Abstract
CoSx/OV–ZnO is prepared by two-step hydro-thermal method. The photocatalytic hydrogen evolution amount of ZnO samples is near to zero. The novel composite photocatalyst CoSx/OV–ZnO have high photocatalytic liveness. It is up to 256 µmol under visible light irradiation for 5 h, which is about increased 30% than CoSx/ZnO. The hydrogen production number of CoSx/OV–ZnO do not reduce obviously after 15 h irradiation, it exhibits high photocatalytic stability. The morphologies, surface features and physical characteristics of catalyst are analysed by scanning electron microscope (SEM), X-ray diffraction, X-ray energy spectrum (XPS), Brunner–Emmet–Teller (BET), and fluorescence spectra etc. The SEM results demonstrates amorphous CoSx is uniformly diffused on the hexagonal wurtzite OV–ZnO. The XPS shows the successful synthesis of ZnO oxygen vacancies. The CoSx/OV–ZnO has better superficial area and pore volume (BET), which is conducive to the adsorption of EY molecules. The ZnO electron transfer and the charge separation are obviously improved (fluorescence and electrochemical). Accordingly, CoSx/OV–ZnO might be a latent new photocatalyst.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the rapid development of industrialization and the increasing greenhouse effects, the environmental pollution has around the corner [1,2,3]. Contaminants were released from traditional fossil fuel, it seriously affected the human health. Absence and harm of traditional energy has become the focus of modern social development. So that, we require looking for a novel green energy sources regarded as a potential substitute for traditional energy. A lot of facts prove that hydrogen energy is a clean, effective and renewable green energy. The hydrogen energy exhibited the high caloric value (39.4 kW h/kg) in all chemical fuels, and do not discharge pollutants in use process [4]. Additionally, solar energy is an inexhaustible clean energy source. At present research illustrates that utilizing solar energy to produce hydrogen has become one of the hot spots. By using solar energy to produce hydrogen requires photocatalysts. Consequently, it is a pivotal step for us to find stable, efficient and sustainable photocatalyst for photocatalytic hydrogen production. Oxide semiconductor (ZnO, WO3, TiO2) has attracted considerable attention among semiconductor materials. They are widely used in photocatalytic field. Amid them, nontoxic ZnO is a more suitable choice due to its abundance and chemical stability [5,6,7]. But zinc oxide is wide-band-gap (3.3 eV) materials [8,9,10], it cannot be excited by visible light, its application was limited under visible light. Another major drawback is low quantum efficiency of ZnO, which is due to fast recombination of photogenic electron–hole pairs [11,12,13,14]. Other researchers have improved the photocatalytic properties of ZnO by modifying chemical substances and morphology in previous reports [15]. In this experiment, we increased the oxygen vacancy on the surface of zinc oxide. For metal oxides, given specific conditions, it will contribute to oxygen detachment in the lattice, resulting in oxygen deficiency, obtaining to oxygen vacancies [16, 17]. Previous reported that the oxygen vacancies metal oxides can improve the active sites of reactivity [18]. It suppresses the electron–hole recombination process and obtains excellent photocatalytic activity in visible light [19,20,21,22,23]. Transition metal sulfide was also the focal point due to excellent electrical and optical properties [24, 25]. Most of all, cobalt sulfur compounds are widely used with narrow band gap, high sunlight utilization efficiency. The previous research results showed that the cobalt sulfur compounds were superior semiconductor materials with excellent conductivity [26, 27]. In this study, first, ZnO is synthesized, oxygen vacancies are introduced into ZnO by NaBH4. Secondly, amorphous cobalt sulfide nanoparticles were successfully modified on the surface of OV–ZnO or ZnO photocatalyst. The preparation methods of CoSx were different from previous reports, CoSx catalyst was prepared by simple hydrothermal method using cheap raw material. And the composite material CoSx/OV–ZnO has not been reported. A new binary CoSx/OV–ZnO composite was prepared by two-step hydrothermal method. The hydrogen production experiment was carried out in the Eosin Y (EY) sensitization system. The composite CoSx/OV–ZnO has higher photocatalytic hydrogen evolution activity than ZnO sample. In addition, the composite catalyst CoSx/OV–ZnO maintained good stability. The experimental results show that this composite catalytic material CoSx/OV–ZnO is expected to become a promising photocatalyst.
