Abstract
Oxidized nickel/silver (NiO/Ag) nanocomposites were prepared via micro-emulsion method. In the method, the NiO nanospheres were dispersed in ethanol including sodium dodecyl sulfonate (SDS) and sonicated to form homogeneous suspension, and then an appropriate amount of AgNO3 aqueous solution was added to this suspension above, forming a micro-emulsion. In the micro-emulsion, the NiO nanospheres were surrounded with many Ag+ ions. With the adding of NaBH4, Ag+ ions were reduced to Ag nanospheres. Finally, a series of NiO/Ag nanocomposites were prepared successfully. The microstructures of as-prepared samples were characterized by the XRD, SEM. The results showed that NiO and Ag nanospheres formed uniform nanocomposites. The capacitance performance of NiO/Ag nanocomposites electrodes with different Ag loading contents was investigated. The results showed that the capacitance performance of NiO/Ag nanocomposites was accordingly increased as the Ag content increases. The capacitance of the NiO/Ag electrodes with 3 % Ag loading was effective to obtain fully reversible and higher specific capacitance of ~322 F g−1 at 1 A g−1 current density.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanomaterials have been attracting great interest due to their potential use in applications such as supercapacitors, batteries, sensors, and solar cells [1, 2]. In electrochemical technology, supercapacitors receive a special place because they bridge the gap between batteries and conventional capacitors, and deliver stored energy more rapidly than batteries, and possess higher density and long cycle life [3, 4]. At present, pseudocapacitors exhibit higher energy density than electric double layer capacitors (EDLCs). The transition metal oxides have been used as pseudocapacitor electrode materials, which undergo fast reversible Faradic redox reaction and result in a specific capacitance 10–100 times higher than that of EDLCs [5, 6].
Metal oxides such as RuO2, MnO2, NiO, Co3O4 have evolved as an important family of anode materials to be a high-capacity alternative to graphite [3, 7–9]. Among these metal oxides, NiO stimulated extensive scientific research due to the potential applications in photocatalysts, electrochromic, magnetic, and catalysis materials [10–13]. Owing to the high surface area of NiO, it is promising in the field of supercapacitors and gas sensors. At present, many scientists are working hard to develop various methods for the synthesis of NiO. It is reported that metal coating or doping on nickel oxide can enhance the supercapacitor performance [14–17]. Cu-doping NiO [14], Fe-doped NiO hollow spheres [15], Au nanoparticles coating on NiO [16] have been prepared by various method. Owing to the excellent conductive capacity and huge specific areas of Ag nanospheres, the capacitance performance of Ag-doped NiO nanocomposites can be enhanced effectively [18]. Several methods have been reported [19–21]. However, most of methods are complicated, time-consuming, and expensive, or require a relatively high temperature. Therefore, the development of a simple and fast synthetic route that can generate a high-quality NiO naocomposites remains an important topic of investigation.
In this article, an attempt was made to prepare NiO/Ag naocomposites via micro-emulsion method. In the method, the NiO nanospheres were dispersed in ethanol including SDS and sonicated to form homogeneous suspension, an appropriate amount of AgNO3 aqueous solution was added to this suspension above. Then, a micro-emulsion formed, in which the NiO nanospheres were surround with many Ag+ ions. With the adding of NaBH4, Ag+ ions were reduced to Ag nanospheres. Finally, a series of NiO/Ag nanocomposites with different percentages quality of Ag were prepared successfully and exhibited superior electrochemical properties than pure NiO.
2 Experimental
2.1 Synthesis of NiO
Nickel sulfate, sodium dodecyl sulfonate (SDS), silver nitrate, potassium hydroxide at analytical reagent grade were obtained from commercial suppliers and used as received. The synthetic process was as follows [22]: 40 mL of 0.02 mol/L KOH aqueous solution was slowly dropped into 50 mL of 0.02 mol/L nickel sulfate solution with 3.5 mg of SDS under an ultrasonic water bath at power 80 W for 6 h. The solution was removed from the sonicator and kept for 24 h at room temperature without stirring. The colloidal precipitate was collected by centrifugation, washed with water and ethanol several times, and dried at 50 °C under vacuum for 24 h. The obtained product was sufficiently ground and then calcined at 350 °C for 2 h.
