Abstract
Ambient air synthesis offers the possibility to reduce the cost of production for perovskite solar cells. We have investigated the morphological and charge transport properties of CH3NH3PbI3 under dark and one sun illumination. Synthesis of CH3NH3PbI3 was carried out using different concentrations of CH3NH3I employing two step ambient air synthesis process in order to find an optimum concentration for such synthesis. Effect of different operating temperatures on photoconductive properties of perovskite films has also been investigated by varying the temperature from 300 to 340 K. Our investigations conclude that compactness of perovskite film plays more crucial role in determining charge transport properties, especially under illumination and at varying temperature. The process parameter i.e. low concentration of CH3NH3I, which leads to large crystal size, also creates more voids/pin holes under ambient air synthesis. Present investigation shows that an optimum concentration of 0.050 M of CH3NH3I achieves best grain size to voids ratio and optimum photoconductive properties.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Organometallic halide perovskites have transformed the renewable energy outlook in recent years [1, 2]. With a certified maximum efficiency at 20.1 [3], it has the potential to meet the energy requirements of our times. Ambipolar transport [4], long diffusion lengths [5], high charge carrier mobilities [6] and high absorption coefficients across broad spectrum [7], [8] ensure that this material has all the right ingredients. Phenomenal research effort has been directed in recent years at unraveling this material and manufacturing has evolved from one step solution processing [9] to two step sequential deposition [10], vacuum assisted growth [11] to dual source evaporation [12] to name a few. Device geometries ranging from planar to mesostructured [11] to mesosuperstructured [4] have been investigated. Ambipolar charge transport and long diffusion lengths led to the breakthrough concept of hole transport layer free devices, achieving impressive performances [13]. Manufacturing has also been reported under ambient air conditions of high humidity, promising ease and flexibility of processing [10].
Sequential deposition method has evolved over other solution processing routes yielding the best device efficiencies [10]. It involves first step deposition of PbI2 films followed by CH3NH3I deposition resulting in the formation of CH3NH3PbI3. While there is a broad consensus on the concentration of PbI2 at 1 M, various concentrations of CH3NH3I have been reported for this method, and a systematic study of the effects of CH3NH3I concentration on device performance has been undertaken [14]. High efficiencies reported for sequential deposition method have invariably been obtained under controlled processing conditions which require energy intensive processes. Reports are also available for the ambient air synthesis of CH3NH3PbI3 solar cells; some of which have achieved impressive efficiencies [10]. Using sequential deposition with some process modification, reliable film formation has been achieved, even in ambient air condition. Effects of ambient air processing on the charge transport and material properties of CH3NH3PbI3 are still open for further investigation.
In the present contribution, we set forth to examine the effect of ambient air synthesis conditions on the material and charge transport properties of CH3NH3PbI3. By varying the concentration of CH3NH3I (in second step) under ambient air conditions, we obtained reliable film growth of CH3NH3PbI3 and characterized its micrographic granularity using AFM/SEM. X-ray diffraction measurements were later carried out for CH3NH3PbI3 films grown using different molar concentrations of CH3NH3I. We have also performed the conductivity measurements of these thin films at different temperatures and both under dark and one sun illumination conditions. In the light of above investigations, we further varied the CH3NH3I concentration in the initial mix to come up with the best concentration for ambient air synthesis of perovskite film with optimum properties. To the best of our knowledge this is the first systematic study on the effect of molar concentration of CH3NH3I on the material and electrical properties of ambient air synthesized CH3NH3PbI3, while catering for operating temperature and light intensity variations.
2 Materials and methods
All materials for this study except CH3NH3I were sourced from Sigma Aldrich and used without further purification. CH3NH3I was purchased from Dyesol. 1 M PbI2 solution in DMF was spin coated on cleaned glass slides at 2500 rpm for 20 s. Lead iodide coated slides were then annealed at 40 °C for 3 min and 100 °C for 5 min in a laboratory oven. CH3NH3I was dissolved in iso-propanol at 6 mg/ml (0.038 M), 7 mg/ml (0.044 M), 8 mg/ml (0.050 M), 9 mg/ml (0.057 M) and 10 mg/ml (0.064 M) respectively. Lead iodide coated slides were pre-heated to 70 °C before spin coating with CH3NH3I solution. We made certain changes to the reported procedure [10] for ambient air synthesis. Before coating CH3NH3I on lead iodide coated glass slide, we soaked the slides with 20 µl of iso-propanol while spinning and simultaneously added few drops of CH3NH3I solution in iso-propanol. The slides attained light brownish shade during this step, suggesting formation of CH3NH3PbI3. Next, we soaked the slides in CH3NH3I solution for 20 s and followed by spinning at 2500 rpm for 20 s, while blowing hot air during the process. The slides were again annealed at 40 °C for 3 min and 100 °C for 5 min.
