Abstract
In this paper, Ce-doped ZnO gas sensors with different Ce/Zn ratios have been fabricated by a facile hydrothermal process, starting from zinc acetate dihydrate, cerium(III) nitrate hexahydrate and PEG. The microstructure, morphologies and components of pure ZnO and Ce-doped ZnO are characterized by X-ray diffraction, scanning electron microscope and energy dispersive spectrometer, respectively. The sensitivities to testing gases are investigated through the static testing system. Compared with pure ZnO, Ce-doped ZnO sensors showed relatively higher sensitivity and faster response/recovery time. Among them, 3 at.% Ce-doped ZnO sensor exhibited a maximum sensitivity of 560 (Ra/Rg) for 300 ppm of 1,2-propanediol at the optimum operating temperature of 260 °C and the response and recovery time is 4 s and 16 s, respectively. Finally, the gas-sensing mechanism of ZnO sensors is discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The gas categories are the key measurands in home, industry and urban atmosphere. Since many decades, with the awareness of protecting environment increasing, the specific demand for gas determination has emerged. Semiconducting metal oxide gas sensors (SMO), such as ZnO, SnO2 and WO3, have drawn considerable attention owing to their attractive features [1, 2]. Among them, ZnO, a representative n-type semiconductor of group II–VI, finds application in the fields of piezoelectric, optoelectronic, gas sensor and solar cell application due to its unique optical, electrical and optoelectronic properties [3,4,5,6]. In the sensor field, ZnO has been regarded as an excellent candidate gas-sensing material for the detection of both reducing gas and oxidizing gas [7]. However, a series of key parameters, such as particle size, surface morphology, microstructure and active centers, affect gas sensing properties [8], thus different preparation methods and the gas-sensing mechanism are needed to be explored. Besides, doping is one of effective ways to tailor gas sensing properties of ZnO as doping can alter the structure, surface-to-volume ratio and grain size. Furthermore, doping can introduce new impurity level and surface defects in semiconductor materials [9]. In the previous studies, noble metal additives (Pt, Pd) [10], transition-metal oxides (CuO, Fe2O3) [10, 11], and main-group metal oxides (In2O3, Bi2O3) [12] have been tried as the dopant for enhancing the sensing properties of ZnO by changing energy-band structure, mending the morphology, increasing surface-to-volume ratio and creating more active centers at the grain boundaries. Especially, doping with rare earth elements can influence gas-sensing properties by cracking (or dehydrogenation) and ring-opening of hydrocarbon [13, 14]. As one of rare earth elements, cerium, as a dopant, has received great attention due to its peculiar properties to enhance the gas sensing properties of the metal oxides arising from the availability of the 4f shell, such as changing energy-band structure, mending the morphology and surface-to-volume ratio, creating more active center at the grain boundaries. Although cerium has two kinds of oxidation states, namely, Ce2O3 and CeO2, in which f electron states are partially occupied or empty respectively, it can be found that cerium almost exists as CeO2. CeO2 has been employed as dopant in other semiconductor-based gas sensor, such as In2O3 [15], due to its chemical stability and high diffusion coefficient resulting from oxygen vacancies. And oxygen vacancies at rare earth (RE) oxide surfaces alter the geometric and electronic structure as well as chemical properties of the host system.
In this work, pure and Ce-doped ZnO samples in different concentrations are synthesized by using hydrothermal method which is a relatively facile method. The sensors based on Ce-doped ZnO sample exhibit excellent sensing performance to 1,2-propanediol. The sensing mechanism is also discussed.
2 Experimental section
All the reagents in the experiment are analytical-grade purity and used without any further purification.
2.1 Preparation
A simple and facile hydrothermal method is used for the synthesis of pure ZnO and Ce-doped ZnO by using zinc acetate dihydrate and cerium(III) nitrate hexahydrate as staring materials and dopant sources, respectively. In a typical synthesis, 0.439 g of Zn(CH3COO)2·2H2O, different amount of Ce(NO3)3·6H2O according to Ce/Zn atomic ratio (0, 1, 2, 3, 4 at.%) and 0.2 g of PEG are first added into 10 mL of deionized water and the solution is stirred for 30 min. At the same time, 10 mL NaOH solution (4 mol/L) is dropped into the above solution slowly accompanied by stirring until the suspension forms. The suspension is subsequently transferred into a Teflon-lined autoclave (50 mL) and kept at 150 °C for 12 h in an oven. The product is collected and washed with distilled water and ethanol several times to remove by-products, and dried at 80 °C for 8 h, finally followed by calcination in air at 500 °C for 1 h.
