Abstract
Pure and La-doped SnO2 nanoparticles were synthesized by ball-milling solid chemical reaction method. The microstructure, morphologies and gas sensing properties of as-synthesized nanoparticles were characterized by X-ray diffraction, scanning electron microscopy, energy dispersive X-ray, transmission electron microscope and gas-sensing measurement device. Compared with pure SnO2 sensor, La-doped SnO2 sensor exhibited excellent formaldehyde sensing properties at the optimum temperature of 240 °C. Among them, 3 at% La-doped SnO2 sensor showed the highest response of 31.5–50 ppm formaldehyde vapor, the response and recovery time were 5 and 26 s, respectively. Moreover, the relationships between response value and HCHO concentration, and the selectivity of pure and 3 at% La-doped SnO2 sensor were investigated. The result indicated that the 3 at% La-doped SnO2 sensor had high response, short response-recovery time and good selectivity to formaldehyde. Finally, the gas-sensing mechanism of SnO2 sensor were discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Formaldehyde is considered as one of the most common toxic volatile organic compounds (VOC) in homes, offices and urban environment [1]. In recent years, the formaldehyde is widely applied in the field of architecture and decoration, which can lead to the slow release of formaldehyde vapor into the environment [2]. Previous study showed that long-term exposure to the air containing toxic VOC such as formaldehyde, benzene, even led to malignant diseases such as cancer, leukemia, etc. [3]. Hence it is necessary to carry out effective ways to monitor formaldehyde gas in the environment.
Metal oxides semiconductor (MOS), such as SnO2, ZnO, TiO2, WO3, In2O3, and so on, is believed to be an ideal material to detect the toxic and flammable gases, and has been developed rapidly because of its low cost, reliability, accuracy and real time gas analyzing [4–8]. Among them, SnO2, an important n-type semiconductor with a wide band gap energy of 3.6 eV [9], has been proven to be a kind of excellent gas-sensing material with high response to ppm (part per million) level and employed for detection of numerous gases [10]. However, there are some disadvantages in the pristine SnO2 gas sensor, such as poor selectivity, long response-time and high working temperature, these defects restrict the further application of SnO2. In order to improve the properties of SnO2 sensor material, introduction of dopants in the host material is commonly adopted. Many studies have reported the effect of doping of different element (Au [11], Cu [12], Cd [13], Yb [14], Pr [15], Pd [16], Ce [17]) on the structure, morphology and gas sensing properties of SnO2. Among them, the rare earth doped tin oxide exhibited excellent gas sensing properties due to the unique catalytic and sensitive. Wu et al. [18] have reported that the sensitivity of single crystal SnO2 nanobelts to ethanediol, ethanol and acetone was enhanced by lanthanum doping. Jinkawa et al. [19] reported the gas-sensing properties of SnO2 were dramatically enhanced after impregnation of La2O3. In general, the doped La2O3 has been shown to effectively promote performance of SnO2 based gas sensors. However, the effects of La-doped SnO2 on the formaldehyde gas sensing properties is less reported.
In this paper, we present a simple ball-milling solid chemical reaction approach to synthesize pure and La-doped SnO2 gas sensor, expecting to enhance the formaldehyde gas sensing properties. The microstructure, morphologies and gas sensing properties of pure and La-doped SnO2 nanoparticles were studied.
2 Experimental
2.1 Materials
All the chemicals were purchased from Kelong Co, Chengdu, China and used without further processing or purification: chloride dihydrate (SnCl2·2H2O, ≥99 %, AR), oxalic acid dihydrate (C2H2O4·2H2O, ≥99 %, AR), Lanthanum nitrate hexahydrate [La(NO3)3·6H2O, ≥99 %, AR], anhydrous ethanol (≥99 %, AR), Ammonia solution (AR).
