Abstract
Pure barium stannate (BaSnO3) nanostructures were prepared via a new facile surfactant-free coprecipitation-calcination route by employing Ba(Sal)2 (Sal = salicylidene) and SnCl2·2H2O as precursors. Ba(Sal)2 was employed as precursor to synthesize BaSnO3 nanostructures for the first time. The as-obtained BaSnO3 nanostructures were analyzed by UV–vis diffuse reflectance spectroscopy, field emission scanning electron microscopy, Fourier transform infrared spectroscopy, energy dispersive X-ray microanalysis, transmission electron microscopy, and X-ray diffraction. Based on the morphological investigations of the as-prepared samples, it was found that the particle size and shape of the BaSnO3 depended on the reaction temperature, precipitator and surfactant. BaSnO3 nanostructures with various shapes and particle sizes were successfully prepared. Furthermore, the photocatalytic properties of as-synthesized BaSnO3 were evaluated by degradation of eriochrome black T (anionic dye) as water contaminant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Research on preparation and characterization of nanostructured materials is well-known as a significant field in recent science and technology [1–5]. One of these nanostructured materials is barium stannate (BaSnO3). Barium stannate as one of the most attractive alkaline earth stannates is well-known for its excellent potential applications, including protective coating, capacitors, catalyst support, photocatalyst, ceramic and sensor [6–12] as well as remarkable and specific dielectric, optical, photovoltaic, and electrical properties [7, 13, 14]. BaSnO3 with perovskite structure has cubic crystal form [15]. Until now, solid state [16], combustion [17], sol–gel [18], reverse micelle [19] and coprecipitation [20] process have been introduced for the preparation of BaSnO3. It is generally accepted that the particle size and shape have important impact on the behavior of nanostructured materials, and therefore they play key and significant role in the final application of nanostructured materials. So, different preparation methods have been exploring to control the particle size and shape of nanostructured materials [21–23].
It has been shown that inorganic or organometallic compounds with special and interesting architecture can be employed as starting materials to prepare nanostructured materials and controlling their size and shape [21–24].
This work describes a new facile coprecipitation-calcination procedure to prepare BaSnO3 nanostructures with the aid of Ba(Sal)2 (Sal = salicylidene) and SnCl2·2H2O as precursors. The synthesis of many inorganic nanostructured materials by coprecipitation–calcination procedure as a favorable, reliable, simple and effective synthetic way becomes increasingly significant owing to the easy control of the purity, particle size and shape. To our knowledge, it is the first time that Ba(Sal)2 (Sal = salicylidene) is utilized as barium source for the synthesis of BaSnO3 nanostructures and the effects of critical preparation factors on the size and shape of the BaSnO3 via a new simple surfactant-free coprecipitation-calcination process are studied.
2 Experimental
2.1 Materials and characterization
All the chemicals employed for the preparation of BaSnO3 nanostructures including SnCl2·2H2O, methanol, Ba(NO3)2, salicylaldehyde (Sal), ethylenediaminetetraacetic acid disodium salt (Titriplex III), tetraethylenepentamine (TEPA), ethylenediamine (EN), triethylenetetramine (Trien) and sodium dodecyl sulphate (SDS) were purchased from Merck Company and were utilized as received. The energy dispersive spectrometry (EDS) analysis was examined by Tescan mira3 microscope. Powder X-ray diffraction (XRD) patterns of as-obtained samples were collected from a Philips diffractometer applying X’PertPro and the monochromatized Cu Ka radiation (l = 1.54 Å). The UV–vis diffuse reflectance spectra of the as-synthesized BaSnO3 nanostructures were obtained on a UV–vis spectrophotometer (Shimadzu, UV-2550, Japan). FE-SEM images of the BaSnO3 samples were obtained on Hitachi S-4160 field emission scanning electron microscope (FESEM). Fourier transform infrared spectra of the as-prepared products were obtained employing KBr pellets on an FT-IR spectrometer (Magna-IR, 550 Nicolet) in the 400–4000 cm−1 range. Transmission electron microscope (TEM) micrographs of BaSnO3 nanostructures were obtained on a JEM-2100 with an accelerating voltage of 200 kV equipped with a high resolution CCD Camera.