2.2 Characterization
The phase composition of the samples is characterized by X-ray diffraction (XRD-6000, SHIMADZU) using Cu-Kα radiation (λ = 1.5418 Å). A continuous scanning mode is used to collect 2θ data from 10° to 80°. Scanning electron microscope (SEM: JEOL JSM-5900LV,Japan) is used to observe the morphology of calcined samples and energy dispersive spectrometer (EDS) technique is used to determine their elemental compositions.
2.3 Fabrication and measurement of gas sensor
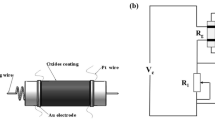
A paste is prepared from a mixture of the as-prepared ZnO with anhydrous ethanol, and then ultrasonic dispersed until it becomes homogeneous. After the paste is coated onto an Al2O3 ceramic tube on each end of which a pair of Au electrodes and four Pt wires have previously been installed, the Al2O3 ceramic tube is heated in air at 500 °C for 1 h to remove anhydrous ethanol. Subsequently, a heater of Ni-Cr wire is inserted into the ceramic tube to regulate the operating temperature (Fig. 1a). Finally, the sensor devices are aged for 5 days in air in order to improve their long-term stability. The sensitivity (S = Ra/Rg, Rg: resistance in gas, Ra: resistance in the air) to 1–300 ppm 1,2-propanediol is measured at different operating temperatures, i.e. 200, 240, 260, 300 and 340 °C, using a WS-30A gas sensitivity instrument (Weisheng Electronics Co, Ltd China). The schematic of the basic measuring electric circuit is shown in Fig. 1b. The response time is defined as time within which the 90% steady response value reaches and the recovery time is defined as the time for dropping to 10% of the steady response value of sensor when testing gases are outlet.
3 Results and discussion
3.1 Characterization
Figure 2a shows the XRD patterns of pure ZnO, 1, 2, 3 and 4 at.% Ce-doped ZnO samples. It is evident that all the diffraction peaks of pure ZnO and Ce-doped ZnO, i.e. (100), (002), (101), (102), (110), (200), (112) and (103), can be indexed as hexagonal wurtzite structure ZnO, which are in good agreement with standard JCPDS data card No. 79-2205. Compared to those of pure ZnO, the XRD patterns of Ce-doped ZnO samples are not obviously changed in the half-width and the positions of diffraction peaks, demonstrating that Ce-doping has no obvious effect on the crystal structure of Ce-doped ZnO. However, the intensity of the diffraction peaks decreases, especially at 2θ of 31.6°, 36.1°, 56.4° and 67.8°, with the concentrations of doped Ce increasing. Meanwhile, a weak peak is found at 28.6° (Fig. 2b), indicating that the cubic structure CeO2 exists in the Ce-doped ZnO according to the standard JCPDS data card No. 78-0694. Hence, in the case of the valency of Ce3+ in the cerium nitrate hexahydrate, there is a variation of valency after calcinations of the samples.
The SEM images in Fig. 3 show the morphologies of pure ZnO and ZnO doped with different concentrations of cerium. As can be seen from the SEM images, the ZnO rods grow in high density and distribute randomly. The typical length of the ZnO rods is in the range of 2–5 μm while the diameter is in the range of 100–500 nm. The surfaces of the pure ZnO and 1 at.% Ce/Zn are comparatively smooth in Fig. 3a, b. However, with the concentrations of doped Ce increasing, CeO2 phases congregate on the surface of the ZnO,leading to the surface of Ce-doped ZnO becoming coarsened. The coarse structure indicates an enhancement in the effective surface area and formation of surface defects for better adsorption of gas molecules. To assess the elemental composition of ZnO samples, the EDS analysis is done and the result is shown in Fig. 3f. In the EDS spectra, apart from Si and Au caused by the detection process, 3 at.% Ce-doped ZnO is mainly composed of zinc (52.72 at.%), oxygen (45.54 at.%) and cerium (1.74 at.%), which means the measured Ce/Zn ratio is roughly equal to the nominal composition.
3.2 Gas sensing properties
In the case of n-type metal-oxide semiconductor surface-controlled gas sensors, it is generally thought that absorbed oxygen, which traps electrons from the conductance band, plays a crucial role in gas sensitivity. For ZnO-based gas sensors, the change of resistance is mainly induced by the adsorption and desorption of oxygen molecules from the surface of ZnO [16]. In the air, oxygen molecules are adsorbed on the surface of ZnO-based sensor as the oxygen ions (O2−, O− and O2 −). The process is shown as follows [17].
When reducing gases (RG) are injected, they react with the oxygen ions. This reaction leads to the exchange of charges between adsorbed gas species and oxygen ions, and then the thickness of depletion will reduce. At the same time, the surface or grain boundary conduction of ZnO will increase [18].