2 Experimental process
2.1 Materials
All Chemicals were used in the contemporary study without any further purification. The reagents were as follows: zinc nitrate hexahydrate [Zn(NO3)2·6H2O], sodium hydroxide (NaOH), sodium borohydride (NaBH4), cobaltous nitrate hexahydrate [Co(NO3)2·6H2O], sodium sulfide nonahydrate (Na2S·9H2O), deionized water was produced by an ultra-pure water machine.
2.2 Sample preparation procedure
2.2.1 Preparation of ZnO by a hydrothermal process
0.02 mol Zn(NO3)2·6H2O and 0.01 mol NaOH were joined in 65 mL deionized water under magnetic stirring 40 min to form a white suspension. It was putt in Teflon lined Stainless Steel autoclave with a capacity of 100 mL, and was heated at 120 °C for 12 h in drying oven. Subsequently, a white ZnO samples were filtered and washed with deionized water. The samples were dried at 80 °C in drying oven.
2.2.2 Increasing the surface oxygen vacancy concentration of ZnO
The oxygen vacancy of ZnO was improved by reducing agent NaBH4. 3.7 mmol ZnO was placed in 60 mL deionized water for 1 h ultrasound. 3.96 mmol NaBH4 was diffused in the ZnO under energetically stirring for 4 h. Samples were filtered through deionized water washing. The final catalyst was dried at 60 °C in vacuum oven, and the sample was collected and was named OV–ZnO [28].
2.2.3 Loading CoSx on ZnO or OV–ZnO
Loading of CoSx was carried out utilizing hydrothermal method. 2.47 mmol ZnO or OV–ZnO catalyst was joined in 50 mL deionized water. 2.5 mmol Na2S·9H2O and 2.5 mmol Co(NO3)2·6H2O were dispersed in 15 mL deionized water, subsequently, the latter is mixed with the former. The mixed liquid was kept at 50 °C for 0.5 h. The obtained liquid was put in a 100 mL Teflon lined Stainless Steel autoclave and was heated at 180 °C for 12 h in vacuum oven. After the accomplish reaction, the samples were filtered and washed with deionized water, and were dried for 80 °C in vacuum oven. The CoSx/ZnO or CoSx/OV–ZnO catalyst was obtained. As a blank control, the pure CoSx was synthesized without adding ZnO or OV–ZnO in the same conditions.
2.3 Characterization
The X-ray diffraction (XRD) designs were used to obtain the composition and crystal structure of the crystalline material which measured using Cu Kα radiation (at 40 kV and 40 mA) and recorded from 10° to 90°. Morphology of the samples was characterized by scanning electron microscopy (SEM) at an accelerating voltage of 5 KV. The chemical state and the element composition were identified by conventional X-ray photoelectron spectroscopy (XPS) using the ultrahigh vacuum VG ESCALAB 250 electron spectrometers. Photoluminescence spectrum (PL) was acquired using a FLUOROMAX-4 spectrophotometer at room temperature. The specific surface areas and the pore diameter nitrogen of the products were carried out at 77 K with an ASAP 2020 M instrument, as well as was recorded of others Brunauer–Emmett–Teller (BET) data.
2.4 Photocatalytic H2 produced reactions measurements
The photocatalytic reaction was investigated by a 5 W LED light (420 nm) underneath magnetic stirring. Photocatalytic trials were monitored in a 65 mL quartz glass container, fitted with a magnetic stirrer. In a typical photocatalytic experiment, 10 mg of catalyst was dissolved in 30 mL 10% triethanolamine (TEOA) aqueous solution (electronic sacrificial agent) and was processed for 10 min by ultrasound, the pH value of the electron sacrificial agent was adjusted by 10%TEOA solution contain 10% 0.1 mol L−1 HCl solution or 10% 0.1 mol L−1 NaOH solution. And furthermore 20 mg EY as sensitizer were suspended in above solution by magnetic stirring for 30 min on a magnetic stirrer. We put nitrogen into the reaction system to remove oxygen outside for 40 min with a needle.
The hydrogen generation number of the sample was surveyed with gas chromatography (SP-2100, 13X column, TCD, N2 carrier) in the course of H2 evolution measurements.