For solar cell fabrication, a 10 cm2 ITO coated glass slide with a resistivity of 15 Ω/cm2 was ultrasonically cleaned with detergent and ultrapure water with subsequent cleaning with iso-propanol. They were then dipped in piranha solution for one min and were then cleaned again with flowing ultra pure water. Sol gel was used to prepare TiO2 sol by mixing 2- methoxyethanol (99.9+ %), titanium isopropoxide (<97 %), and ethanolamine (99+ %) in 4:1:0.5 molar ratios in a three necked flask and refluxing in inert environment for 1 h. Temperature was raised to 80 °C and then to 120 °C with stirring under inert environment for 2 h at 120 °C. Sol thus prepared was spin coated on pre cleaned ITO coated slides at 2500 rpm for 20 s. Films were annealed in oven at 500 °C for 2 h. Optimum concentration of CH3NH3PbI3 was deposited using the synthesis protocol as described above.
All characterization of CH3NH3PbI3 was carried out immediately after synthesis to guard against degradation effects, as the slides were not encapsulated. Fresh samples were prepared every time a characterization technique was employed, while rigorously following the above defined processing protocol to ensure good correlation of measured data. Atomic Force Microscopy (AFM) was performed on JEOL SPM 5200 using NSC35 micro fabricated cantilevers tip in non contact mode. Samples were studied under ambient air conditions. Scanning Electron Microscopy (SEM) was performed on thermionic emission JEOL JSM6490A employing tungsten tip. X-Ray diffraction (XRD) studies were carried on STOE Stadi MP using Cu Ka source. Step size was kept at 0.04° and 3 s dwell time in theta 2 theta mode to achieve maximum intensity at all angles. The conductivity measurements were made using ECOPIA HMS-5000 apparatus employing gold coated four probes. Contacts were established by silver paste and the measurements were recorded at temperatures varying between 300 and 340 K with a step size of 5 K, both under one sun illumination and under dark. Completed solar cells were characterized at 100 mW/cm2 irradiance intensity using Newport 67005 solar simulator operated with an AM 1.5G filter and a Keithley 2400 source meter.
3 Results and discussion
Two step spin coating procedure is an improvement of the two step dipping procedure because of the precise control over the quantities involved. The size of the CH3NH3PbI3 cuboids decreases with increase in the concentration of the CH3NH3I solution used during second step. The cuboids granular size is found to be decreasing from ~2820 nm for 0.038 M to ~490 nm for 0.064 M. The roughness profile determined through AFM measurements also confirms this decreasing trend of the cuboids size with increasing concentration of the CH3NH3I solution. Both size and roughness distribution is graphically presented in Fig. 1. The vertical thickness of the film is approximated at 300–400 nm, suggesting preferential orientation subsequently confirmed thorough XRD. Large crystal growth at lower concentration of the CH3NH3I with sparse density suggests fewer nucleation centers allowing for the growth of larger cuboids. At higher concentrations, the crystal growth is impeded by higher nucleation density resulting in high particle density and smaller crystals.
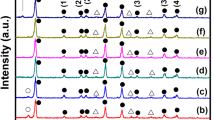
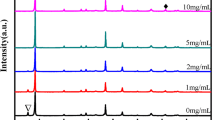
XRD profiles recorded for different concentrations are presented in Fig. 2 where peaks associated with CH3NH3PbI3 are marked as * and those associated with un-reacted PbI2 are marked as #. A clear trend can be observed for CH3NH3PbI3 signature, increasing in intensity with increasing concentration of the CH3NH3I in the solution and then falling with the maximum concentration employed in the study. Strongest intensity for CH3NH3PbI3 is observed for a CH3NH3I concentration of 0.050 M. This trend can be attributed to increasing grain population with intermediate concentrations and the fall at the maximum concentration is due to the small size grains owing to higher nucleation centers competing for growth and resulting in small size grains. PbI2 signature also shows a decreasing trend with increasing concentration of CH3NH3I. At the highest CH3NH3I concentration the lead iodide signature almost vanishes as should be expected for the increased concentration resulting in complete conversion of PbI2 to CH3NH3PbI3. These trends have resulted in all likelihood to give highest grain size to voids ratio of CH3NH3PbI3 film at intermediate concentration of CH3NH3I. Characteristic peaks for CH3NH3PbI3 occur at 14.0, 24.3, 28.4, 31.7, 40.5, 43.0, and 50.3o in Fig. 2, corresponding to reflections from (110), (202), (220), (310), (224),(314), and (404) planes, confirming tetragonal phase of absorber. Preferred orientation along (110) plane evident from these diffractograms, validates the blocky appearance in SEM images, confirming preferred growth along c axis than a and b axis.
