Abstract
Transparent conducting aluminum (i.e. 2 at.%) doped zinc oxide (AZO) thin films were prepared on glass substrates by sol–gel dip coating technique using different solvents. This inexpensive dip coating method involves dipping of substrate consecutively in zinc solution and tube furnace for required cycles. Prepared films were investigated by XRD, SEM, PL, Raman spectroscopy optical and electrical studies. From the XRD studies, it confirmed the incorporation of aluminum in ZnO lattice. The prepared samples are polycrystalline nature, and these films reveal hexagonal wurtzite arrangement with (002) direction. The structural parameters such as crystallite size, dislocation density, micro strain, texture coefficient and lattice constant were investigated. SEM study showed well defined smooth and uniformed ganglia shaped grains are regularly distributed on to the entire glass substrate without any pinholes and cracks, and the average grain size is 75 nm. From the optical studies, the observed highest transmittance is 93% in the visible range and the band gap (Eg) is 3.26 eV. Room temperature PL spectra exhibited strong UV emission peak located at 386 nm for all the films. The electrical properties of the AZO thin films were studied by Hall-Effect measurements and found as n-type conductivity with high carrier concentrations (n), 2.76 × 1019 cm− 3 and low resistivity (ρ), 7.56 × 10− 3 Ω cm for the film deposed using methanol as solvent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In general transparent conducting oxide films (TCOs) play important roles in the optoelectronic devices because of its suitable properties such as high transparency and excellent electrical conductivity. Last few years the transition-metal oxides and their alloys have been unique physical and chemical properties such as Eg of 3.37 eV at ambient temperature, large exciton binding energy of 60 meV. ZnO play a major role for many optoelectronic devices and applications because it has the properties of high electron mobility, excellent transparency, strong room-temperature luminescence and wide band gap [1]. ZnO is an II-VI group semiconductor family, and it adopts a hexagonal wurtzite crystal structure. It has been used for solar cell, photodetector and LED [2], Femtosecond laser direct writing of microholes on roughened ZnO for output power enhancement of InGaN light-emitting diodes [3], Enhanced photoresponse of self-powered perovskite photodetector based on ZnO nanoparticles decorated CsPbBr3films [4]. Also, it has unique benefits compare to another challenger, e.g.: low material costs, richness in the earth crust, non-toxicity, chemical stability, high transparency, etc. [5, 6]. Along with all above-mentioned applications, ZnO also is used in some other purposes such as gas sensing, dye sensitization and perovskite solar cells [7].
Depending on the doping scheme, ZnO can be an n-type and a p-type semiconductor [8, 9]. However, p-type doping in ZnO may be possible by substituting either group-I elements (Li, K, Na, etc.) for Zn-atoms or group-V elements (P, N, Sb, As, etc.) and for oxygen atoms [5, 8, 10]. Similarly, for n-type ZnO doping with group-III elements (B, Al, Ga, In), as substitution elements for Zn, has been attempted by many researchers [1, 8, 9, 11, 12]. As a result of this a high transparency, high quality and highly conductive ZnO films as this substitution of divalent Zn2+ by a trivalent ion generates a free electron. Predominantly, Al-doped ZnO (AZO) thin films have attracted an extensive amount of interest due to their low optical loss with good electrical conductivity. Further, the Al doped ZnO (AZO) is a good alternative to indium tin oxide (ITO) because it has low-cost material and also thermally and chemically stable compared to ITO.
In recent years, many researchers have been prepared highly transparent and conducting AZO thin films by various techniques. Many physical and chemical methods, such as magnetron sputtering [13], pulsed laser deposition [14], hydrothermal [15], chemical vapor deposition [16], spray pyrolysis [17], SILAR method [18] and sol–gel coating [19]. Among these techniques, the sol–gel process presents many advantages compared to other techniques such as excellent homogeneity, low-cost, thickness control and possibility of coating vast and complex surfaces and low-temperature processing. Nowadays, the sol–gel methods have been extensively used to obtain various kinds of functional oxide films. Especially, the dip coating method is a good choice for the formation of large area thin film for various optoelectronic applications due to its significant advantage in making homogeneous films.
In previous researchers have studied pure ZnO thin film, and also discussed the effect of two different solvents using ethanol (C2H6O) and 2-methoxyethanol (2-ME) by sol–gel dip coating method [20, 21].
