Abstract
We fabricated a series of Y3Al5O12:Mn4+ and Y1−yLuyAl5O12:Mn4+ phosphors by a solid state reaction. The phase and the optical properties of the synthesized phosphors were investigated. Under the excitation at 465 nm, Y3Al5O12:Mn4+ phosphors show emission bands locating at deep red regions, which is induced by the spin- and parity-forbidden 2Eg → 4A1g transitions of Mn4+. The substitution of Y3+ by Lu3+ decreases the lattice parameter and thus strengthens the crystal field strength, which gives rise to the blue shift of emission band for Y1−yLuyAl5O12:Mn4+ phosphors. Due to the compensation of red light by Y3Al5O12:Mn4+ or Y1−yLuyAl5O12:Mn4+ phosphor, the values of correlated-color-temperature for fabricated LEDs are decreased, which leads to the suitable application for them in indoor illumination.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The well known two drawbacks of the white light emitting diodes generated by the blue chip and Y3Al5O12:Ce3+ phosphors are the high correlated-color-temperature (CCT) and poor color-rendering index (CRI) [1, 2]. And these two drawbacks are mainly induced by the absence of red spectral contribution. To improve the light properties of white light emitting diodes, one of widely used methods is to mix a highly efficient red phosphor showing strong blue absorption with Y3Al5O12:Ce3+ yellow phosphor [3,4,5,6,7,8]. In past years, most of attempts for the red phosphors always focus on rare earth ions doped phosphors. But we know that the storage of rare earth elements are lacking. As a result, researchers try their best to look for elements with abundant storage those can be used in red phosphors. And in recent years, Mn4+ is found to be a good candidate because that Mn4+ activated red phosphors are much cheaper and possess desirable spectral features [7].
Over the last several years, Mn4+ doped aluminate garnet (Ln3Al5O12, Ln = Lu, Y, Gd) phosphors have been paid more and more attentions [8,9,10]. It is known that Mn4+ prefers to occupy octahedral site to obtain high luminescence efficiency. In Ln3Al5O12 hosts, Mn4+ priors to substitute octahedral Al3+ site due to the little distinction of radius between Al3+ (0.535 Å) and Mn4+ (0.530 Å), which gives large opportunities for Ln3Al5O12:Mn4+ phosphors to maintain the rigid garnet structure, decrease defect formation and guarantee the high luminescence efficiency of Mn4+ [8]. In this work, we synthesized a series of Ln3Al5O12:Mn4+ (Ln = Y and Lu) phosphors by the solid state reaction and investigated the luminescent properties of the synthesized phosphors.
2 Materials and method
A series of Y3Al5O12:x%molMn4+ (x = 0.1, 0.2, 0.3 and 0.4) and Y1−yLuyAl5O12:0.3%molMn4+ (y = 1, 2 and 3) phosphors were synthesized by a solid state reaction. The chemical reagents of Y2O3 (99.9%), Lu2O3 (99.9%), Al2O3 (99.9%) and MnO2 (99.99%) were used as raw materials in the synthesis of Ln3Al5O12:Mn4+ (Ln = Y and Lu) phosphors. In the synthesis, chemical reagents with stoichiometric ratio were weighted and mixed in an agate mortar with the addition of a little amount of ethyl alcohol. After a thorough blending, the mixture was transferred into a platinum crucible and calcined at 1500 °C for 6 h in a muffle furnace. After the system cooled to room temperature naturally, the product was ground for subsequent measurements.
The phase was confirmed by X-ray powder diffraction (XRD) technique in a D8 advance X-ray diffractometer (Bruker, German) using Cu Kα radiation (λ = 0.15418 nm). The ultraviolet–visible (UV–vis) absorption spectra of the phosphors were recorded on a Cary 5000 UV–vis spectrophotometer. The excitation and emission spectra were measured by an Edinburgh Instrument FLS920 spectrophotometer equipped with a 150 W xenon lamp as the excitation source. The emission spectra of the fabricated LEDs were measured by a PMS-80 LED spectrophotocolorimeter.