2.5 Photoelectrochemical measurements
ITO was cleaned and soaked through anhydrous ethanol, and ITO was dried. The catalyst powder (12 mg) was added in anhydrous ethanol and scattered by ultrasound, and until the anhydrous ethanol evaporate to a sticky liquid at room temperature. Subsequently, the above sticky liquid was uniformly applied to the electrode surface (ITO) by a pipette. The obtained working electrode was dried at room temperature.
The measurement was detected on an electrochemical analyzer (Chenhua CHI 660E) in a standard three-electrode cell. Pt electrode was used as the counter electrode, and a saturated calomel electrode (SCE) as the reference electrode. The supporting electrolyte was 0.2 M Na2SO4 solutions. The immersion district of the working electrode in the electrolyte was about 0.95 cm2. A 300 W Xe lamp furnished with a filter of 420 nm to filter off ultraviolet light. Photocurrent response testing of the photoanodes with on and off cycles was carried out.
3 Results and discussion
3.1 SEM presentation
The surface microtopography of OV–ZnO and CoSx/OV–ZnO samples were observed by SEM, and the results were showed in Fig. 1. Figure 1a was a scanning electron micrograph (SEM) of oxygen ZnO. It results revealed that OV–ZnO crystals was the morphology of hexagonal wurtzite structure. It had a good crystallinity. Figure 1b, c were a SEM images of a composite catalyst 50%CoSx/OV–ZnO. It can be seen from the diagram that the CoSx nanoparticles had good dispersivity, which was consistent with the XRD results. It was uniformly diffused on the OV–ZnO surfaces. This dispersion structure provided more active sites, which enhanced the photocatalytic activity.
3.2 TEM presentation
The composite catalyst CoSx/OV–ZnO was detected by the transmission electron microscope (TEM). In Fig. 2a, the crystalline black massive structure was OV–ZnO. The grayish white substance was CoSx adjacent to OV–ZnO, the CoSx catalyst retained amorphous structure. Figure 2b showed the existence of OV–ZnO. The lattice fringes of OV–ZnO can be clearly noticed, which was 0.18 nm and 0.2 nm in lattice pitch, corresponding to the (100) plane and (101) plane of OV–ZnO, respectively, it was consistent with the XRD results of Fig. 3a. Figure 2c was an EDS. It revealed the presence of S, Co, O and Zn elements in 50% CoSx/OV–ZnO composite catalyst [29, 30]. Furthermore, Cu element of copper carriers was tested too.
3.3 XRD description
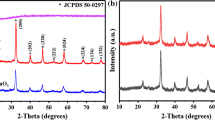
The XRD figures (Fig. 3) of ZnO, OV–ZnO, 50% CoSx/ZnO and 50% CoSx/OV–ZnO samples were observed. In Fig. 3a, the XRD peak of ZnO samples and hexagonal wurtzite phase ZnO (JCPDS no.36-1451) keep good concordance. The ZnO displayed diffraction peaks at 2θ degrees of 31.895, 34.588, 36.430, 47.697, 56.767, 63.003, 68.105 corresponded to the (100), (002), (101), (102), (110), (103) and (112) planes of the hexagonal wurtzite phase ZnO (JCPDS no.36-1451), respectively. As you can see from Fig. 3a, ZnO no additional impurity peak, and the ZnO catalyst had good crystallinity. The diffraction peak intensities of ZnO gradually decreased by oxygen vacancies and CoSx increasing. The particle size of ZnO is 43.70–58.38 nm; the particle size of OV–ZnO is 37.58–55.19 nm; the particle size of CoSx/ZnO is 34.81–51.09 nm; and the particle size of CoSx/OV–ZnO is 29.08–47.39 nm. From the calculated grain size, it can be seen that the smaller the grain size, the higher the dispersion, which is conducive to the photocatalytic hydrogen production reaction, which is consistent with the XRD diagram. In Fig. 3b, the XRD pattern exhibited that the loaded CoSx were amorphous on the ZnO or OV–ZnO surface [31], which was in line with the TEM analysis results of Fig. 3a. So, there was same peak position as pure ZnO in all samples (Fig. 3a), there are no observed obvious diffraction peaks about CoSx in composite catalyst.
