)
V. Femandes, J.J. Klein, N. Mattoso et al., Phys. Rev. B 75, 121304 (2007)
E.M. Waleed, A.A. Al-Ghamdia, F.A. Al-Agelc et al., Mater. Res. Bull. 72, 154–159 (2015)
P. Sumalin, P. Supree, M. Santi, J. Appl. Phys. 112, 113904 (2012)
J.R. Mcbride, K.C. Hass, B.D. Poindexter, W.H. Weber, J. Appl. Phys. 76, 2435–2441 (1994)
B. Choudhury, A. Choudhury, Curr. Appl. Phys. 13, 217–223 (2013)
M.S. Anwar, S. Kumar, F. Ahmed, N. Arshi, G.S. Kil, D.W. Park et al., Mater. Lett. 65, 3098–3101 (2011)
M. Venkatesan, C.B. Fitzgerald. J.M.D. Coey, Nature 430, 630–630 (2004)
J.M.D. Coey, A.P. Douvalis, C.B. Fitzgerald et al., Appl. Phys. Lett. 84, 1332–1334 (2004)
F. Meng, C. Zhang, Q.H. Bo et al., Mater. Lett. 99, 5–7 (2013)
Y. Jiang, J.B. Adams, M. van Schilfgaarde, J. Chem. Phys. 123, 064701 (2005)
W. Lee, S.Y. Chen, Y.S. Chen, C.L. Dong, H.J. Lin, C.T. Chen, A. Gloter, J. Phys. Chem. C 118, 26359–26367 (2014)
Acknowledgements
This work was supported by the National Natural Science Foundation of China under Grant Nos. 61176010 and 61172027.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, S., Jiang, Z. & Zhang, Y. Effect of additional Zn2+ dopant on the ferromagnetic properties of Co-doped CeO2 nanoparticles prepared by sol–gel method. J Mater Sci: Mater Electron 29, 7952–7956 (2018). https://doi.org/10.1007/s10854-018-8797-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-8797-6