Sensitivity of pure ZnO and various Ce-doped ZnO based sensors towards 10 ppm 1,2-propanediol is investigated at different testing temperature, as shown in Fig. 4. Pure ZnO and Ce-doped ZnO based sensors exhibit maximum response to 1,2-propanediol at 260 °C. Therefore, 260 °C is chosen as the optimum operating temperature for 1,2-propanediol detection. Noticeably, 3 at.% Ce/Zn sensor showed the maximum response to 1,2-propanediol at 260 °C, which was almost 6 times as high as that of pure ZnO based sensor. The changes in sensitivity at different operating temperature can be attributed to the fact that the adsorption type of oxygen molecules becomes chemisorption at higher temperature from physisorption at lower temperature. With the adsorption attraction of chemisorption enhancing, the concentration of adsorbed oxygen will increase. Hence, the sensitivities are improved with the operating temperature increasing. However, when the operating temperature exceeds 260 °C, the reduction in sensitivity is due to the fact that the adsorption reaction is proverbially exothermic. The reaction \(\left( {{{\text{O}}_{{\text{2}}({\text{ads}})}}+{{\text{e}}^ - } \to {\text{ O}}_{{{\text{2}}({\text{ads}})}}^{ - },{\text{ O}}_{{{\text{2}}({\text{ads}})}}^{ - }+{{\text{e}}^ - } \to {\text{ 2O}}_{{({\text{ads}})}}^{ - },{\text{ O}}_{{({\text{ads}})}}^{ - }+{{\text{e}}^ - } \to {\text{ O}}_{{({\text{ads}})}}^{{{\text{2}} - }}} \right)\) will proceed to reverse direction above 260 °C, which leads to the decrease of the trapped electrons from the conductance band [16]. In addition, CeO2 phase, formed on the surface of ZnO particles, has both positive and negative effects on the gas-sensing properties. On one hand, it can promote dehydrogenation reaction, on the other hand, CeO2 can function as an insulator and reduce the available sites, leading to worsen gas-sensing properties [6]. When appropriate amount of CeO2 congregates on the surface of the ZnO, the positive effect dominates against the negative effect. Therefore, appropriate amount of CeO2 is an effective measure to enhance the ZnO sensing property to 1,2-propanediol.
In order to discuss the sensing performance of Ce-doped ZnO based sensor, the response to 1,2-propanediol concentration is measured in the range from 1 to 300 ppm 1,2-propanediol ambient. And the response and recovery characteristics of Ce-doped ZnO based sensors are shown in Fig. 5 at the operating temperature of 260 °C. In the range of 1–50 ppm, the relative responses increase linearly with the increase of 1,2-propanediol concentration and the detection limit to 1,2-propanediol is lower than 1 ppm. When the 1,2-propanediol concentration reaches above 50 ppm, the response increases slowly with further increase of the 1,2-propanediol concentration. Eventually, the sensor reaches saturation at about 300 ppm.
The selectivity, besides sensitivity, is another key property of gas sensors. The selectivity of a gas sensor to particular gas may be quantitatively described as \({\text{SE}}{{\text{L}}_{\text{i}}}~=~\left( {{{\text{S}} \mathord{\left/ {\vphantom {{\text{S}} {\sum {{\text{S}}_{\text{i}}}}}} \right. \kern-0pt} {\sum {{\text{S}}_{\text{i}}}}}} \right) \times {\text{1}}00\%\), where Si is the sensitivity of a sensor to gas i and ∑Si represents the sum of sensitivities of a sensor to different target gases at the same concentrations [19]. Based on this definition, the selectivity to C3H8O2, C2H5OH, CH3COOH and C3H6O may then be described in this study as \({\text{SE}}{{\text{L}}_{{\text{i}}~}}=\left[ {{{\text{S}}_{\text{i}}}/\left( {{{\text{S}}_{{\text{C3H8O2}}}}+{\text{ }}{{\text{S}}_{{\text{C2H5OH}}}}+{\text{ }}{{\text{S}}_{{\text{CH3COOH}}}}+{\text{ }}{{\text{S}}_{{\text{C3H6O}}}}} \right)} \right] \times {\text{1}}00\%\), where i represents one of the gases as C3H8O2, C2H5OH, CH3COOH or C3H6O. Figure 6 shows the response of sensors to 100 ppm of various gases at the operating temperature of 260 °C. The results demonstrate that the 3 at.% Ce-doped ZnO sensor exhibits good selectivity for 1,2-propanediol (about 66.7%). Compared with the selectivity to 1,2-propanediol, the selectivity to other testing gases is much lower (<20%), suggesting that 3 at.% Ce-doped ZnO sensor be good gas sensors to selectively detect 1,2-propanediol.