However, to the best of our knowledge, ZnO thin films have not been reported using many solvents (three or more) by dip coating method. In this study, we investigate for the first time the effect of four different solvents such as ethanol (C2H6O), methanol (MeOH), isopropyl alcohol (IPA) and 2-methoxyethanol are used to prepare AZO thin films. After preparation of highly conductive and transparent AZO thin films were characterized by structural, morphological, optical and electrical properties.
2 Detailed experimental condition
2.1 Precursor materials used
For the preparation of pure and doped films analytical reagents like host precursor zinc acetate dehydrate (Zn(CH3COO)2·2H2O), dopant precursor aluminum chloride hexahydrate (AlCl3·6H2O) were used. Further, stabilizer agent monoethanolamine (MEA) also the organic solvents such as, 2-methaxyethanol (C3H8O2), Methanol (CH3OH), Ethanol (C2H6O) and Isopropyl alcohol (C3H8O) were used to prepare the precursor solution.
2.2 Solution preparation
The key steps for sol–gel solution preparation are as follows: Firstly starting material, 0.2M of zinc acetate dihydrate [Zn(CH3COO)2·2H2] was separately dissolved in (50 ml) of various solvents namely: 2-methaxyethanol (C3H8O2), Isopropyl alcohol (C3H8O), Ethanol (C2H6O) and Methanol (CH3OH). Secondly, the dopant material, 2 at.% of Aluminium chloride hexahydrate (AlCl3·6H2O) was dissolved into the various zinc complex solution. Mixed both host and dopant precursor solution in the same beaker; further, the solution contained beaker was placed on the magnetic stirrer at 60 °C for 15 min. Thirdly the stabilizing agent, monoethanolamine (MEA) solution was adding drop by drop until Zn and MEA reach a molar ratio of 1:1. Monoethanolamine (C2H7NO) has been used to control the pH of condensation reaction in sol–gel solution. The resultant solution was continuously stirred at 60 °C for 2 h to yield a clear and homogeneous solution. Further, the stirred solution was kept at room temperature i.e. 300 K for 5 days. Finally, we get the aged sol–gel solution, and it is used for coating process. Figure 1 represents the photographed image of different solvents aged AZO solution named as A, B, C and D. From the image one can observe that all the solutions were clear and homogeneous. Especially, methanol solvent solution to yield a clear and transparent solution compared to other solvents.
2.3 Preparation of AZO thin films
7.5cm × 2.5 cm glass substrates (Blue star) was firstly cleaned in detergent and rinsed into the heated chromic acid for 2 h. Further, the substrate was cleaned using acetone and then dried in hot air oven for 10 min. The AZO thin film prepared from four different sol solutions by using the dip-coating method. Schematic diagrams of sol–gel dip coating setup as shown in Fig. 2. Sol–gel dip coating method was excellent to produce uniformly homogeneous AZO films with controlled film thickness. The cleaned glass substrate was dipped into the sol solution at room temperature and then withdrawn at a constant speed of 5 cm/min by using automated computer controlled dip coater setup. After the dipping process, the substrates were vertically dried using tube furnace at 280 °C for fixed time to evaporate the solvent and remove organic residuals. Both dip coating and drying process were repeated seven times until the desired film thickness. Finally, the prepared film was annealed at 500 °C in air for 2 h. This same procedure was used to prepare all the four different solvents AZO films. The prepared films were mentioned as A, B, C and D, corresponding to 2-methaxyethanol, Isopropyl alcohol, Ethanol and Methanol respectively.
2.4 Characterization tools of prepared films
To study the effects of solvents on AZO thin films, several fabrication tools were used to characterize the films. The structural properties of the AZO films were characterized by XRD using a PANalytical X-Pert Powder with CuKα radiation of wavelength λ = 0.1540 nm, in the range of 2θ = 20°–80°. The SEM microstructures were obtained with a Nova Nano SEM from (SIES system). The optical characterization of the prepared films was measured by (A PerkinElmer 45 UV–Vis spectrometer) in the wavelength ranges of 370–1100 nm. PL spectra of NZO thin films were recorded at room temperature (RT) at an excitation wavelength of 325 nm using a He–Cd laser (325 nm) as light source. The electrical properties of the samples were investigated with an Ecopia HMS-3000 Hall effect measurement system.