2.2 Synthesis of NiO/Ag composites
First, 200 mg NiO was dispersed in 20 mL ethanol and sonicated for 1.5 h at room temperature. After that, an appropriate amount of SDS was added and stirred for another 1.5 h. Then, an appropriate amount of AgNO3 aqueous solution (2.86 × 10−3 mol L−1) was added to this suspension. After that, 2.86 × 10−3 mol L−1 NaBH4 solution was slowly dropped into the suspension above and stirred for 2 h. The product was collected by centrifugation, washed with water and ethanol several times, then dried at 50 °C under vacuum for 24 h. The Ag nanoparticles were prepared by the same preparation procedure except the adding of NiO. The samples prepared under different mass percentage of Ag are denoted as NiO/Ag0.5 (0.5 %), NiO/Ag1 (1 %), NiO/Ag1.5 (1.5 %), NiO/Ag2 (2 %) and NiO/Ag3 (3 %).
2.3 Materials characterization
X-ray diffraction studies were performed by using D/max-2400 diffraction X-ray diffractometer (Rigaku) with Cu Ka as radiation source. Scanning electron microscope (SEM; Hitachi, Japan, JEOL, JSM-6330F) was used to observe the morphologies of the materials. Prior to the characterization, the specimen were coated with a very thin layer of gold atoms. The observations were carried out after retrieving the slices onto Cu grids.
2.4 Electrochemical measurements
The working electrodes were prepared according to the following steps. The typical mass load of the electrode materials is 10 mg. 75 wt% of active materials was mixed with 7.5 wt% of acetylene black and 7.5 wt% of conducting graphite in an agate mortar until a homogeneous black powder was obtained. To this mixture, 10 wt% polytetrafluoroethylene was added with a few drops of ethanol. The resulting paste was pressed at 10 MPa to a stain less-steel grid that served as a current collector. Each electrode contained about 5 mg of electroactive material and had a geometric surface area of about 1 cm2. A typical three-electrode experimental cell equipped with a working electrode, a platinum foil counter electrode, and a saturated calomel reference electrode (SCE) was used for measuring the electrochemical properties of the working electrode and its performance as a Faradaic ECs. All electrochemical measurements were carried out on CHI860D electrochemical working station in KOH aqueous solution as electrolyte at 25 °C.
3 Results and discussion
3.1 The structural and morphology of composites
XRD diffraction data for the polycrystalline materials provided information about the phase composition and the degree of crystallinity of the nanoparticle [18]. The mean crystallite dimension, or size, of the coherent crystalline domain and the lattice imperfections can be estimated from peak broadening [22]. The full width at half maximum (FWHM) implies the crystal sizes of the samples [23]. Figure 1 shows XRD patterns of the NiO nanospheres and NiO/Ag nanocomposites as-prepared. Both samples show typical diffraction at 37.28°, 43.23° and 62.78°, which are indexed to the (111), (200) and (220) crystal planes of cubic NiO phase (JCPDS 47-1049), respectively. The samples have not exhibited the diffraction angles of 30.28° and 50.28°, indicating not presence of a Ni2O3 structure. The broad FWHM of NiO reveals that the grain size of NiO may be very small. For the NiO/Ag nanocomposites, besides the typical peaks of NiO, some new peaks at 2θ = 38.14°, 44.33°, 64.51°, 77.46° appeared, which can be assigned to (111), (200), (220) and (311) crystal planes of Ag nanospheres (JCPDS 89-3722), respectively. It is evident that the NiO/Ag nanocomposites are composed of cubic NiO and Ag. Owing to the well crystallinity of Ag, the FWHM of NiO/Ag nanocomposite seems decreased compared with NiO nanospheres.