3.4 X-ray photoelectron spectroscopy of ZnO and OV–ZnO
The element valence of ZnO, OV–ZnO and CoSx/OV–ZnO were detected by XPS. And the results are recorded in Fig. 4. As appeared in Fig. 4a, the XPS full spectrum indicate that the catalyst CoSx/OV–ZnO mainly consists in Zn, O, S and Co elements. In Fig. 4b, it shows a comparison between OV–ZnO and ZnO′s full spectrum graphs, and their difference is small. In Fig. 4c, In ZnO, the peak center of Zn 2p3/2 and Zn 2p1/2 are founded to 1021.45 eV and 1044.52 eV, respectively, which is assigned to the Zn2+ of ZnO [32,33,34]. The binding energy of Zn 2p3/2 and Zn 2p1/2 are situated to 1021.36 and 1044.5 eV, which is shifted towards high power of OV–ZnO. This peak migration of ZnO and OV–ZnO catalyst could be ascribed to the reduction of the surface band bending [28]. In Fig. 4d, the O 1s spectra of ZnO and OV–ZnO have three constituents. There are O 1s binding energy peaks at 530.1 eV, 531.6 eV and 532.3 eV. They are originated as the core electrons of lattice oxygen, O2− around the oxygen-defect areas and surface hydroxyl, respectively [28, 35]. In Fig. 4e, the Co 2p spectra of CoSx have four diffraction peaks, the peak at 781.87 eV and 786.17 eV are attributed to Co 2p3/2, while the peak at 797.98 eV and 802.99 eV are ascribed to Co 2p1/2 [36]. In Fig. 4f, it is S 2p spectra, the peaks at 168.84 and 169.91 eV were ascribed to S 2p3/2 and S 2p1/2 of CoSx. S is mainly in the form of S2− in CoSx. Relative ratio of peak area can be determined to increase from 0.34 of ZnO to 0.42 of OV–ZnO about oxygen vacancy (Table 1). The XPS analysis results further confirm that the ZnO and OV–ZnO photocatalyst is successfully prepared.
3.5 UV-Vis analysis
As shown Fig. 5, the optical properties of the different samples are determined by UV–Vis diffuse reflectance spectroscopy. The absorption edge of oxygen vacancy ZnO sample is more than ZnO in Fig. 5a. With the addition of CoSx, the absorption range of visible light in CoSx/ZnO and CoSx/OV–ZnO are increased (Fig. 5b). It shows relatively strong visible light absorption in CoSx/OV–ZnO than CoSx/ZnO. And Fig. 5c–f corresponds to the band gap images of ZnO, OV–ZnO, CoSx/ZnO and CoSx/OV–ZnO catalysts, respectively. The band gap of the ZnO is 3.04 eV, when the oxygen vacancy of ZnO is improved, which the band gap is 2.98 eV [37]. And when the CoSx is successfully loaded on the surface of ZnO and OV–ZnO, the band gap of the composite CoSx/OV–ZnO decreases 1.05 eV than ZnO. We can know that the oxygen vacancies and CoSx can extend the visible light absorption of ZnO [38, 39]. Under the visible light, the reduction of the photocatalyst band gap is conducive to the separation of electron–hole pairs, thus improving the activity of photocatalytic hydrogen evolution.
3.6 Brunauer–Emmett–Teller (BET) analysis
In order to analyze the physical characteristics of four samples, such as BET surface area and pore size, and they are investigated using physical adsorption apparatus. Figure 6a shows the nitrogen adsorption–desorption isotherms of ZnO, OV–ZnO, CoSx/ZnO and CoSx/OV–ZnO samples. The curves of CoSx/ZnO and CoSx/OV–ZnO are type III isotherms with H3 typical hysteresis loop. At the high relative pressure range from 0.75 to 1.0 (P/Po), the CoSx/OV–ZnO shows high adsorption, indicating the presence of accumulational pores [40]. The SBET of ZnO, OV–ZnO, CoSx/ZnO, CoSx/OV–ZnO is 4.04, 4.08, 23.13 and 52.21 m2/g in Table 2, respectively. The CoSx/OV–ZnO sample has better specific surface area and pore volume after introducing oxygen vacancy and CoSx in ZnO, this indicates that oxygen vacancies and COSx have obvious effects on ZnO. Which will be more favorable for the adsorption of dye molecules, leading to enhance the photocatalytic activity of the composite catalyst [41, 42]. In Fig. 6b depicts that ZnO, OV–ZnO, CoSx/ZnO and CoSx/OV–ZnO samples displays a similar pore size distributions curves, which consists of micropores, mesopores and micropores. The average pore size is located in the mesopores. This will be more conducive to the catalytic reaction. In Fig. 6c depicts that BET surface area plots along with corresponding regression coefficient values [43].