3 Results and discussion
3.1 X-ray diffraction studies
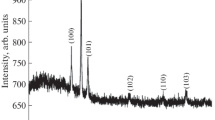
Figure 3 shows the XRD patterns of sol–gel dip coated AZO thin films with different solvents such as 2-methaxyethanol, Isopropyl alcohol, Ethanol and Methanol are namely as A, B, C and D films, respectively. The XRD peaks appeared at 2θ values of approximately 31.64, 34.44, 36.18, 47.41, 56.72 and 62.96 were corresponding to the (100), (002), (101), (102), (110) and (103) diffraction peaks of the ZnO. All the observed diffraction peaks are well-suited with the standard (JCPDS file no: 89-0510) indicating that the deposited films have polycrystalline hexagonal wurtzite structure. No other secondary phases related to either Al or Al2O3 are observed even changing different solvents, which means that the aluminium ions were easily reacted in all the solvents, as well as Al3+ ions are incorporated into the ZnO lattice. So, all the deposited AZO films by using different solvents have been same hexagonal wurtzite structure. From the XRD spectrum, the strong intensity of the peak indicates that the preferred orientation along (002) plane with c-axis perpendicular to the substrate of all the thin films. The preferential orientation is caused by the lower internal stress and surface energy, so the (002) plane had the lowest surface energy density as it attains highest X-ray intensity. Generally, the preferential orientation of the growing films takes place along the lowest surface energy of crystal plane, more details can be seen in recent report by Mondal et al. [22]. Secondary, low intense peaks (100) and (101) are also reasonably observed in the XRD spectrum. The intensities of the (100), (002), (101) peaks tend to increase with changing different solvents, indicating well-defined crystallinity of the films. Moreover, a sharp increase for the (002) plane is observed compared to others. It can be clearly understood that the (methanol solvent-D) film exhibits a higher crystalline quality when compared with A, B and C films.
The size of the crystallites for AZO films was determined by the Scherer formula. The (002) orientation was used to calculate the crystallite size [23,24,25].
where D is the crystallite size, λ is the X-ray wavelength (λ = 1.54 Å), β is the full width at half maximum (FWHM), K is a shape factor, and θ is the Bragg angle of (002) peak. The FWHM value of the primary peak (002) was reduced for to change in solvents, and it is indicated that the increase in crystallite size for the deposited AZO films. The increase in film crystallinity and crystallite size may be related to the hydrolysis and thermal decomposition of zinc acetate during annealing process [26]. Other phenomena of enhancement in the crystallite size can be explained by the film thickness increases also surface energy decreases.
The micro strain and dislocation density of the AZO thin films were determined using the following equations [27, 28].
where (β) is Full Width at Half Maximum value and (D) is the size of crystallites. Figure 4 and Table 1 shows the variations of structural parameters such as crystallite size, dislocation density, micro strain and texture coefficient. In general, good crystallization of the film had large crystallite size and small dislocation density and lower micro strain values. Hence, the dislocation density and micro strain values exhibit decrease with the change in solvents from A to D, which indicates that methanol solvent (D) has low crystal lattice imperfections. The reduction of lattice defects is due to decrease in strain and dislocation density. The lower micro strain and dislocation density also may be attributable to the texturing of planes along high density (002) plane.
The texture coefficient (TC) values of (002) plane was determined by using following equation [29].
where I(hkl) is the observed intensity, I0(hkl) is the JCPDS standard intensity and N is the number of diffraction planes. The texture coefficient values support the discussion on the preferred orientation along (002) plane. In general, a film to have a preferential orientation at any (hkl) plane, the texture coefficient must be at least one. For our observed TC(hkl) value is greater than (TC > 1) in all the films, which represents the preferred orientation and are related to the abundance of grains in a given (hkl) direction.
The lattice constants of AZO thin films can be calculated by using the following relation [30].
where (h k l) are the Miller indices of the particular planes, a = b and c are the lattice parameters, and dhkl is interplanar distance. The observed lattice constants and cell volumes for the AZO films are typically equal to the standard values (JCPDS file No. 89-0510). Moreover, the calculated lattice constants (a) and cell volume (v) for the (film-D) had higher than that of other films. It can be said that the methanol solvent (film-D) was produced higher crystallite size and high crystalline quality of the film compared to other solvents. In Table 1 the lattice constants and cell volume values are significantly increased with respect to solvents is may be due to different stain level in the films.