It is evident from the SEM and AFM profiles in Fig. 3 that the voids also decrease with increasing concentrations of the CH3NH3I in the solution. For working with hole transport layer (HTL) free devices, it is imperative to obtain complete coverage of the underlying electron selective contact to avoid short circuiting the device. Larger particle sizes have also been attributed to contribute to light scattering [14], resulting in lower light absorption efficiencies.
Optical absorption spectrum of the films developed by varying the concentration of MAI is presented in Fig. 4. Absorption increases with the increase in the molarity of the solution from 0.038 M and the maximum absorption is recorded for films based on 0.050 M MAI solution.
Un-reacted lead iodide forms a needle like morphology as is evident form SEM and AFM micrographs in Fig. 5. This corresponds to the 2H poly-type consistent with earlier reports for lead iodide films [15]. These films convert from needle like morphology to dense closely packed CH3NH3PbI3 crystals of block like appearance (upon CH3NH3I treatment). This block like appearance is an indication of preferential orientation of the CH3NH3PbI3 grains along (110) plane, confirmed by XRD study as well. Intercalation of CH3NH3I in lead iodide crystal structure and the associated volume change is responsible for this change in appearance. Unit cell volume changes from 124 Å3 of to Lead iodide to 990 Å3 of CH3NH3PbI3 upon reaction with CH3NH3I. The unit cell volume for perovskite phase CH3NH3PbI3 is 248 Å3, almost twice that of lead iodide. This change is unit cell volume is responsible for the dense and rough appearance of CH3NH3PbI3 under SEM, a change from needle like appearance of lead iodide.
Low magnification large area SEM images (Fig. 5) reveal uniform coverage for CH3NH3PbI3 particles, an indication of the efficiency of the synthesis protocol under high humidity. Suitable humidity levels have been identified as being a prerequisite for obtaining void free films rendering optimum optoelectronic properties. Films prepared under nitrogen environment are reported to introduce grain boundaries and voids causing energetic disorder, hampering the photovoltaic performance. On the contrary, films deposited under ambient air conditions obtained large grain size, decreasing grain boundaries and improving charge transport [16, 17]. Proper humidity levels result in grain boundary creep generating large grains. Increased grain size and decreased voids render improved photovoltaic performance owing to enhanced carrier life times. Humidity in the ambient air synthesis may help in generating additional charge carriers by autoionization of adsorbed water molecules.
Both the grain size and compactness of CH3NH3PbI3 films are important in determining optoelectronic properties. Improved light absorption and greater charge carrier generation occur for compact CH3NH3PbI3 films, while large sized grains result in better carrier transport as well as greater charge collection efficiencies. The defects and mis-orientation at grain boundaries cause increased local recombination of electron–hole pairs and enhanced carrier scattering, hence charge transport and collection is adversely affected. The best compromise in grain size and compactness in our two step ambient air spin coating protocol is obtained at intermediate concentrations of CH3NH3I solution.
To validate the optoelectronic properties of resultant CH3NH3PbI3 films, the resistivity (conductivity) measurements were performed both under dark and one sun illumination at a range of temperatures to study detailed behavior. Figure 6 summarizes the results of our investigations. The scatter values of three independent measurements are plotted in all cases, which are obtained using three similar CH3NH3PbI3 films prepared under each CH3NH3I concentration. The scatter values are directly plotted to underline the high process quality and minimal variations in our two step spin coating method. The resistivity under dark conditions is higher than under one sun illumination for all CH3NH3PbI3 films, a result which is expected since additional free charge carriers are generated from light absorption. The best value of conductivity (inverse of resistivity) under one sun illumination occurs for CH3NH3PbI3 film prepared using highest molar concentration i.e. 0.064 M of CH3NH3I solution. This result is verified from the fact that despite smaller grain size, most compact CH3NH3PbI3 film is formed under this molar concentration.