3 Results and discussion
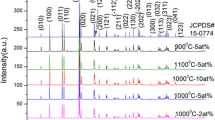
Figure 1 presents the XRD patterns of the synthesized Y3Al5O12:0.3mol%Mn4+, Y2LuAl5O12:0.3mol%Mn4+, YLu2Al5O12:0.3mol%Mn4+, Lu3Al5O12:0.3mol%Mn4+ phosphors and the standard data of Y3Al5O12 (JCPDs card no. 88-2047) and Lu3Al5O12 (JCPDs card no. 73-1369). The ionic radius of Y3+ (1.019 Å) is larger that of Lu3+ (0.977 Å), which induces the decrease of lattice parameter with the increase of Lu3+ and the shifts of diffraction peaks to larger 2θ angles. In the XRD patterns of the synthesized phosphors, there are no diffraction peaks corresponding to other phase, suggesting that Lu3+ and Mn4+ have entirely incorporated into the structure of Y3Al5O12. Herein, Lu3+ and Mn4+, respectively, substitute Y3+ and Al3+ sites due to the similar ionic radii.
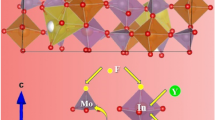
The luminescence of a phosphor has close relation with the crystal structure information of the host. Both Y3Al5O12 and Lu3Al5O12 have the cubic phase and belong to the space group of \({\text{Ia}}\bar {3}{\text{d}}(230)\). The parameters for Y3Al5O12 and Lu3Al5O12 are a = b = c = 12.008 Å and a = b = c =11.906 Å, respectively. Figure 2 shows the crystal structures of Y3Al5O12 and Lu3Al5O12 of the 1 × 1 × 1 unit cell. In Ln3Al5O12 (Ln = Y and Lu), Ln3+ ions coordinate with 8 O2− ions and form the polyhedral shape, but Al3+ ions have two different lattice sites and coordinate with 6 and 4 O2− ions to form AlO6 octahedral and AlO4 tetrahedral shapes. The AlO6 octahedral and AlO4 tetrahedral shapes connect with each other by means of sharing the same O2− ion. And in Y3Al5O12 and Lu3Al5O12, the bond lengths between Mn and ligand O are 1.921 and 1.864 Å, respectively. As the atom number per cell keeps constant, the decrease of lattice parameter means the shortening of distance between atoms. This weakens the Mn interaction between atoms. So, Mn4+ will experience a stronger crystal field as the content of Lu increases.
Figure 3 shows the absorption spectra of Y3Al5O12:x%molMn4+ (x = 0.1, 0.2, 0.3 and 0.4) phosphors in the UV–vis range. Y3Al5O12:Mn4+ phosphors shows obvious absorption bands in UV–vis range. There are a strong absorption band in the range of 225–400 nm and a relatively weak band in the range of 425–525 nm. The wide and strong absorption band in the range of 225–400 nm originates from the Mn4+ → O2− charge transfer, the spin-allowed 4A2g → 4T1g and 4A1g → 4T2g transitions, but the absorption band in the range of 425–525 nm corresponds to the 4A2g → 4T2g transition [11,12,13]. The absorption intensity increases in UV range and the absorption edge in UV range shifts to longer wavelength with the increase of Mn4+ doping concentration. Unlike in UV range, the absorption intensity increases with the increasing Mn4+ doping concentration but the absorption edge does not vary.
Figure 4 presents the excitation spectrum of Y3Al5O12:0.3mol%Mn4+ phosphor and emission spectra of Y3Al5O12:x%molMn4+ (x = 0.1, 0.2, 0.3 and 0.4) phosphors. In the excitation spectrum monitored at 667 nm, there are two obvious excitation bands peaking at about 324 and 465 nm, which originates from the 4A2g → 4T1g and 4A1g → 4T2g transitions of Mn4+ in the octahedral coordination. The wide range of 275–525 nm for the excitation spectrum of Y3Al5O12:Mn4+ suggests that the phosphor can be excited by the lights in UV, near-UV and blue regions. It is also worth noting that the excitation band of Y3Al5O12:Mn4+ hardly overlaps with the emission band of Y3Al5O12:Ce3+ (broad yellow emission band peaking at 530–540 nm) [2, 14, 15], suggesting that the risk of photon re-absorption usually taking place between the nitride red phosphor and the Y3Al5O12:Ce3+ yellow phosphor is highly reduced. Under the excitation at 465 nm, Y3Al5O12:x%molMn4+ (x = 0.1, 0.2, 0.3 and 0.4) phosphors show emission bands locating at deep red light region, which comes from the spin- and parity-forbidden 2Eg → 4A1g transitions of Mn4+. The emission intensity increases firstly with the increasing Mn4+ concentration up to x = 0.3 and then decreases with the further increasing Mn4+ concentration. The decrease of emission intensity is induced by the concentration quenching. Concentration quenching mechanism leads to the inability to increase the emission intensity by simply increasing Mn4+ ions. Moreover, it can be seen that the peaks of emission bands do not shift with the changing Mn4+ concentration. It is known that Mn4+ substitutes the site of Al3+ and these two ions have similar ionic radii in Y3Al5O12. The similar sizes keep the lattice without any constriction or expansion. As a result, there is the invariability of the field splitting and thus the locations of emission peaks do not shift.