3.2 Raman spectroscopy
Raman spectroscopy is a useful instrument to investigate the phase information of a material, and the effect of molar concentration were also studied by using Raman scattering. Figure 5 shows the Raman spectrum of the deposited AZO thin films with different solvents in the range of 200–1500 cm− 1. From the figure, it is obviously observed that two leading and one broad characteristic peaks located at 571, 1095 and 805 cm− 1 respectively, for all the four films. In the Raman spectrum, it having a same number of peaks was observed for different solvents, which confirms the absence of other impurity phases in spite of changing solvents; it agree with our XRD spectrum. This A1(LO) mode at 571 cm− 1 was also observed for Fe, Sb, and Al-doped ZnO thin films [31]. From literature, there are two phonon longitudinal A1(LO) modes of ZnO was observed in this spectral range of 575 and 583 cm− 1 [31]. These two modes is attributed to defects of oxygen vacancies and free charge carriers. The high intense A1(TO) mode was observed at 1095 cm− 1 is related to the defects zinc interstitial on the ZnO lattice. The E2 (high) mode at 437 cm− 1 is indicates the ZnO wurtzite structure, and also it is not observed on top of the band in the Raman spectra. A broad peak observed at 805 cm− 1, which is emitted from the glass substrate as a result of high transmittance in the visible range for AZO thin films (see Fig. 7).
3.3 Surface morphological study
Figure 6a–d shows the SEM images of dip coated AZO thin films with various solvents A, B, C and D, respectively. The surface morphology of the films was found to be strongly dependent on the solvent. In the present work, change the remarkable solvent changes the particle size and shape of the AZO nanoparticles. Particle size estimated using SEM micrograph is found to be lying in the range from 62 to 93 nm, for different solvents. This indicates that the optical transmittance was high for the AZO thin films. Figure 6a shows that the formation of cobweb shaped grains are homogeneously distributed in the entire film surface and the size of each grain around 62 nm. Figure 6b, the SEM image shows very well nanostructure with the grain size about 85 nm. It is clearly revealed that the film shows well-defined wrinkle shaped grains are uniformly present onto the film surface without any pinholes and porous nature. This SEM image was good agreement with Srinivasan et al. [32]. In Fig. 6c SEM image shows interconnected cluster formed Botanic root shaped grains with some porous throughout the film surface with the grain size of around 93 nm. It indicates that the film can easily affect the transmittance because the film thickness is high compared to others (see UV graph). Finally, Fig. 6d image, it clearly shows the excellent particles are regularly distributed on to the entire glass substrate, and grain size is 70 nm. Furthermore, the surface of the (film-D) exhibits well defined smooth and uniformed ganglia shaped grains without any pinholes and cracks when compared with other films. The obtained SEM image was perfectly matched with previously reported image [33] by dip coated ZnO film. This result can be connected with the discussion related to the observed better crystalline quality of (film-D) presented in structural studies.
Figure 6e shows the EDAX spectrum of Al doped ZnO thin film, and it confirmed the presence of aluminium doped zinc oxide. From the EDAX exhibits the presence of all core elements i.e. Zn, O, and Al. The EDAX measurement shows the quantitative values of Zn, O, and Al are represents 46.3, 52.1 and 1.6%, respectively. This indicates that the Al ions are successfully incorporated into ZnO lattice. So this incorporation factor was helpful for the influencing structural, optical and electrical properties.
3.4 Optical properties
Thickness is the most important tool for film parameter and it is controls the film properties. The thickness of the film (t) was carried out by stylus profilometer. The variation of film thickness with different solvents was listed in Table 2.
Figure 7 shows the optical transmittance spectra of AZO thin films deposited from sol gel dip coating with the various solvents in the wavelength range of 350–1100 nm. It was seen from the transmittance spectra that all the films exhibited a good transmittance in the visible and near infrared region as shown in Fig. 7 and Table 2. The transmittance spectra linearly increases with the increasing wavelength for all the films with a sharp fundamental absorption edge in the wavelength of around 375–395 nm. The sharp fall in the transmittance near the fundamental absorption edge is an identification of the good crystallinity of the films [34]. In the present study, the average transmittance of all the deposited films varies from 67 to 93% in the visible range. Particularly, film (A) has maximum transmittance is 93% in the visible region compared to other films (B, C and D).The observed transmittance of the AZO films initially decreased and then significantly increases with the change of different solvents is due to dependent the film thickness. It can be ascribed to the formation of larger particles on the surface of AZO thin films for solvents which cause by the scattering of light. The reduction of transmittance (film-B and C) may be attributed to the increased scattering of photons by an increase of the roughness of the film surface morphology. Furthermore, the increase in transmittance (film-D) may be due to the enhancement of film crystallinity and reduction of surface roughness of the films (see XRD and SEM).