3.7 The photocatalytic performance of catalysts and pH (TEOA) effecting for hydrogen evolution
Photocatalytic hydrogen production was evaluated under solar light irradiation using TEOA (sacrificial agents) and EY dye (sensitizer) aqueous solution. As follows, the ZnO oxygen vacancy is improved, and CoSx is introduced, the consequence is displayed in Fig. 7. Figure 7a displays the loading amount of CoSx in the composite catalyst. With the loading amount of CoSx increase, the H2 evolution amount of the composite catalyst is improved from 139 µmol. The increase may be due to the CoSx has excellent electron acceptance and transfer capabilities, and photoelectrons can be collected and returned to ZnO, further preventing photoelectrons from binding to holes. When the loading amount of CoSx on OV–ZnO reached 50%, and the maximum hydrogen production amount reached 256 µmol about 5 h. The photocatalytic activity of CoSx/OV–ZnO was obviously decreased when the excess CoSx was increased. This is due to the excessive CoSx can mask the active sites of OV–ZnO and block the contact with the solution: excessive CoSx shields the incident light, inhibit the light absorption of OV–ZnO and reduces the generation of light charge; high load CoSx can be used as the charge recombination center.
It is worth noting that pH (TEOA) has a grand influence on the photocatalytic performance in Fig. 7b. While pH is 8, the activity of hydrogen evolution is low, and hydrogen production is simply 23 µmol. With the increase of pH value, the activity of hydrogen production continues to increase and hydrogen production amount attained the maximum at pH is11. The amount of CoSx/OV–ZnO photocatalytic hydrogen production attained 256 µmol. After that, we see that the number of hydrogen evolution decreases from 11 to 12. At pH 11 (TEOA solution), and EY can validly adsorb on the composite catalyst surface to part in the catalytic reaction. While pH is 12, this is due to the strongly alkaline conditions lead to low proton (H+) concentration in the solution, the thermodynamic driving force for H2 evolution from water decreased due to the lower concentration of H+ and EY can be reductively quenched by TEOA completely at pH 12 [44], therefore, the hydrogen production activity is affected.
3.8 The H2 evolution performance and the stability testing
As in Fig. 8a, ZnO displays a low photocatalytic hydrogen evolution activity. After that, we increase oxygen vacancy of ZnO. It displays an impact in increasing the hydrogen production performance of ZnO. It is worth mentioning that photocatalytic performance is greatly enhanced as introducing CoSx on OV–ZnO, the amount of photocatalytic hydrogen production reached 256 µmol in 5 h, increased 30% than CoSx/ZnO. So, oxygen vacancy and CoSx can substantially improve photocatalytic activity. In Table 3, we provide comparison of photocatalytic activity.
In order to prove the stability of the composite catalyst, we require doing stability experiment. The stability trials are carried out in TEOA aqueous solution. As shown in Fig. 8b, there are total 15 h in this experiment, and subsequently every 5 h became a cycle with three processes. After each cycle, the catalyst solution requires to be centrifuged. We require doing rejoin TEOA and EY, and furthermore oxygen is removed in the outside to enter the next cycle. When the three stages are accomplished, the amount of hydrogen production decreased slightly than the first cycle, which is due to the catalyst decreases during centrifugation. From the above discuss, the hydrogen production performance did not reduce obviously after 15 h irradiation, and it has good hydrogen production stability in the composite catalyst system [45].
3.9 Electrochemical analysis
Prove the vital role of oxygen vacancies and CoSx together improving charge separation for enhancing H2 evolution performance. Transient photocurrent measurements of ZnO, OV–ZnO, CoSx/ZnO and 50% CoSx/OV–ZnO samples are carried out. They reveal that catalysts have a photocurrent response in the course of three on–off cycles under visible light irradiation. In Fig. 9a, the light density of samples is the same as the trend observed in their photocatalytic hydrogen evolution performance. The highest photocurrent density can be seen in the CoSx/OV–ZnO sample underneath visible light irradiation, which reflects a better visible-light response. At the same time, the ZnO shows the lowest photocurrent intensity. The pure ZnO has low electron migration efficiency under visible light irradiation, resulting in the rapid recombination of photoelectrons and holes. After increasing the oxygen vacancy of ZnO and the amorphous CoSx, the light density is rapidly improved and more efficient charge separation [46]. This consequence indicates activity of the CoSx/OV–ZnO photocatalytic H2 evolution is attributed to the oxygen vacancy and the amorphous CoSx. The photoelectric test results are in good agreement with the hydrogen production.