2.2 Synthesis of Ba(Sal)2
To synthesize Ba(Sal)2, a salicylaldehyde solution (6 mmol in 60 mL of methanol) was added drop-wise to an aqueous Ba(NO3)2 solution (3 mmol in 60 mL of distilled water) under magnetic stirring. The Ba(Sal)2 was obtained after refluxing the mixture for about 3 h.
2.3 Synthesis of BaSnO3 nanostructures
BaSnO3 nanostructures were synthesized by novel coprecipitation-calcination way. In a typical experiment, 1 mmol of Ba(Sal)2 and 1 mmol of SnCl2·2H2O were dissolved in 30 mL hot distilled water (60 °C) separately. SnCl2·2H2O solution was added into a solution of Ba(Sal)2 drop-wise under magnetic stirring. The pH of the resultant solution was adjusted to 13 by adding EN solution drop-wise and final solution was heated at 60 °C for 1 h under constant stirring. The yellow precipitate was filtered, washed out with distilled water for three times, air-dried and calcined at 700 °C for 4 h (sample no. 1). Schematic diagram of the synthesis of the BaSnO3 nanostructures is illustrated in Scheme 1. To examine the surfactant influence, a stoichiometric amount of the surfactant was dissolved in 4 mL distilled water and added after mixing SnCl2·2H2O and Ba(Sal)2 solutions. The influence of the reaction temperature, precipitator and surfactant on the particle size and shape of the BaSnO3 were investigated and the obtained results illustrated in Table 1.
2.4 Photocatalytic test
The photocatalytic characteristics of as-prepared BaSnO3 nanostructures were evaluated by utilizing eriochrome black T (anionic dye) solution. The Solution including 0.001 g of the eriochrome black T and 0.004 g of the as-synthesized BaSnO3 in the quartz reactor was utilized to carry out the photocatalytic test. After aerating for 30 min, the mixture was subjected to the irradiation of the UV light from the 400 W mercury lamps. The eriochrome black T photodegradation percentage was estimated as follow:
where At and A0 are the obtained absorbance value of the eriochrome black T solution at t and 0 min by a UV–vis spectrometer, respectively.
3 Results and discussion
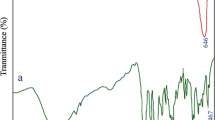
It has been shown that FT-IR spectroscopy is desirable and suitable device for understanding the functional group of any organic compound. The infrared spectra of the Ba(Sal)2 and sample no. 3 after washing steps and after calcination are illustrated in Fig. 1a–c. In the FT-IR spectrum of the Ba(Sal)2 (Fig. 1a), the bands located at 1524 and 1637 cm−1 are corresponding to the C–O stretching vibrations and the band centered at 1434 cm−1 is corresponding to the C–C stretching vibration of the Sal compound. The C–O stretching vibrations and C–C stretching vibration of the free Sal compound appears at 1680 and 1660 cm−1 as well as at 1490 cm−1. Upon Ba(Sal)2 preparation, these vibrations shifted to lower regions [25]. The absorption peak located at 3306 cm−1 in the FT-IR spectrum of the Ba(Sal)2 is corresponding to the stretching vibrations of the physisorbed water molecules. Absorption peak at 466 cm−1 may correspond to Ba–O band, there is no peak nearby this point in Sal compound. The peaks located at 856 and 1449 cm−1 in Fig. 1b can be related to carbonate group [20]. Besides the peak seen at 550 cm−1 may be related to the Sn–O stretching vibration [26]. In the case BaSnO3 nanostructures (sample no. 3 after calcination), the absorption peak seen at 3425 cm−1 are corresponding to the v(OH) stretching vibration of the surface adsorbed water molecules [22]. The characteristic peak of the BaSnO3 located at 645 cm−1 [27] (Fig. 1c).