The absorption spectrum of AZO thin films with different solvents is shown in Fig. 8. From the absorption spectra, the absorbance of all the AZO thin film decreased with the increase of wavelength. Optical absorbance depends on surface roughness, band gap and impurity centers in materials [35]. Film (A) has low absorption compared to other films; this is due to smooth surface and low film thickness. In this work, the sharp absorption peak located at 385 nm for the AZO films, which may be attributable to the excitation of electrons from valence band to conduction band [36]. Also, the sharp absorption peak is revealed in the 385 nm, which is close to the bulk band gap of ZnO (3.25 eV). This sharp absorption peak of the AZO films concludes good crystalline nature with low defect density; thus it is suitable for optoelectronic devices [37]. The absorption edge for all the films in the wavelength range of 385–410 nm indicates the absorption properties of the AZO films in the UV region.
Direct allowed optical band gap of AZO thin films have been determined from the relation between the incident photon energy (hν) and the absorption coefficient (α) given in equation [38,39,40,41,42].
where α is the absorption coefficient, h is Planck’s constants, Eg is the optical band gap, ν is the frequency of an incident photon, and A is a constant. Figure 9 shows the plots of (αhν)2 versus hν for the dip coated AZO thin films with different solvents. From this plots, the linear nature indicates that the present samples are showing direct allowed band (Eg) with approximate values of 3.26, 3.10, 3.05 and 3.15 eV for the A, B, C and D films, respectively. The observed band gap was initially decreased and then sharply increases of change in different solvents, this is due to the variation of thicknesses of the deposited films (see Table 2) and also on grain size [40, 42]. As clear from the data tabulated in Table 2 that the initially the thickness is increasing and decreased for final film which is in agreement with the obtained values of band gap. This calculated band gap value is perfectly matched with previously reported value [43].
3.5 Photoluminescence spectroscopy
Photoluminescence is one of the most important studies to identify the film quality, impurity level of the material and estimate the film disorder [44].
The room temperature photoluminescence spectrum of AZO is shown in Fig. 10. All the four films are excited wavelength at 325 nm. The PL spectra of all the films exhibit four different emission peaks. Firstly, strong and high intention peak located at 386 nm corresponds to the UV emission is due to near NBE emission of ZnO material [45], which is mainly dependable for crystalline quality of the film. The observed peak at 386 nm corresponds to the band gap (3.20 ev) of AZO thin films, which is recommended to the recombination of free exciton–exciton collision process [46, 47]. Secondly, one strong and one weak emission peaks are also observed at 413 nm (2.98 eV) and 436 nm (2.87 eV) corresponds to near blue emissions, these two blue emissions is attributed to zinc interstitials (Zn2+) and zinc vacancies, respectively. Thirdly, a weak blue-green emission peak was obtained at 481 nm (2.56 eV), is attributed to single ionized oxygen vacancies (VO +) also it may be due to the surface defects present in the films [48]. Finally, an unidentified weak peak located at 522 nm corresponds to green emission is endorsed to the electron transition from the ionized oxygen vacancies to the valence band [49, 50].The observed peak intensities are high in case of methanol solvent (film-D)compare than other solvents (film-A, B and C), so methanol solvent has a high quality of AZO thin film. It is good to agree with our XRD result.
3.6 Electrical properties
The electrical properties such as film conductivity, carrier concentration, and mobility are a major role for TCO thin films. Usually, AZO, the thin film has low resistivity since the interstitial incorporation of Al3+ ions into the ZnO lattice. Consequently, one free electron was formed in the ZnO lattice during incorporation Al3+ ion.
The variations in ρ, n and μ for respect to different solvents as shown in Fig. 11, and the values of sheet resistance (Rsh) was given in Table 2. In the present work, the electrical properties were mostly depending on the solvents. From the Table 2, the observed sheet resistance (Rsh) decreases with changing different solvents, and it reaches a minimum value while the methanol solvent (film-D). As shown in this Fig. 11, the resistivity values were decreased caused by an increase in carrier concentration for different solvents. The increasing carrier concentration, while the decreasing grain boundaries and crystal deficiencies of the films. However, the observed carrier mobility decreases with changing solvents, caused by ionized impurity scattering mechanism [51]. The observed low resistivity and high carrier concentration values are 7.56 × 10− 3 (Ω cm) and 2.76 × 1019 (cm)−3 respectively, for the methanol solvent (film-D). It may be due to the Al3+ ions have been completely substituted and incorporation of Zn sites and ZnO lattice, respectively. Also, from the XRD studies, film (D)has the high crystallinity of the AZO film compared to other films, so it is one of the reasons of higher carrier concentration and lower resistivity of the film (see the Fig. 3; Table 2).