In addition, Fig. 9b is a linear sweep voltammetry (LSV) scan image of ZnO, OV–ZnO, CoSx/ZnO and CoSx/OV–ZnO. As shown in the diagram, the H+ is reduced a cathode current of H2, the current density is not very different at the range of 0 to − 0.3 applied voltage. The possible reason is that the reduction of H+ to H2 requires a minimum applied voltage in the TEOA solution [47]. From − 0.3 to − 0.6 voltage range, composite catalyst 50%CoSx/OV–ZnO shows higher reduction current density. The result can promote the catalytic hydrogen production activity of ZnO catalyst. This consequence is in good accord with the above test results. It will be showed that 50% CoSx/OV–ZnO catalyst has a good foreground of application.
3.10 Photoluminescence properties
Fluorescence spectra is used to demonstrate the crucial role of CoSx and OV–ZnO in promoting photoelectron transfer. Fluorescence spectroscopy of EY and EY sensitized ZnO, OV–ZnO, CoSx/ZnO and CoSx/OV–ZnO are followed in Fig. 10. Which mainly investigates the transfer of photo-generated electrons and the mechanism of photocatalytic hydrogen production [48, 49]. It can be seen the EY aqueous solution shows an intensive emission peak centered at 535 nm. With the modification of Zinc Oxide, the peak intensity of EY decreases slightly. With the increase of the ZnO surface oxygen vacancy, EY exhibits a broad emission and the peak intensity decreases significantly, which is originated from oxygen vacancy of ZnO crystals [50]. Additionally, the fluorescence intensity of EY decreases after adding CoSx. Especially, when oxygen vacancies and CoSx are added together, the fluorescence intensity and emission peak of EY reduced seriously. The reason is that two types of semiconductors combine tightly together to form a heterostructure, which contributes to the transmission of photoelectrons and it inhibits the recombination of photogenerated electrons and hole pairs [51, 52].
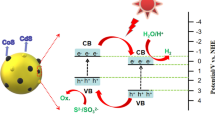
3.11 Mechanism of photocatalytic H2 evolution
Such as in Fig. 11, the mechanism of photocatalytic H2 evolution is conjectured in system. The hydrogen production activity of CoSx/OV–ZnO increases significantly because the heterogeneous structure of CoSx and oxygen vacancy ZnO, which the interface helps to enhance the photoelectrons into conduction of the catalyst. Therefore, the recombination of photoelectrons and hole pairs is suppressed, thereby enhancing the photocatalytic activity. EY (sensitizers) and TEOA (electronic sacrificial agent) play a role. As follows, under visible light irradiation, the EY molecules on the superficial OV–ZnO and CoSx, it absorbs photon energy to become excited singlet state EY1*. The singlet excited EY1* quickly turn out to be the triplet excited state EY3* by a valid inter system crossing (ISC). EY3*can be reduced to EY−· by TEOA. The EY−· releases electrons to the CoSx–OV–ZnO active surface to participate in proton reduction reaction, the electrons of some EY−· species is passed to the OV–ZnO conduction band and then pass to CoSx, and H+ is reduced to H2 [53,54,55]. EY−· is reduced to ground state by TEOA and continues to absorb photon energy to take park in the reaction. In addition, OV–ZnO can also absorb photon energy to excite electron–hole pairs, which are transferred directly to CoSx to reduce protons (H+) for hydrogen production.
4 Conclusion
We have illustrated that the amorphous CoSx modified OV–ZnO catalyst by a simple hydrothermal means. The oxygen vacancies and the amorphous CoSx are considered as an effective interfacial mediator to boost the charge transfer, which induce an efficient charge separation. The amount of 50% CoSx/OV–ZnO photocatalytic hydrogen evolution has been greatly improved. The number of the ZnO sample hydrogen production is zero basically, and the hydrogen production amount of composite catalyst 50% CoSx/OV–ZnO reached 256 µmol in 5 h, increased 30% than CoSx/ZnO. The composite catalyst system has a better hydrogen generation stability. Electrochemical and fluorescence test is in good agreement with the hydrogen production. Additionally, the mechanism reveals that amorphous CoSx mainly promoted the H+ reduction to H2 due to the exposure of abundant active sites. As a consequence of composite catalyst have high activity and low cost. So, the CoSx/OV–ZnO has a good application prospect in photocatalyst filed.