To evaluate the morphological features of the samples, SEM of the NiO nanospheres, Ag nanospheres and NiO/Ag nanocomposites have been studied. Figure 2a shows the SEM image of NiO with low resolution (10,000×), and Fig. 2b is the image with high resolution (80,000×). From Fig. 2a, b, it can be seen that the morphology of NiO nanospheres was the thin flake aggregated by uniform spherical nanoparticles with the average size of 30 nm. The surface of flake was so rough that some holes appeared. Energy dispersive spectroscopy (EDS) of the NiO nanospheres is shown in Fig. 3a. It revealed that it is consists of nickel and oxygen besides carbon and cuprum from a C-coated Cu grid. In the method of NiO nanospheres, SDS plays a crucial role in preventing the random aggregation in the synthesis of nanoparticles and helps to develop the specific morphology [24–26].
Figure 2c, d show the Ag nanospheres with average sizes of 5–12 nm, which is smaller than the NiO nanospheres. These Ag nanospheres are easily to unite each other to form flat and smooth thin flakes, which is thinner than NiO flakes. Due to the small size of Ag nanospheres, the specific areas of Ag nanospheres may be huge. Energy dispersive spectroscopy (EDS) for the Ag nanoparticles is exhibited in Fig. 3b. It is clear that the as-prepared Ag flakes are consists of Ag besides carbon and cuprum from a C-coated Cu grid.
Figure 2e, f show the SEM micrograph of the as-prepared NiO/Ag nanocomposites with different resolutions. It is evident from Fig. 2f that the morphology of NiO/Ag nanocomposites is very consistent with the pure NiO nanospheres. However, the NiO/Ag nanocomposites become more uniform than NiO nanospheres. Those uniform nanospheres were adhered to form a flat and smooth surface, as shown in Fig. 2e. It is obviously that these holes appeared in NiO (Fig. 2b) was rare in the NiO/Ag nanocomposites, which may be caused by the Ag nanospheres. The small Ag nanospheres can effectively prevent the reunion and overgrowth of the NiO nanosphere, resulting in the homogeneous NiO/Ag nanocomposites. Not only that, but the small Ag nanospheres increase the ion/electron transfer and the contact chance between electrolyte and active materials. EDS for the NiO/Ag nanocomposites is exhibited in Fig. 3c. It is clear that the as-prepared NiO/Ag nanocomposites are consists of Ni, O, Ag besides carbon and cuprum from a C-coated Cu grid.
3.2 The formation mechanism for the NiO/Ag nanocomposites
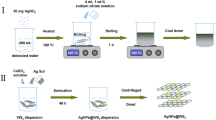
Based on the XRD and SEM analysis, the NiO/Ag nanocomposites were prepared via micro-emulsion method. The process can be described more clearly through the schematic illustration in Fig. 4. In the process, the homogeneous NiO nanospheres ethanol suspension was formed firstly by ultrasound. As an appropriate amount of anionic surfactant SDS was added, the carbon chains of SDS attached on the surface of NiO nanospheres while the negative electrons of sulfonate groups were surrounded with the NiO nanospheres. Then, AgNO3 aqueous solution was added to the suspension above. A large number of Ag+ ions were attracted by the negative electrons and aggregated in the surround of NiO nanospheres. Finally, a stable micro-emulsion formed, in which the NiO nanospheres were surround with many Ag+ ions. With the adding of NaBH4, Ag+ ions were reduced to small and uniform Ag nanospheres. These Ag nanospheres aggregated in the surround of NiO nanospheres to prevent the reunion and overgrowth of the NiO nanosphere, resulting in the homogeneous NiO/Ag nanocomposites. Furthermore, Ag nanospheres can increase the ion/electron transfer and the contact chance between electrolyte and active materials. So, the electrochemical property with higher specific capacitance was enhanced, resulting in high discharge/charge current density [18].