3.6.1 Figure of merit
The quality of TCO aluminium doped ZnO thin films can be determined by the quality factor (or) figure of merit (ϕ).The figure of merit (ϕ) values can be calculated using optical transmittance and sheet resistance by Haacke [52].
where, T and Rsh are the optical transmittance in particular wavelength (700 nm) and the electrical sheet resistance, respectively. The calculated figure of merit (ϕ) values as listed in Table 2. The best value of the figure of merit (ϕ) was obtained for the film deposited from methanol solvent (film-D) and its value is 4.62 × 10− 4 (Ω/cm)−1 at the particular wavelength of 700 nm. Thus, it can be concluded that the methanol solvent can perform the better role as an optoelectronic device and also window layer in the solar cell. In common a TCO thin film has both the electrical conductivity and optical transmittance should be high as possible for optoelectronic and solar cell applications. In this report, we observed both transmittance and conductivity values are high so that it can be easily used for optoelectronic applications.
4 Conclusion
In this study, highly transparent AZO films were successfully deposited on glass substrates by sol–gel dip coating technique by four different solvents. Following conclusions were learned from this study.
-
1.
The XRD patterns of all the AZO films showed polycrystalline nature with hexagonal wurtzite crystal structure, and also aluminium atoms are completely incorporated into the ZnO lattice, because there is no (Al) related phase was observed in the XRD spectrum.
-
2.
According to SEM and optical analyses, the deposited films are well defined uniformly distributed grains, and also it has very high transmittance (93%) values in the visible region.
-
3.
Mainly, the room temperature PL studies confirms the deposited AZO thin films have good quality.
-
4.
Electrical resistivity and figure of merit values are presented in Table 2, and also the best electrical resistivity and figure of merit values are 7.56 × 10− 3 (Ω cm) and 4.62 × 10− 4 (Ω/cm)−1, respectively be observed for methanol solvent (film-D).
-
5.
Finally, the observed optical and electrical values of the deposited film using methanol solvent make them suitable for many applications such as opto-electronics device and solar cell, etc.
References
A. Janotti, C.G. Van de Walle, Rep. Prog. Phys. 72, 1–29 (2009)
Z. Zang, A. Nakamura, J. Temmyo, Optics Express 21(9), 11448–11451 (2013)
Z. Zang, X. Zeng, J. Du, M. Wang, X. Tang, Opt. Lett. 41(15), 3463–3466 (2016)
C. Lia, C. Hana, Y. Zhang, Z. Zanga, M. Wanga, X. Tanga, J. Dua, Sol. Energy Mater. Sol. Cells 172, 341–346 (2017)
K. Ellmer, A. Klein, ZnO and its applications, in Transparent Conductive Zinc Oxide: Basics and Applications in Thin Film Solar Cells, Springer Series in Materials Science, vol 104, ed. by K. Ellmer, A. Klein, B. Rech (Springer-Verlag, Berlin, 2008), pp. 1–33
J.L. Chen, D. Chen, Z.H. Chen, Optimization of the process for preparing Al-doped ZnO thin films by sol-gel method. Sci. China Ser. E Tech. Sci. 52, 88–94 (2009)
F. Ahmed, N. Arshi, M.S. Anwar, R. Danish, B.H. Koo, Thin Solid Films 547, 168–172 (2013)
Y.K. Tseng, G.J. Gao, S.C. Chien, Synthesis of c-axis preferred orientation ZnO:Al transparent conductive thin films using a novel solvent method. Thin Solid Films 518, 6259–6263 (2010)