Response-recovery characteristics are also important parameters of a gas sensor. The typical response-recovery curves of pure ZnO and 3 at.% Ce–ZnO sensors for 50 ppm 1,2-propanediol at the operating temperature 260 °C are shown in Fig. 7. As seen in Fig. 7b, the response time and recovery time of 3 at.% Ce–ZnO sensor are 8 and 21 s, respectively, shorter than that of pure ZnO sensor (14 and 25 s). These results reveal that the response and recovery time towards 1,2-propanediol can be significantly improved by decorating CeO2 onto the ZnO surface to form rough structure, which will enhance the adsorption of target gas and lead to more active sites available for reaction [20].
4 Conclusion
In summary, pure ZnO and various Ce-doped ZnO samples are synthesized by using a simple hydrothermal methods and followed by calcination. Their microstructure, sensing performance and sensing mechanism are investigated. Compared with the pure ZnO sensor, 3 at.% Ce-doped ZnO sensor demonstrates the highest response and rapidest response/recovery time at 260 °C. Especially, the sensor can detect 1,2-propanediol down to 1 ppm with obvious response (3.47). For the same concentration of other testing gases, 3 at.% Ce-doped ZnO based sensor shows excellent selectivity for 100 ppm 1,2-propanediol (about 66.7%) at the operating temperature 260 °C. Hence, 3 at.% Ce-doped ZnO sensor may be considered as a promising sensing material for selective detection of 1,2-propanediol.
References
H. Tang, M. Yan, H. Zhang, S. Li, X. Ma, M. Wang, D. Yang, Sens. Actuators B 114, 910 (2006)
C. Liangyuan, B. Shouli, Z. Guojun, L. Dianqing, C. Aifan, C.C. Liu, Sens. Actuators B 134, 360 (2008)
H. Xu, A.L. Rosa, T. Frauenheim, R.Q. Zhang, S.T. Lee, Appl. Phys. Lett. 91, 031914 (2007)
S.R. Mahmoodi, B. Raissi, E. Marzbanrad, N. Shojayi, A. Aghaei, C. Zamani, Procedia Chem. 1, 947 (2009)
S.P. Huang, H. Xu, I. Bello, R.Q. Zhang, J. Phys. Chem. C 19, 114 (2010)
N.F. Hamedani, A.R. Mahjoub, A.A. khodadadi, Y. Mortazavi, Sens. Actuators B 169, 67 (2012)
F. Grasset, Y. Molard, S. Cordier, F. Dorson, M. Mortier, C. Perrin, M. Guilloux-Viry, T. Sasaki, H. Haneda, Adv. Mater. 20, 1710 (2008)
X. Song, D. Zhang, M. Fan, Appl. Surf. Sci. 255, 7343 (2009)
H. Chen, H. Yu, S. Cui, J. Xu, Y. Zhang, C. Liu, Physica B 516, 36 (2017)
G.S.T. Rao, D.T. Rao, Sens. Actuators B 55, 166 (1999)
J.H. Yu, G.M. Choi, Sens. Actuators B 75, 56 (2001)
B.L. Zhu, D.W. Zeng, J. Wu, W.L. Song, C.S. Xie, J. Mater. Sci. 14, 521 (2003)
B.A. Watson, M.T. Klein, R.H. Harding, Appl. Catal A 160, 13 (1997)
X.L. Zhang, A.M. Zhu, X.H. Li, W.M. Gong, Catal Today 89, 97 (2004)
D. Han, P. Song, S. Zhang, H. Zhang, Q. Xu, Q. Wang, Sens. Actuators B 216, 488 (2015)
C. Ge, C. Xie, S. Cai, Mater. Sci. Eng. B 137, 53 (2007)
X. xiang, D. zhu, D. wang, J. Mater. Sci. 27, 7425 (2016)
S. Pearton, Prog. Mater. Sci. 50, 293 (2005)
G. Li, S. Kawi, Sens. Actuators B 59, 1 (1999)
G.X. Wan, S.Y. Ma, X.B. Li, F.M. Li, H.Q. Bian, L.P. Zhang, W.Q. Li, Mater. Lett. 114, 103 (2014)
Acknowledgements
The authors are grateful for the Project 2014GZ0090 supported by the Science and Technology Support Program in Sichuan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
lu, W., zhu, D. & xiang, X. Synthesis and properties of Ce-doped ZnO as a sensor for 1,2-propanediol. J Mater Sci: Mater Electron 28, 18929–18935 (2017). https://doi.org/10.1007/s10854-017-7846-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-017-7846-x