3.3 Electrochemical studies
Cyclic voltammetry (CV) is used to evaluate the electrochemical properties and quantify the specific capacitance of as-prepared NiO/Ag electrodes. The electrochemical performance of NiO/Ag nanocomposites with different Ag mass percent content is characterized by CV in the potential range from −0.3 to 0.5 V, as shown in Fig. 5a–e. It is clear that the redox peak was observed at all NiO/Ag nanocomposites CV curves. As the scan rate increased, the CV curves of NiO/Ag nanocomposites showed light compression. The degree of compression is correlated with the rate capability of the electrode system, which indicates prolonged duration, which is required to charge the capacitor due to increased time constant [27]. These electrodes display significant rate capability as they are able to maintain shape under fast scan rate without any pronounced shape distortion.
The NiO capacitor in an alkaline solution relies on charge storage in the electric double layer at the electrode–electrolyte interface and charge storage in the host material through redox reactions on the surface and hydroxyl ion diffusion in the host material [28, 29]. Figure 5f displays the CV curves of NiO/Ag nanocomposites with various Ag loadings (0–3 %) at the 5 mV s−1 scan rate in 1.0 mol L−1 KOH aqueous electrolyte. It is clear that the redox peaks reveal the Faradaic pseudocapacitive property of the NiO/Ag nanocomposites.
The capacitance of NiO/Ag nanocomposites with 0.5, 1, 1.5, 2, 3 % and unloaded NiO along with the specific capacitance delivered by NiO and Ag are plotted, as shown in Fig. 6. The specific capacitance (SC) is calculated based on the charge–discharge curves using the following equation [18, 21]:
where i is the discharge current in amperes, Δt is the discharge time in seconds corresponding to the voltage difference (ΔE) in volts excluding iR drop, and m is the mass of the active electrode material in grams. The obtained values are 211, 277, 322 and 121 F g−1 for NiO/Ag0.5, NiO/Ag1.5 and NiO/Ag3 and NiO electrodes, respectively. Because the specific capacitance values are normalised by the total weight of the composite, the capacitance contributed by NiO decreases steadily with increasing “Ag component”. The specific capacitance of NiO is only 121 F g−1. It can be clearly observed that the capacitance of NiO/Ag nanocomposites remarkably increases from 121 to 211 F g−1 when the Ag loading increases to 0.5 wt%. The results show that low loading of Ag nanospheres loading can also enhance obviously the specific capacitance. As the Ag loading on NiO increased gradually, the specific capacitance of NiO/Ag nanocomposites increased accordingly.
We compared the electrochemical behavior of a NiO/Ag3 nanocomposite in the different concentrations electrolytes. Figure 7 shows the charge–discharge curve of NiO/Ag3 obtained in the 1 and 6 mol L−1 KOH at a current density of 1 A g−1. The capacitance of the NiO/Ag3 nanocomposite from the charge–discharge analysis was 322 F g−1 in the 1 mol L−1 KOH, which is close to that tested in the 6 mol L−1 KOH (345 F g−1). The results indicate that the capacitor electrode of the NiO/Ag3 nanocomposite is stable in different concentrations of KOH electrolytes and that the NiO/Ag3 nanocomposite in the 6 mol L−1 KOH appears to be higher capacitance than 1 mol L−1 KOH. The result shows that the NiO/Ag nanocomposite can be applied to more wide fields.
4 Conclusions
In summary, a series of NiO/Ag nanocomposites have been synthesized via micro-emulsion method which is simple convenience and easy to application. The formation of micro-emulsion leads the small and uniform Ag nanospheres attached on the surface of NiO, forming the NiO/Ag nanocomposites. These small Ag nanospheres around NiO prevent the reunion and overgrowth of the NiO nanosphere, resulting in the homogeneous NiO/Ag nanocomposites. Because Ag nanospheres can increase the ion/electron transfer and make contact between electrolyte and active materials sufficient, the electrochemical properties with higher specific capacitance was enhanced. The results show that the capacitance performance of NiO/Ag nanocomposites increased accordingly with the Ag content increasing. The capacitance of the NiO/Ag nanocomposites electrodes with 3 % Ag loading were effective to obtain fully reversible and higher specific capacitance of ~322 F g−1 at 1 A g−1 current density.