Ü. Özgür, I. AlivovYa, C. Liu, A. Teke, M.A. Reshchikov, S. Doğan, V. Avrutin, S.J. Cho, H. Morkoç, A comprehensive review of ZnO materials and devices. J. Appl. Phys. 98, 041301:1–041301:103 (2005)
C. Bundesmann, R. Schmidt-Grund, M. Schubert, Optical properties of ZnO and related compounds, in Transparent Conductive Zinc Oxide—Basics and Applications in Thin Film Solar Cells (Springer Series in Materials Science), vol 104, ed. by K. Ellmer, A. Klein, B. Rech (Springer-Verlag, Berlin, 2008), pp. 79–124
H. Von Wenckstern, H. Schmidt, M. Brandt, A. Lajn, R. Pickenhain, M. Lorenz, M. Grundmann, D.M. Hofmann, A. Polity, B.K. Meyer et al., Anionic and cationic substitution in ZnO. Prog. Solid State Chem. 37, 153–172 (2009)
F. Khan, V.S.N. Singh, M. Husain, P.K. Singh, Sol-gel derived hydrogen annealed ZnO:Al films for silicon solar cell application. Sol. Energy. Mater. Sol. Cells 100, 57–60 (2012)
J.I. Nomoto, T. Hirano, T. Miyata, T. Minami, Thin Solid Films 520, 1400–1406 (2011)
A.C. Galca, M. Secu, A. Vlad, J.D. Pedarnig, Thin Solid Films 518, 4603 (2010)
M. Farbod, M. Zargar Shoushtari, S. Parhoodeh, J Physica B 406, 205–210 (2011)
W.H. Kim, W.J. Maeng, M.K. Kim, H. Kim, J. Electro. Chem. Soc. 158, D495 (2011)
K. Mahmood, S.B. Park, Electron. Mater. Lett. 9, 161 (2013)
K. Deva Arun Kumar, S. Valanarasu, A Study of aluminium doped ZnO (AZO) thin film by SILAR method, in Journal of Latest Research in Engineering and Technology (IJLRET), pp. 17–19 (2016)
J. Zhang, W. Que, J. Solar Energy Mater. Solar Cells 94, 2181–2186 (2010)
C. Boukaous, A. Telia, D. Horwat, M.S. Aida, B.B.S. Ghanem, Eur. Phys. J. Appl. Phys. 65, 20302 (2014)
C. Boukaous, A. Telia, D. Horwat, S. Ghanem, P. Miska, Effect of solvents on the properties of ZnO thin layers obtained by sol gel dip coating process. J. New Technol. Mater. JNTM 04(01), 94–98 (2014)
P. Mondal, D. Das, Phys. Chem. Chem. Phys. 18, 20450–20458 (2016)
N.S. Sabri, A.K. Yahya, M.K. Talari, AIP Conf. Proc. 1250, 436 (2010)
M. Shkir, I.S. Yahia, S. AlFaify, M.M. Abutalib, S. Muhammad, J. Mol. Struct. 1110, 83–90 (2016)
M. Shkir, I.S. Yahia, V. Ganesh, H. Algarni, S. AlFaify, Mater. Lett. 176, 135–138 (2016)
D. Guo, K. Satob, S. Hibinob, T. Takeuchi, H. Bessho, K. Kato, Low-temperaturepreparation of (002)-oriented ZnO thin films by sol–gel method. Thin Solid Films 550, 250–258 (2014)
R. Mariappan, M. Ragavendar, V. Ponnuswamy, J. Alloys Compd. 509, 7337–7343 (2011)
Z.R. Khan, M.S. Khan, M. Zulfequar, M.S. Khan, Mater. Sci. Appl. 2, 340 (2011)
C. Barret, T.B. Massalski, Structure of Metals, (Pergamon, Oxford, 1980), p. 923
M. Ren, Z. Mal, Y. Lu, The effect of the thermal annealing on ZnO thin films grown by pulsed laser deposition, J. Appl. Phys. 88(1), 498–502 (2000). doi:10.1063/1.373685
C. Bundesmann, N. Ashkenov, M. Schubert, D. Spemann, T. Butz, E.M. Kaidashev, M. Lorenz, M. Grundmann, Appl. Phys. Lett. 83, 1974–1983 (2003)
G. Srinivasan, R.T. Rajendra Kumar, J. Kumar, Influence of Al dopant on microstructure and optical properties of ZnO thin films prepared by sol–gel spin coating methodOptical. Materials 30, 314–317 (2007)
N.V. Kaneva, C.D. Dushkin, A.S. Bojinova, ZnO thin films preparation on glass substrates by two different sol-gel methods. Bulgarian Chem. Commun. 44, 63–69 (2012)