The variation of resistivity of CH3NH3PbI3 films with temperature, provide more interesting insight into electronic behaviour. The resistivity under dark for all the samples of CH3NH3PbI3 stays in the range of 1.5–2.0 × 105 Ωcm, less for CH3NH3PbI3 film prepared with highest molar concentration of CH3NH3I, which shows a slight decreasing trend with temperature. This might be the outcome of better charge hopping transport [18, 19, 19] across grain boundaries for this compact film, which increases with increase in the temperature. The resistivity under one sun illumination, however, shows a different behaviour for each CH3NH3PbI3 film, with the photoconductivity (inverse of resistivity) decreasing with temperature for CH3NH3PbI3 films of higher grain size (but more voids), while showing an increasing trend with temperature for moderate size CH3NH3PbI3 grains. However when the resistivity under light becomes an order of magnitude less compared with its value under dark, i.e. for most compact CH3NH3PbI3 film (but with lowest grain size), it is insensitive to change in temperature and stays constant. In summary, photoconductivity of perovskite films is strongly modulated by the variation in temperature, compared with the conductivity measured under dark.
Thus resistivity (conductivity) measurements of CH3NH3PbI3 films prepared using two step spin coating method under simple ambient air conditions, shows that compactness of perovskite film which guarantees improved optoelectronic performance occurs for smaller grain size. At growth conditions, conducive to large crystal growth i.e. lower concentrations of CH3NH3I, voids becomes predominant in the film, adversely affecting charge transport properties, advantage that can be gained by large sized grains.
Based on the indentified optimized concentration of MAI, solar cells were prepared employing a simplified hole transport layer free geometry operating under ambient air conditions. The structure of completed device was ITO (~100 nm)/TiO2 (~200 nm)/CH3NH3PbI3 (~400 nm)/Au (~100 nm). With an active area of 0.11 cm2 it achieved a short circuit current density of 12.31 mA/cm2, fill factor (FF) of 48 % and an open circuit voltage (Voc) of 0.63 V resulting in power conversion efficiency (PCE) of 3.74 % (Fig. 7).
4 Conclusions
Both the morphological and photoconductive properties of perovskite CH3NH3PbI3 films prepared using two step solution syntheses in ambient air conditions are studied to arrive at the optimum concentration of CH3NH3I for these processing conditions. It has been found that the photoconductivity under dark remains constant for all the concentrations of CH3NH3I used in this study but they vary with temperature under one sun illumination. For small concentrations they have a decreasing trend with increasing temperature while for higher concentration the conductivity slightly increases with increase of temperature or stays invariant to the change in temperature. Morphology studies establish that the grain size decrease with increasing concentration of CH3NH3I in the solution and the compactness increases. It has been found that while intermediate molar concentration (0.050 M) gives the best grain size to void ratio of CH3NH3PbI3 film, the photoconductivity is strongly modulated by temperature and best results of charge transport under illumination are obtained for most compact film of CH3NH3PbI3, obtained using 0.050 M CH3NH3I solution in our method. This emphasizes the role of compactness of perovskite film in determining optoelectronic properties of this class of absorber using ambient air processing conditions.