References
P. Wang, S. Zhan, H. Wang, Y. Xia, Appl. Catal. B 230, 210–219 (2018)
J. Low, J. Yu, M. Jaroniec, S. Wageh, A.A. Alghamdi, Adv. Mater. 29, 20 (2017)
S.K. Jin, J.W. Oh, S.I. Woo, Catal. Today 293–294, 8–14 (2017)
J.F.D. Costa-Serra, R. Guil-López, A. Chica, Int. J. Hydrog. Energy 35, 6709–6716 (2010)
R.M. Kakhki, R. Tayebee, F. Ahsani, J. Mater. Sci. Mater. Electron. 28(8), 1–12 (2017)
Z. Wu, Y. Li, L. Gao, S. Wang, G. Fu, Dalton Trans. 45, 11145–11149 (2016)
J. Wang, Y. Xia, H. Zhao, G. Wang, Appl. Catal. B 206, 406–416 (2017)
B.M. Rajbongshi, S.K. Samdarshi, B. Boro, J. Mater. Sci. Mater. Electron. 26(1), 377–384 (2015)
M. Salavati-Niasari, F. Davar, M. Mazaheri. Mater. Lett. 62(12–13), 1890–1892 (2008)
M. Salavati-Niasari, F. Davar, A. Khansari, J. Alloys Compd. 509, 61–65 (2011)
M. Faisal, A.A. Ismail, A.A. Ibrahim, H. Bouzid, S.A. Al-Sayari, Chem. Eng. J. 229, 225–233 (2013)
C.J. Chang, K.L. Huang, J.K. Chen, K.W. Chu, J. Taiwan. Inst. Chem. Eng. 55, 82–89 (2015)
Z. Dong, Y. Wu, N. Thirugnanam, Appl. Surf. Sci. 430, 293–300 (2017)
K. Pandiselvi, H. Fang, X. Huang, J. Wang, X. Xu, L. Tao, J. Hazard. Mater. 314, 67–77 (2016)
S.B. Narendranath, S.V. Thekkeparambil, L. George, S. Thundiyil, R.N. Devi, J. Mol. Catal. A 415, 82–88 (2016)
Q. Hu, L. Yang, G. Fan, F. Li, J. Catal. 340, 184–195 (2016)
J. Li, M. Zhang, Z. Guan, Q. Li, C. He, J. Yang, Appl. Catal. B 206, 300–307 (2017)
X.Y. Feng, P.F. Wang, J. Hou, J. Qian, J. Hazard. Mater. 351, 196–205 (2018)
V. Etacheri, M.K. Seery, S.J. Hinder, S.C. Pillai, Adv. Funct. Mater. 21, 3744–3752 (2011)
Z. Liu, Z. Zhao, Y. Wang, S. Dou, D. Yan, D. Liu, Adv. Mater. 29, 1606207 (2017)
L. Zhuang, L. Ge, Y. Yang, M. Li, Y. Jia, X. Yao, Adv. Mater. 29, 1606793 (2017)
W. Xu, F. Lyu, Y. Bai, A. Gao, J. Feng, Z. Cai, Nano Energy 43, 110–116 (2017)
B. Archana, K. Manjunath, G. Nagaraju, K.B.C. Sekhar, N. Kottam, Int. J. Hydrog. Energy 42, 5125–5131 (2017)
T. Jiang, S. Yang, P. Dai, X. Yu, Z. Bai, M. Wu, Electrochim. Acta 261, 143–150 (2018)
Y. Yang, F. Li, W. Li, W. Gao, H. Wen, J. Li, Int. J. Hydrog. Energy 42, 6665–6673 (2017)
L. Zhu, S.B. Jo, S. Ye, K. Ullah, Z.D. Meng, W.C. Oh, J. Ind. Eng. Chem. 22, 264–271 (2015)
X. Ma, J. Li, C. An, J. Feng, Y. Chi, J. Liu, Nano Res. 9(8), 2284–2293 (2016)
Y.P. Xie, Y. Yang, G. Wang, G. Liu, J. Colloid Interface Sci. 503, 198–204 (2017)
S. Zinatlooajabshir, M.S. Morassaei, M. Salavatiniasari, J. Colloid Interface Sci. 497, 298–308 (2017)
M.S. Morassaei, S. Zinatloo-Ajabshir, M. Salavati-Niasari, J. Mater. Sci. Mater. Electron. 27(11), 1–9 (2016)