2.2 Preparation of pure SnO2 and La-doped SnO2 nanoparticles
Pure and La doped SnO2 were obtained by the ball-milling solid chemical reaction method at room temperature. The mechanochemical reaction was done in a 250 cm3 agate ball milling jar containing agate ball (ball-to-reagent mass ratio of 10:1). Specimens in this experiment were synthesized as follows: SnCl2·2H2O, certain amount of La(NO3)3·6H2O (0, 1, 2, 3, 4 at%) and C2H2O4·2H2O were put into an agate ball milling jar. In addition, the anhydrous ethanol was added to the above system as grinding aid. Subsequently, the milling jar was rotated at 400 rpm in a QM-3SP2 planetary ball mill for 4 h. Meanwhile, ammonia solution was used to adjust the pH of the system per half an hours until pH ≥ 6.5. The product of the mechanochemical reaction (precursor) was ultrasonic washed with distilled water and then ethanol. After washed, the precursor was dried at 70 °C for 12 h. Then, the dry precursor was ground into powder and thermal decomposed at 600 °C for 2 h in muffle furnace to form SnO2. The possible formation process of SnO2 samples can be expressed as following chemical equations:
2.3 Characterization
The X-ray diffraction (XRD) patterns of pure and La-doped SnO2 were obtained by X-ray diffractometer (XRD-6000, SHIMADZU) using Cu-Kα radiation (λ = 1.5418 Å). A continuous scan mode was used to collect 2θ data from 20° to 80°. Scanning electron microscope (SEM: JEOL JSM-5900LV, Japan) was used to observe the morphology of calcined samples and the energy dispersive X-ray (EDX) technique was used to determine their elemental compositions. Transmission electron microscope (TEM) images were taken by using JEOL JEM-100CX (Japan).
2.4 Fabrication and measurement of gas sensor
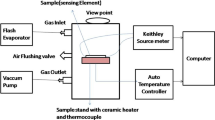
The basic fabricated process of gas sensor is as follows: The as-prepared SnO2 powders and a suitable amount of anhydrous ethanol were mixed and then ultrasonic dispersed to form a paste. Then the paste was coated on ceramic tubes, on which a pair of Au electrodes and four Pt wires were previously installed at each end. The coated ceramic tubes were calcined at 300 °C for 2 h. Subsequently, a heater of Ni–Cr wire was inserted into the ceramic tube to regulate the working temperature (Fig. 1a). The sensor devices were aged for 5 days in air in order to improve the long-term stability.
The gas-sensing properties were measured by a WS-30A gas sensitivity instrument (Weisheng Electronics Co., Ltd China). Figure 1b displayed the basic measuring electric circuit of gas sensing characteristics. The sensor response was defined as S = R a /R g , where Ra and Rg are separately the electrical resistance of the sensor in air and test gas. The response time was defined as time reaching the 90 % steady response value and the recovery time was defined as time attaining within 10 % of the initial response value [15].
3 Results and discussion
3.1 Structural and morphological characteristics
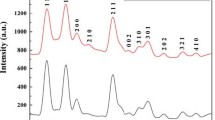
Figure 2a shows the typical XRD patterns of pure and La-doped SnO2 sample, all diffraction peaks can be indexed to tetragonal rutile SnO2 phase (JCPDS NO. 41-1445, a = b = 4.7382 Å, c = 3.1871 Å) [20, 21]. However, no diffraction peaks of La can be obviously observed, which is probably because addition of La is low so as to dissolve in SnO2 lattice [14]. The average size estimated by full width at half maximum (FWHM) of (110) using Scherrer’s formula is 9.1, 8.3, 7.9, 8.7, 10.7 nm, corresponding to the La-doped content of 0, 1, 2, 3 and 4 %. To further discuss the effect of La doping on structure characteristics of SnO2, the (110) and (101) diffraction peaks of sample are magnified in Fig. 2b. For all the La-doped sample, it can be seen that the 2θ diffraction peaks shift to the bigger angle side compared to the pure SnO2. According to the Bragg’s law and the tetragonal rutile structure of SnO2 system, the lattice constant a of samples can be calculated and simplified as the following formula [22]:
where λ and θ are separately the X-ray wavelength (0.15418 nm) and diffraction angle of (110) crystal lattice plane. Table 1 shows the 2θ position of prominent diffraction peaks and lattice parameter calculated from Eq. (3). It is observed that diffraction angles of the (110), (101) and (211) peaks increase and the lattice constant a gradually decreases with the increase of La doping. Both the shift of diffraction peaks and decrease of lattice constant a probably prove that La doping can cause lattice mismatch of SnO2. Figure 2c shows the corresponding EDX pattern of 3.0 at% La-doped SnO2 powder. As can be seen, the as-synthesized product is consisted of O, Sn and La elements. The additional peaks of C, Si and Au are caused in the detection process. All of these suggest that the lanthanum ions have been doped into SnO2 lattice.
Figure 3a–e displays the SEM images of SnO2 powder doped with different amount of La. It can be seen that the La doping has little impact on morphology of SnO2. This is possibly because in the ball-milling solid chemical reaction, mechanical force makes the refinement degree of precursor particles tend to be consistent. Both the pure and La-doped SnO2 powders consist of aggregation of spherical shaped particles and the size of aggregations is in range of 50–500 nm.