References
M.A. Green, A. Ho-Baillie, H.J. Snaith, The emergence of perovskite solar cells. Nat. Photonics 8(7), 506–514 (2014)
M.M. Lee, J. Teuscher, T. Miyasaka, T.N. Murakami, H.J. Snaith, Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science 338(6107), 643–647 (2012)
W.S. Yang, J.H. Noh, N.J. Jeon, Y.C. Kim, S. Ryu, J. Seo, S.I. Seok, High-performance photovoltaic perovskite layers fabricated through intramolecular exchange. Science 348(6240), 1234–1237 (2015)
G. Xing, N. Mathews, S. Sun, S.S. Lim, Y.M. Lam, M. Grätzel, S. Mhaisalkar, T.C. Sum, Long-range balanced electron- and hole-transport lengths in organic–inorganic CH3NH3PbI3. Science 342(6156), 344–347 (2013)
S.D. Stranks, G.E. Eperon, G. Grancini, C. Menelaou, M.J.P. Alcocer, T. Leijtens, L.M. Herz, A. Petrozza, H.J. Snaith, Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science 342(6156), 341–344 (2013)
H.-S. Kim, I. Mora-Sero, V. Gonzalez-Pedro, F. Fabregat-Santiago, E.J. Juarez-Perez, N.-G. Park, J. Bisquert, Mechanism of carrier accumulation in perovskite thin-absorber solar cells. Nat. Commun. 4, 2242 (2013)
S. De Wolf, J. Holovsky, S.-J. Moon, P. Löper, B. Niesen, M. Ledinsky, F.-J. Haug, J.-H. Yum, C. Ballif, Organometallic halide perovskites: sharp optical absorption edge and its relation to photovoltaic performance. J. Phys. Chem. Lett. 5(6), 1035–1039 (2014)
X. Ziang, L. Shifeng, Q. Laixiang, P. Shuping, W. Wei, Y. Yu, Y. Li, C. Zhijian, W. Shufeng, D. Honglin, Y. Minghui, G.G. Qin, Refractive index and extinction coefficient of CH_3NH_3PbI_3 studied by spectroscopic ellipsometry. Opt. Mater. Express 5(1), 29 (2014)
S. Ye, W. Sun, Y. Li, W. Yan, H. Peng, Z. Bian, Z. Liu, C. Huang, CuSCN-based inverted planar perovskite solar cell with an average PCE of 15.6 %. Nano Lett. 15(6), 3723–3728 (2015)
H.-S. Ko, J.-W. Lee, N.-G. Park, 15.76 % efficiency perovskite solar cell prepared under high relative humidity: importance of PbI2 morphology in two-step deposition of CH3NH3PbI3. J. Mater. Chem. A 3(16), 8808–8815 (2015)
R. Sheng, A. Ho-Baillie, S. Huang, S. Chen, X. Wen, X. Hao, M.A. Green, Methylammonium lead bromide perovskite-based solar cells by vapour-assisted deposition. J. Phys. Chem. C 119(7), 150127132618007 (2015)
J. Borchert, H. Boht, W. Fränzel, R. Csuk, R. Scheer, P. Pistor, Structural investigation of co-evaporated methyl ammonium lead halide perovskite films during growth and thermal decomposition using different PbX 2 (X = I, Cl) precursors. J. Mater. Chem. A 3(39), 19842–19849 (2015)
A. Mei, X. Li, L. Liu, Z. Ku, T. Liu, Y. Rong, M. Xu, M. Hu, J. Chen, Y. Yang, M. Gratzel, H. Han, A hole-conductor-free, fully printable mesoscopic perovskite solar cell with high stability. Science (80-.) 345(6194), 295–298 (2014)
J.-H. Im, I.-H. Jang, N. Pellet, M. Grätzel, N.-G. Park, Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nat. Nanotechnol. 9(11), 927–932 (2014)
J. Burschka, N. Pellet, S.-J. Moon, R. Humphry-Baker, P. Gao, M.K. Nazeeruddin, M. Grätzel, Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499(7458), 316–319 (2013)
H. Zhou, Q. Chen, G. Li, S. Luo, T.-B. Song, H.-S. Duan, Z. Hong, J. Hou, Y. Liu, Y. Yang, Z. Hong, T.-B. Song, L. Meng, Y. Liu, C. Jiang, H. Zhou, W.-H. Chang, G. Li, Y. Yang, Moisture assisted perovskite film growth for high performance solar cells. Appl. Phys. Lett. 345(18), 542–546 (2014)
H. Zhou, Q. Chen, G. Li, S. Luo, T.-B. Song, H.-S. Duan, Z. Hong, J. You, Y. Liu, Y. Yang, Interface engineering of highly efficient perovskite solar cells. Science (80-.) 345(6196), 542–546 (2014)
V. Coropceanu, J. Cornil, D.A. da Silva Filho, Y. Olivier, R. Silbey, J.-L. Brédas, Charge transport in organic semiconductors. Chem. Rev. 107(4), 926–952 (2007)
N. Karl, Charge carrier transport in organic semiconductors. Synth. Met. 133–134, 649–657 (2003)
Acknowledgments
This research was funded by Higher Education Commission Pakistan through Grant Number 213-58732-2EG2-014(50023541).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ahmed, M.I., Butt, H.T., Hussain, Z. et al. Effects of ambient air processing on morphology and photoconductivity of CH3NH3PbI3 . J Mater Sci: Mater Electron 27, 12028–12035 (2016). https://doi.org/10.1007/s10854-016-5351-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-5351-2