References
J. Warnan, J. Gardner, L.L. Pleux, J. Petersson, Y. Pellegrin, E. Blart, L. Hammarstrom, F. Odobel, Multichromophoric sensitizers based on squaraine for NiO based dye-sensitized solar cells. J. Phys. Chem. C 118, 103–113 (2014)
S. Vijayakumar, S. Nagamuthu, G. Muralidharan, Porous NiO/C composites as electrode material for electrochemical supercapacitors. ACS Sustain. Chem. Eng. 1, 1110–1118 (2013)
K.K. Purushothaman, I.M. Babu, B. Sethuraman, G. Muralidharan, Nanosheet-assembled NiO microstructures for high-performance supercapacitors. ACS Appl. Mater. Interfaces 5, 10767–10773 (2013)
D.T. Dam, X. Wang, J.-M. Lee, Graphene/NiO nanowires: controllable one-pot synthesisand enhanced pseudocapacitive behavior. ACS Appl. Mater. Interfaces 6, 8246–8256 (2014)
G.P. Wang, L. Zhang, J.J. Zhang, A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 41, 797–828 (2012)
B.E. Conway, Transition from supercapacitor to battery behavior in electrochemical energy storage. J. Electrochem. Soc. 138, 1539–1548 (1991)
A. Farkas, F. Hess, H. Over, Experiment-based kinetic monte carlo simulations: CO oxidation over RuO2 (110). J. Phys. Chem. C 116, 581–591 (2012)
M. Kan, J. Zhou, Q. Sun, Y. Kawazoe, P. Jena, The intrinsic ferromagnetism in a MnO2 monolayer. J. Phys. Chem. Lett. 4, 3382–3386 (2013)
C.Y. Ma, Z. Mu, J.J. Li, Y.G. Jin, J. Cheng, G.Q. Lu, Z.P. Hao, S.Z. Qiao, Mesoporous Co3O4 and Au/Co3O4 catalysts for low-temperature oxidation of trace ethylene. J. Am. Chem. Soc. 132, 2608–2613 (2010)
J.J. Zou, C.J. Liu, Y.P. Zhang, Control of the metal-support interface of NiO-loaded photocatalysts via cold plasma treatment. Langmuir 22, 2334–2339 (2006)
E.O. Zayim, I. Turhan, F.Z. Tepehan, N. Ozer, Sol-gel deposited nickel oxide films for electrochromic applications. Sol. Energy Mater. Sol. Cells 92, 164–169 (2008)
K. Karthik, S.G. Kalai, M. Kanagaraj, S. Arumugam, J. Victor, Particle size effect on the magnetic properties of NiO nanoparticles prepared by a precipitation method. J. Alloys Compd 509, 181–184 (2011)
Y. Kobayashi, J. Horiguchi, S. Kobayashi, Y. Yamazaki, K. Omata, D. Nagao, M. Konno, M. Yamada, Effect of NiO content in mesoporous NiO–Al2O3 catalysts for high pressure partial oxidation of methane to syngas. Appl. Catal. A Gen. 395, 129–137 (2011)
L. Zhao, G. Su, W. Liu, L. Cao, J. Wang, Z. Dong, M. Song, Optical and electrochemical properties of Cu-doped NiO films prepared by electrochemical deposition. Appl. Surf. Sci. 257, 3974–3979 (2011)
H.J. Kim, K. Choi, K.M. Kim, C.W. Na, J.H. Lee, Highly sensitive C2H5OH sensors using Fe-doped NiO hollow spheres. Sens. Actuators B 171–172, 1029–1037 (2012)
D. Buso, M. Guglielmi, A. Martucci, G. Mattei, P. Mazzoldi, C. Sada, M.L. Post, Growth of cookie-like Au/NiO nanoparticles in SiO2 sol-gel film and their optical gas sensing properties. Cryst. Growth Des. 8, 744–749 (2008)