V. Senthamilselvi, K. Saravanakumar, N. Jabena Begum, R. Anandhi, A.T. Ravichandran, B. Sakthivel, K. Ravichandran, J.Mater. Sci. 23, 302–308 (2012)
M. Arshad, A. Azam, A.S. Ahmed, S. Mollah, A.H. Naqvi, J. Alloy. Compd. 506, 8378–8381 (2011)
M. Bouloudenine, N. Viart, S. Colis, A. Dinia, Catal. Today 113, 240–244 (2006)
K. Ravichandran, R. Mohan, N. Jabena Begum, S. Snega, K. Swaminathan, C. Ravidhas, B. Sakthivel, S. Varadharajaperumal, Vacuum 107, 68–76 (2014)
J. Tauc, Amorphous and Liquid Semiconductors. (Plenum Press, New York, 1974)
M. Shakir, S.K. Kushwaha, K.K. Maurya, G. Bhagavannarayana, M.A. Wahab, Solid State Commun. 149, 2047–2049 (2009)
M. Shkir, H. Abbas, Z.R. Khan, J. Phys. Chem. Solids 73, 1309–1313 (2012)
M. Shkir, V. Ganesh, S. AlFaify, I.S. Yahia, J. Mater. Sci. 28, 10573–10581 (2017)
M. Shkir, S. Alfaify, S. Muhammad, M. Nasir, N. Vijayan, S.K. Jat, M. Zulfequar, S. Rubio, E. Dieguez, Materials Focus 4, 202–207 (2015)
W.-W. Zhong, F.-M. Liu, L.-G. Cai, P. Ding, C.-C. Zhou, J. Alloys Compd. 509, 3847–3851 (2011)
N. Jabena Begum, R. Mohan, K. Ravichandran, Superlatt. Micro Struct. 53, 89–98 (2013)
L. Ma, S. Ma, H. Chen, X. Ai, X. Huang, Microstructuresand optical properties of Cu-doped ZnO films prepared byradio frequency reactive magnetron sputtering. Appl. Surf. Sci. 257, 10036–10041 (2011)
Y.C. Kong, D.P. Yu, B. Zhang, W. Fang, S.Q. Feng, Ultraviolet-emitting ZnO nanowires synthesized by a physical vapor deposition approach. Appl. Phys. Lett. 78, 407–409 (2001)
S.C. Lyu, Y. Zhanga, H. Ruh, H.J. Lee, H.W. Shim, E.K. Suh, C.J. Lee, Low temperature growth and photoluminescence of well-aligned zinc oxide nanowires. Chem. Phys. Lett. 363, 134–138 (2002)
N.L. Tarwal, P.R. Jadhav, S.A. Vanalakar, S.S. Kalagi, R.C. Pawar, J.S. Shaikh, S.S. Mali, D.S. Dalavi, P.S. Shinde, P.S. Patil, Photoluminescence of Zinc oxide nanopowder synthesized by a combustion method. Powder Technol. 208, 185–188 (2011)
R. Mariappan, V. Ponnuswamy, P. Suresh, Effect of dopingconcentration on the structural and optical properties of pureand tin doped zinc oxide thin films by nebulizer spray pyrolysistechnique. Superlattices Microstruct. 52, 500–513 (2012)
Q.P. Wang, X.J. Zhang, G.Q. Wang, S.H. Chen, X.H. Wu, H.L. Ma, Influence of excitation light wavelength on the photo-luminescence properties for ZnO films prepared by magnetron sputtering. Appl. Surf. Sci. 254, 5100–5104 (2008)
H.L. Shen, H. Zhang, L.F. Lu, F. Jiang, C. Yang, Progress in natural science. Mater. Int. 20, 44–48 (2010)
G. Haacke, J. Appl. Phys. 47, 4086 (1976)
Acknowledgements
The authors would like to express their gratitude to deanship of scientific research, King Khalid University, Saudi Arabia for providing the financial support under the Project Number R.G.P. 2/3/38.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declares that there is no conflict of interest in the current article.
Rights and permissions
About this article
Cite this article
Deva Arun Kumar, K., Ganesh, V., Shkir, M. et al. Effect of different solvents on the key structural, optical and electronic properties of sol–gel dip coated AZO nanostructured thin films for optoelectronic applications. J Mater Sci: Mater Electron 29, 887–897 (2018). https://doi.org/10.1007/s10854-017-7985-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7985-0