Figure 4 gives the TEM image of 3 at% La-doped SnO2 powder. We can see that the aggregations mentioned above are gathered from the nanoparticles with a size of about 14 nm. The crystallite size from TEM is in good with the XRD results.
3.2 Gas sensing properties
Many studies have proven that the gas sensitivity is greatly influenced by the operating temperature [23–26]. In order to determine the optimum operating temperature for formaldehyde detection, the responses of pure and La-doped SnO2 sensors to 100 ppm HCHO at different operating temperature are tested as shown in Fig. 5. As the operating temperature rises, the response performances of all sensors are enhanced at first and reach the maximum value at 240 °C, then decrease gradually with a further rising of temperature. Therefore, the optimum working temperature of La-doped SnO2 sensors towards formaldehyde is 240 °C.
Figure 6 shows the response and recovery characteristic curves of pure and La-doped SnO2 sensors to formaldehyde vapor at 240 °C. The concentration range of formaldehyde vapor is 10–800 ppm, and the relative humidity is 55 % RH. It can be seen from the figure that the response cycles corresponding to the 10, 50, 100, 200, 500 and 800 ppm, are recorded successively. The continuity of response and recovery characteristic curve is good, demonstrating the sensors have good repeatability and stability [12]. In addition, the response value of undoped SnO2 sensor is obviously lower than that of those sensors with different amount of La-doping. It shows that La doping can effectively improve the HCHO gas sensing properties of SnO2 sensor. Among all these La-doped SnO2 sensors, the one with 3 at% La-doped shows the highest response value to different concentrations of formaldehyde gas. Therefore, the optimum amount of La-doping to HCHO gas-sensing for this work is 3.0 at%. Moreover, the response value and response-recovery time (Tres/Trec) of (0, 3 at%) La-doped SnO2 sensors to 50, 100, 200, and 500 ppm formaldehyde vapor are listed in Table 2. The average response and recovery time of (0, 3 at%) La-doped SnO2 sensors is about 25, 5 and 100, 26 s, respectively, which indicates that the doping of La can significantly shorten the response and recovery time.
Figure 7 shows the response of 3 at% La-doped SnO2 sensor versus formaldehyde concentration from 5 to 6000 ppm at 240 °C. When exposed to 5 ppm HCHO, the response value of 3 at% La-doped SnO2 sensor is 4.2, suggesting it has good gas sensing properties in the lower formaldehyde concentration and the detectable minimum limit is about 5 ppm. On the other hand, the response rises up rapidly with increasing formaldehyde concentration, especially in the range of 0–200 ppm, the relationship between the sensor response and HCHO concentration is linear ranging (as shown in the inset of Fig. 7). But the rising rate is reduced when the concentration of formaldehyde vapor exceeds 800 ppm, which illustrates that the sensor tends to saturation gradually [21]. Furthermore, Fig. 8 exhibits the corresponding dynamic resistances change of pure and 3 at% La-doped SnO2 sensor at 240 °C in the logarithmic coordinates. The resistance of pure and 3 at% La-doped SnO2 sensor is about 481 and 9625 kΩ in air. When the formaldehyde is introduced, the resistance drops sharply.
Besides, the selectivity is one of the key parameters for appraising the properties of gas sensors [27–29]. In order to clarify the selectivity of our sample, the pure and 3 at% La-doped SnO2 sensors are tested in various gases, such as ethanol (EtOH), acetone (CH3COCH3), ammonia (NH3) and formaldehyde (HCHO) of 500 ppm at 240 °C. As shown in Fig. 9. It can be observed clearly that the 3 at% La-doped SnO2 sensor shows a higher response to all the tested gas than pure SnO2. Moreover, the response of 3 at% La-doped SnO2 sensor to HCHO is about 233, which is much higher than that to other test gases. The result demonstrates the 3 at% La-doped SnO2 sensor has a good selectivity to formaldehyde.
To sum up, 3 at% La-doped SnO2 sensor has high response, short response-recovery time and good selectivity to formaldehyde. A comparison of this work with other formaldehyde sensors based on SnO2 material using various methods is presented in Table 3. The result shows that the 3 at% La-doped SnO2 is a promising candidate for formaldehyde sensor related applications.
3.3 Gas sensing mechanism
As a kind of surface-control type sensing material, gas sensing performance of SnO2 is mainly influenced by its surface characteristics. Monitoring of the target gas concentration by SnO2 gas sensor is dependent on the change of surface resistance. When pure SnO2 is exposed to air, oxygen molecules tend to be adsorbed on the surface, and then the partially adsorbed oxygen will capture electrons from the surface to generate ions in the form of O −2(ads) , O −(ads) and O 2−(ads) . The process is as follows [10, 18, 28]:
These adsorbed oxygen ions can cause a reduction of electron concentration in the conduction band, resulting in the formation of potential barrier [2]. Thus, the resistivity of SnO2 in air is higher than the intrinsic resistivity.