Figure 5 shows the emission spectra of Y1−yLuyAl5O12:0.3%molMn4+ (y = 0, 1, 2 and 3) phosphors under the excitation at 465 nm. All of phosphors show emission bands corresponding to the spin- and parity-forbidden 2Eg → 4A1g transitions of Mn4+. The Lu3+ substitution does not lead to the obvious change of emission band shape but induces the slightly blue shifts of emission peaks. For Y3Al5O12:0.3%molMn4+, the peak of the strongest emission band locates at 667 nm. But for Y2LuAl5O12:0.3%molMn4+, YLu2Al5O12:0.3%molMn4+ and Lu3Al5O12:0.3%molMn4+ phosphors, the peaks of the strongest emission bands shift to 665, 664 and 662 nm, respectively. Generally, the Mn4+ luminescence has a close relation with the crystal strength and site symmetry of the host. Energy of the Mn4+: 2Eg → 4A2g transition is mainly determined by the nephelauxetic effect which is correlated with the wave-function overlap between the Mn4+ ions and the ligand [16,17,18]. As discussed above, the Lu3+ substitution decreases the lattice parameter and shortens the band length between Mn4+ and ligand O2−. That is to say, Mn4+ experiences a stronger crystal field in Y1−yLuyAl5O12:0.3%molMn4+ (y = 1, 2 and 3) phosphors. The stronger crystal field strength induced by the shorter band length between Mn4+ and ligand O2 makes the energy-level splitting more, which gives rise to the blue shift of emission band for Lu3+ substituted Y3Al5O12:Mn4+ phosphors.
The LEDs based on GaN blue chips were fabricated by mixing Y3Al5O12:Ce3+ phosphor (GaN-YAG:Ce3+ LED) with Y3Al5O12:Mn4+ phosphor (GaN-YAG:Mn4+ LED) or Lu3Al5O12:Mn4+ phosphor (GaN-LuAG:Mn4+ LED). Figure 6 gives the electro-luminescence spectra of the fabricated LEDs under the excitation of 20 mA current. All of electro-luminescence spectra consist of emission bands locating at blue and yellow regions, which, respectively, originate from the blue chip and the Y3Al5O12:Ce3+ phosphor. The emission bands locating at deep red region for GaN-YAG:Mn4+ and GaN-LuAG:Mn4+ LEDs are induced by the mixed Y3Al5O12:Mn4+ phosphor or Lu3Al5O12:Mn4+ phosphor. The CIE coordinates for GaN-YAG:Ce3+, GaN-YAG:Mn4+ and GaN-LuAG:Mn4+ LEDs are (0.325, 0.338), (0.359, 0.327) and (0.366, 0.319), respectively. And the CCT values for GaN-YAG:Ce3+, GaN-YAG:Mn4+ and GaN-LuAG:Mn4+ LEDs are 5841, 4290 and 3928, respectively. These results demonstrate that the mixed Y3Al5O12:Mn4+ phosphor or Lu3Al5O12:Mn4+ phosphor can decrease the CCT values of fabricated LEDs. Generally, the warm white light is suitable for home applications as the value of CCT less than 5000 K and the cold white light is suitable for commercial lighting purposes if the value of CCT higher than 5000 K [19, 20]. Therefore, we can conclude that Y3Al5O12:Mn4+ phosphor or Lu3Al5O12:Mn4+ phosphor is the promising commercial red phosphor in warm white LEDs.
4 Conclusion
Y3Al5O12:Mn4+ and Y1−yLuyAl5O12:Mn4+ phosphors were synthesized successfully by a solid state reaction. In Y3Al5O12 or Y1−yLuyAl5O12 hosts, Mn4+ ions substituted Al3+ sites and formed solid solutions. Under the excitation at 465 nm, the synthesized Y3Al5O12:Mn4+ and Y1−yLuyAl5O12:Mn4+ phosphors show emission bands corresponding to the spin- and parity-forbidden 2Eg → 4A1g transitions of Mn4+. Due to the smaller ionic radius of Lu3+, the substitution of Y3+ by Lu3+ decreases the lattice parameter and thus strengthens the crystal field strength, which gives rise to the blue shift of emission band for Lu3+ substituted Y3Al5O12:Mn4+ phosphors. The electro-luminescence spectra of LEDs fabricated by mixing Y3Al5O12:Ce3+ phosphor with Y3Al5O12:Mn4+ or Y1−yLuyAl5O12:Mn4+ show obvious emission band locating at red region, which decreases the correlated-color-temperature of white light. Y3Al5O12:Mn4+ and Y1−yLuyAl5O12:Mn4+ phosphors are suitable for applications in warm white LEDs due to the compensation of red light.