Y. Huang, Q. Zhang, J. Xi, Z. Ji, Transparent conductive p-type lithium-doped nickel oxide thin films deposited by pulsed plasma deposition. Appl. Surf. Sci. 258, 7435–7439 (2012)
H. Wu, D. Lin, R. Zhang, W. Pan, Facile synthesis and assembly of Ag/NiO nanofibers with high electrical conductivity. Chem. Mater. 19(8), 1895–1897 (2007)
J.B. Wu, Z.G. Li, Y. Lin, Porous NiO/Ag composite film for electrochemical capacitor application. Electrochim. Acta 56(5), 2116–2121 (2011)
J. Song, L. Xu, R. Xing, W. Qin, Q. Dai, H. Song, Ag nanoparticles coated NiO nanowires hierarchical nanocomposites electrode for nonenzymatic glucose biosensing. Sens. Actuators B Chem. 182, 675–681 (2013)
F. Delogu, Mechanochemical decomposition of Ag and Ni oxalates. Mater. Chem. Phys. 147(3), 629–635 (2014)
W. Sun, L. Chen, S. Meng, Y. Wang, H. Li, Y. Han, N. Wei, Synthesis of NiO nanospheres with ultrasonic method for supercapacitors. Mater. Sci. Semicond. Process. 17, 129–133 (2014)
A. Caballero, L. Hernan, J. Morales, Z. González-Granados, A.J. Sánchez-Herencia, B. Ferrari, A high capacity anode for lithium batteries consisting of mesoporous NiO nanoplatelets. Energy Fuels 27, 5545–5551 (2013)
J. Denayer, G. Bister, P. Simonis, P. Colson, A. Maho, P. Aubry, R. Cloots, Surfactant-assisted ultrasonic spray pyrolysis of nickel oxide and lithium-doped nickel oxide thin films, toward electrochromic applications. Appl. Surf. Sci. 321, 61–69 (2014)
L. Xu, Y.S. Ding, C.H. Chen, L. Zhao, C. Rimkus, R. Joesten, S.L. Suib, 3D flowerlike α-nickel hydroxide with enhanced electrochemical activity synthesized by microwave-assisted hydrothermal method. Chem. Mater. 20, 308–316 (2008)
Y.Z. Zheng, H.Y. Ding, M.L. Zhang, Preparation and electrochemical properties of nickel oxide as a supercapacitor electrode material. Mater. Res. Bull. 44, 403–407 (2009)
K. Sankaranarayanan, V. Hakkim, B.U. Nair, A. Dhathathreyan, Nanoclusters of nickel oxide using giant vesicles. Colloids Surf. A Physicochem. Eng. Aspects 407, 150–158 (2012)
S.K. Chang, Z. Zainal, K.-B. Tan, N.A. Yusof, W.M.D.W. Yusoff, S.R.S. Prabaharan, Nickel-cobalt oxide/activated carbon composite electrodes for electrochemical capacitors. Curr. Appl. Phys. 12, 1421–1428 (2012)
H. Pang, Y. Shi, J. Du, Y. Ma, G. Li, J. Chen, J. Zhang, H. Zheng, B. Yuan, Porous nickel oxide microflowers synthesized by calcination of coordination microflowers and their applications as glutathione electrochemical sensor and supercapacitors. Electrochim. Acta 85, 256–262 (2012)
Acknowledgments
The authors are grateful for financial aid from the National Natural Science Foundation of China (51563022), Gansu Science and Technology Support Project (1504GKCA093).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Su, Q. Synthesis of NiO/Ag nancomposites by micro-emulsion method and the capacitance performance as electrodes. J Mater Sci: Mater Electron 27, 4752–4759 (2016). https://doi.org/10.1007/s10854-016-4355-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-016-4355-2