However, when SnO2 is exposed to a reducing gas such as HCHO, the reducing gas molecules will react with adsorbed oxygen ions and release the trapped electrons to conduction band [14]. As a result, the conductivity increases and potential barrier height reduction. The process may be carried out by Eq. (8) [2, 16, 24].
As the working temperature rises, the carrier concentration in the conduction band of SnO2 and the chemical activation increase lead to an improvement of response value. But when the temperature is too high, the adsorbed reducing gas molecules may escape from the surface of the sensor [15], resulting in a poor response. Therefore, the gas sensors exhibited the highest response at 240 °C,
According to the XRD analysis mentioned above, as well as the ionic radii of La3+ (0.103 nm) is larger than that of Sn4+ (0.071 nm), it is reasonable to consider that the lanthanum ions doped into the SnO2 lattice induced lattice mismatch. This could provide more active sites on the surface of SnO2 to adsorb more oxygen, which greatly raises the resistivity of SnO2 in air, this result can be demonstrated from Fig. 8. On the other hand, the increase of active sites is beneficial to the decomposition of HCHO, which makes more trapped electrons released, resulting in the decrease of resistivity in reducing gas. Ultimately, the response of SnO2 sensors is enhanced. In addition, La2O3, as the ultimate doping production, is favorable to produce O 2−2 on its surface. The O 2−2 could trigger an H-abstraction chemical reaction with hydrocarbon compound [35]. Therefore, when formaldehyde gas is introduced, the reaction energy of oxidation of carbon-hydrated is reduced and the response of La-doped SnO2 sensor is enhanced. Finally, with the concentration of formaldehyde gas increasing, more electrons are released, and the response value rises continuously. Until adsorbed oxygen ions are depleted, the sensors become saturation, as shown in the Fig. 7.
When the lanthanum doping concentration exceeds 3 at%, the response value decreases, this may be because the redundant doping will lead to the formation of many clusters such as La–La or La–O, reducing the electric transduction of SnO2 nanoparticles [14]. In addition, an excess of lanthanum doping may cause the abnormal growth of SnO2 crystallite and the decrease of specific surface area, it can be seen in the results of XRD. This may be another reason why the response value decreases in the 4 at% La-doped SnO2 sensor.
4 Conclusions
In conclusion, pure and La-doped SnO2 nanoparticles are successfully synthesized by ball-milling solid chemical reaction method and studied for the detection of formaldehyde vapor. We find that at the optimum operating temperature of 240 °C, the La-doped SnO2 sensors exhibit the enhanced HCHO sensing properties comparing with the pure SnO2 sensor. Especially, 3 at% La-doped SnO2 sensor shows the highest response to formaldehyde gas. The improvement of gas-sensing properties of SnO2 sensor is attributed to the increase of active sites and the production of O 2−2 ion. Furthermore, compared with previous studies, the 3 at% La-doped SnO2 sensor shows high response, short response-recovery time and good selectivity to formaldehyde, indicating that 3 at% La-doped SnO2 can be a promising candidate for formaldehyde sensor.