Y. Lei, J. Hou, F. Wang, X. Ma, Z. Jin, J. Xu, Appl. Surf. Sci. 420, 456–464 (2017)
M. Salavati-Niasari, F. Davar, Z. Fereshteh, Chem. Eng. J. 146(3), 498–502 (2009)
X. Hao, J. Zhou, Z. Cui, Y. Wang, Y. Wang, Z. Zou, Appl. Catal. B 229, 41–51 (2018)
Z. Guan, P. Wang, Q. Li, Y. Li, X. Fu, J. Yang, Chem. Eng. J. 327, 397–405 (2017)
W. Liu, X. Tang, Z. Tang, J. Appl. Phys. 114, 123911 (2013)
G. Ai, R. Mo, H. Li, J. Zhong, Nanoscale 7, 6722–6728 (2015)
M.S. Morassaei, S. Zinatloo-Ajabshir, M. Salavati-Niasari, J. Mol. Liq. 220, 902–909 (2016)
H. Khojasteh, M. Salavati-Niasari, A. Abbasi, J. Mater. Sci. Mater. Electron. 27(2), 1261–1269 (2016)
H. Safajou, H. Khojasteh, M. Salavatiniasari, J. Colloid Interface Sci. 498, 423–432 (2017)
X. Hao, Z. Jin, H. Yang, G. Lu, Y. Bi, Appl. Catal. B 210, 45–56 (2017)
D. Liu, Z. Jin, H. Li, G. Lu, Appl. Surf. Sci. 423, 255–265 (2017)
S. Zinatloo-Ajabshir, M.S. Morassaei, M. Salavati-Niasari, J. Clean. Prod. 198, 11–18 (2018)
S. Kumar, N.L. Reddy, A. Kumar, V. Krishnan, Int. J. Hydrog. Energy 43, 3988–4002 (2017)
H. Yu, J. Xu, H. Guo, Y. Li, Z. Liu, Z. Jin, RSC Adv. 7, 56417–56425 (2017)
H. Yu, X. Huang, P. Wang, J. Yu, J. Phys. Chem. C 120(7), 3722–3730 (2016)
X. Hao, Y. Wang, J. Zhou, Z. Cui, Y. Wang, Z. Zou, Appl. Catal. B 221, 302–311 (2018)
H. Yang, Z. Jin, H. Hu, Y. Bi, G. Lu, Appl. Surf. Sci. 427, 587–597 (2018)
M.S. Morassaei, S. Zinatloo-Ajabshir, M. Salavati-Niasari, Adv. Powder Technol. 28, 697–705 (2017)
M.S. Morassaei, A. Salehabadi, A. Akbari, S.H. Tavassoli, J. Alloys Compd. 769, 732–739 (2018)
B. Sambandam, R.J. Michael, P.T. Manoharan, Nanoscale 7, 13935 (2015)
S. Kumar, N.L. Reddy, H.S. Kushwaha, A. Kumar, K. Bhattacharyya, A. Halder, ChemSusChem. 10(18), 3588–3603 (2017)
H. Safardousthojaghan, M. Salavatiniasari, J. Clean. Prod. 148, 31–36 (2017)
P. Wang, T. Wu, C. Wang, J. Hou, J. Qian, Y. Ao, Acs. Sustain. Chem. Eng. 5, 7670–7677 (2017)
X. Hao, Z. Jin, S. Min, G. Lu, RSC Adv. 6(28), 23709–23717 (2016)
S. Min, Y. Lei, H. Sun, J. Hou, F. Wang, Mol. Catal. 440, 190–198 (2017)
Acknowledgements
This work was supported by Ningxia Higher Institutions Scientific Research Program (NGY2016146), and the Graduate Student Innovation Project at North MinZu University (YCX18083).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Li, Y., Xu, J., Liu, Z. et al. Performance of amorphous CoSx/oxygen vacancies ZnO heterojunction photocatalytic hydrogen evolution. J Mater Sci: Mater Electron 30, 246–258 (2019). https://doi.org/10.1007/s10854-018-0287-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-018-0287-3