References
J.K. Sheu, S.J. Chang, C.H. Kuo, Y.K. Su, L.W. Wu, Y.C. Lin, W.C. Lai, J.M. Tsai, G.C. Chi, R.K. Wu, IEEE Photon. Technol. Lett. 15, 18 (2003)
Y. Yang, J. Li, B. Liu, Y. Zhang, X. Lv, L. Wei, X. Wang, J. Xu, H. Yu, Y. Hu, H. Zhang, L. Ma, J. Wang, Chem. Phys. Lett. 685, 89 (2017)
P. Pust, V. Weiler, C. Hecht, A. Tücks, A.S. Wochnik, A.K. Henb, D. Wiechert, C. Scheu, P.J. Schmidt, W. Schnick, Nat. Mater. 13, 891 (2014)
G. Mo, W. Wang, K. Wang, G. Wen, M. Zhu, J. Wang, J. Mater. Sci. Mater. Electron. 28, 8155 (2017)
Y. Wu, Z. Chi, T. He, J. Mater. Sci. Mater. Electron. 28, 14591 (2017)
F. Baur, T. Jüstel, J. Lumin. 177, 354 (2016)
B. Wang, H. Lin, F. Huang, J. Xu, H. Chen, Z. Lin, Y. Wang, Chem. Mater. 28, 3515 (2016)
Y. Chen, K. Wu, J. He, Z. Tang, J. Shi, Y. Xu, Z.-Q. Liu, J. Mater. Chem. C 5, 8828 (2017)
J. Long, Y. Wang, R. Ma, C. Ma, X. Yuan, Z. Wen, M. Du, Y. Cao, Inorg. Chem. 56, 3629 (2017)
D. Chen, Y. Zhou, W. Xu, J. Zhong, Z. Ji, W. Xiang, J. Mater. Chem. C 4, 1704 (2016)
A. Fu, L. Zhou, S. Wang, Y. Li, Dyes Pigments 148, 9 (2018)
Z. Liu, H. Li, K. Liu, H. Yu, K. Cheng, Sol. Energy 142, 61 (2017)
J. Liu, Y. Li, J. Ke, S. Wang, L. Wang, H. Xiao, Appl. Catal. B Environ. 224, 705 (2018)
Y. Kim, K.B. Shim, M. Wu, H.-K. Jung, J. Alloys Compd. 693, 40 (2017)
W.T. Hong, J.H. Lee, J.W. Son, Z. Lee, H.J. Park, H.S. Kim, J.S. Lee, H.K. Yang, Ceram. Int. 42, 2204 (2016)
M.G. Brik, S.J. Camardello, A.M. Srivastava, ECS J. Solid State Sci. Technol. 4, R39 (2015)
M.G. Brik, A.M. Srivastava, ECS J. Solid State Sci. Technol. 2, R148 (2013)
Z. Liu, H. Li, G. Cao, Int. J. Environ. Res. Public Health 14, 857 (2017)
Z. Liu, W. Xu, X. Zhai, C. Qian, X. Chen, Renew. Energy 101, 1131 (2017)
V. Neharika, J. Kumar, V.K. Sharma, O.M. Singh, H.C. Ntwaeaborwa, Swart, J. Electron. Spectrosc. 206, 52 (2016)
Acknowledgements
The work is supported by the National Natural Science Foundation of China (no. 11602066), the China Postdoctoral Science Foundation on the 56th Bath of Surface Founds the Project (no. 2014M561327), the National Science Foundation of Heilongjiang Province of China (nos. QC2015058 and 42400621-1-15047) and the Foundation Research Funds for the Central Universities (nos. HEUCF130214 and HEUCFM170204).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, ZW., Sun, XY. & Wang, ZQ. Ln3Al5O12:Mn4+ (Ln = Y and Lu): non-rare-earth doped red phosphor for improving light properties of white light emitting diodes. J Mater Sci: Mater Electron 29, 5282–5286 (2018). https://doi.org/10.1007/s10854-017-8492-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-8492-z