References
C.J. Weschler, Atmos. Environ. 43, 153 (2009)
Y. Lin, Y. Wang, W. Wei, L.H. Zhu, S.P. Wen, S.P. Ruan, Ceram. Int. 41, 7329 (2015)
J.Y. Chen, Y. Huang, G.Y. Li, T.C. An, Y.K. Hu, Y.L. Li, J. Hazard. Mater. 302, 395 (2016)
D.Z. Zhang, J.J. Liu, H.Y. Chang, A.M. Liu, B.K. Xia, RSC Adv. 5, 18666 (2015)
S. Sarkar, D. Basak, Sens. Actuators B Chem. 176, 374 (2013)
N. Kılınç, E. Şennik, Z.Z. Öztürk, Thin Solid Films 520, 953 (2011)
X.H. Ding, D.W. Zeng, S.P. Zhang, C.S. Xie, Sens. Actuators B Chem. 155, 86 (2011)
S. Yi, S.Q. Tian, D.W. Zeng, K. Xu, S.P. Zhang, C.S. Xie, Sens. Actuators B Chem. 185, 345 (2013)
S.C. Yeow, W.L. Ong, A.S.W. Wong, G.W. Ho, Sens. Actuators B Chem. 143, 295 (2009)
L.P. Chikhale, J.Y. Patil, A.V. Rajgure, F.I. Shaikh, I.S. Mulla, S.S. Suryavanshi, Ceram. Int. 40, 2179 (2014)
F.C. Chung, R.J. Wu, F.C. Cheng, Sens. Actuators B Chem. 190, 1 (2014)
W.X. Jin, S.Y. Ma, Z.Z. Tie, J.J. Wei, J. Luo, X.H. Jiang et al., Sens. Actuators B Chem. 213, 171 (2015)
Z. Tianshu, P. Hing, Y. Li, Z. Jiancheng, Sens. Actuat B Chem. 60, 208 (1999)
T.T. Wang, S.Y. Ma, L. Cheng, J. Luo, X.H. Jiang, W.X. Jin, Sens. Actuators B Chem. 216, 212 (2015)
W.Q. Li, S.Y. Ma, Y.F. Li, X.B. Li, C.Y. Wang, X.H. Yang et al., J. Alloys Compd. 605, 80 (2014)
N. Ma, K. Suematsu, M. Yuasa, K. Shimanoe, ACS Appl. Mater. Interfaces 7, 15618 (2015)
W.F. Qin, L. Xu, J. Song, R.Q. Xing, H.W. Song, Sens. Actuators B Chem. 185, 231 (2013)
Y.M. Wu, H. Zhang, Y.K. Liu, W.W. Chen, J. Ma, S.H. Li et al., Sensors 15, 14230 (2015)
T. Jinkawa, G. Sakai, J. Tamaki, N. Miura, N. Yamazoe, J. Mol. Catal. A: Chem. 155, 193 (2000)
W. Zeng, H. Zhang, Y.P. Li, W.G. Chen, Z.C. Wang, Mater. Res. Bull. 57, 91 (2014)
W.X. Jin, S.Y. Ma, Z.Z. Tie, W.Q. Li, J. Luo, L. Cheng, X.L. Xu et al., Appl. Surf. Sci. 353, 71 (2015)
F.C. Yang, Z.G. Guo, J. Colloid Interface Sci. 448, 265 (2015)
H. Yu, S.M. Wang, C.H. Xiao, B.X. Xiao, P. Wang, Z.F. Li et al., CrystEngComm 17, 4316 (2015)
C.S. Prajapati, A. Kushwaha, P.P. Sahay, Appl. Phys. A 113, 651 (2013)
S.Q. Tian, X.H. Ding, D.W. Zeng, S.P. Zhang, C.S. Xie, Sens. Actuators B Chem. 186, 640 (2013)
J.J. Wu, D.W. Zeng, S.Q. Tian, K. Xu, D.G. Li, C.S. Xie, J. Mater. Sci. 50, 7725 (2015)
J.R. Huang, L.Y. Wang, C.P. Gu, Mater. Lett. 136, 371 (2014)
J.P. Du, R.H. Zhao, Y.J. Xie, J.P. Li, Appl. Surf. Sci. 346, 256 (2015)
N. Duc Chinh, N. Van Toan, V. Van Quang, N. Van Duy, N. Duc Hoa, N. Van Hieu, Sens. Actuators B Chem. 201, 7 (2014)
R.K. Mishra, A. Kushwaha, P.P. Sahay, RSC Adv. 4, 3904 (2014)
Y.L. Cao, Y.Z. Li, D.Z. Jia, J. Xie, RSC Adv. 4, 46179 (2014)
Y.G. Zheng, J. Wang, P.J. Yao, Sens. Actuators B Chem. 156, 723 (2011)
J. Wang, P. Zhang, J.Q. Qi, P.J. Yao, Sens. Actuators B Chem. 136, 399 (2009)
H.Y. Du, J. Wang, M.Y. Su, P.J. Yao, Y.G. Zheng, N.S. Yu, Sens. Actuators B Chem. 166–167, 746 (2012)
G.Z. Zhang, S.P. Zhang, L. Yang, Z.J. Zou, D.W. Zeng, C.S. Xie, Sens. Actuators B Chem. 188, 137 (2013)
Acknowledgments
The authors are grateful for the Project 2014GZ0090 supported by the Science and Technology Support Program in Sichuan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
xiang, X., zhu, D. & wang, D. Enhanced formaldehyde gas sensing properties of La-doped SnO2 nanoparticles prepared by ball-milling solid chemical reaction method. J Mater Sci: Mater Electron 27, 7425–7432 (2016). https://doi.org/10.1007/s10854-016-4718-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4718-8