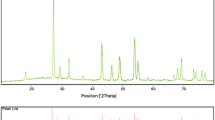
To determine the crystal structure, composition and mean crystallite diameter of the as-obtained samples, XRD analysis was performed. Figure 2a, b exhibits XRD patterns of the sample no. 3 after washing steps and after calcination, respectively. All of the diffraction peaks observed in Fig. 2a can be indexed to orthorhombic BaCO3 with space group of Fd3 m (JCPDS card 44-1487). FT-IR and XRD results (Figs. 1b, 2a) demonstrate that the precipitate formed at the coprecipitation procedure containing BaCO3 and Sn(OH)2 (after washing steps). It seems that the separated Ba2 + ions from Ba(Sal)2 in the basic condition (pH 13) has high affinity to the atmospheric CO2, thus BaCO3 phase forms [27]. Besides, Sn(OH)2 is amorphous and therefore, no its diffraction peaks appears in Fig. 2a. All the diffraction peaks in XRD pattern of the sample no. 3 after calcination can be indexed to pure cubic BaSnO3 with Pm-3 m space group (JCPDS 15-0780). No impurities are illustrated in this pattern. Utilizing Scherrer formula [22], the mean crystallite size of the prepared BaSnO3 nanostructures (sample no. 3) from the XRD results was estimated to be about 20 nm.
In order to study the purity level and chemical composition of the as-synthesized BaSnO3 nanostructures (sample no. 3), EDS technique was employed. Figure 3 reveals the EDS spectrum of the sample no. 3. The EDS spectrum demonstrates that this sample containing Ba, Sn and O elements. So, the EDS and XRD results confirm the high purity of the as-obtained BaSnO3 nanostructures.
FESEM technique was applied to examine the effects of the critical preparation factors on the size and shape of the BaSnO3. The precipitator sort effect on the particle size and shape of the BaSnO3 was investigated (Fig. 4a–c). For this propose, the reactions were performed in presence of the EN, Trien and TEPA (sample nos. 1–3). The FESEM images demonstrate that irregular micro/nanostructures, not uniform sphere-like nanostructures and uniform spherical nanoparticles are prepared in the presence of the EN, Trien and TEPA, respectively. Among these applied precipitator sorts, TEPA has the highest steric hindrance influence (Scheme 2). TEPA can play as both precipitator and co-capping agent role and hinder from the aggregation of the formed nanoparticles. Furthermore, FESEM images illustrate that the increase in the steric hindrance influence causes the grain size becomes small. It seems that the nucleation to be happened rather than the particle growth by increasing the steric hindrance influence. It can be deduced that TEPA was the best precipitator sort for BaSnO3 nanoparticles with uniform spherical shape and small grain size (Fig. 4c).
To examine the effect of the presence of Ba and Sn on the shape and particle size of the products, two reactions was carried out in the absence of Ba and Sn. Figure 5 exhibits the FESEM images of the sample nos. 4 and 5 prepared in the absence of Ba and Sn, respectively. From the FESEM images, it can be observed that bulk structures decorated by not uniform nanoparticles and high agglomerated particles/bulk structures are obtained in the absence of Ba and Sn, respectively (Fig. 5a, b). According to the XRD results illustrated in Fig. 6, BaCO3 and SnO2 are prepared in the absence of Sn and Ba. The proposed formation mechanism of BaSnO3 nanostructures employing Ba(Sal)2 (Sal = salicylidene) and SnCl2·2H2O in the presence of TEPA can be summarized as follows:
By calcining the precipitate formed at the coprecipitation procedure containing BaCO3 and Sn(OH)2, at 700 °C for 4 h, the pure cubic BaSnO3 nanostructures prepare as follows [27]:
The obtained FT-IR and XRD results (Figs. 1, 2, 6) confirm this proposed mechanism for BaSnO3 nanostructures formation.
The reaction temperature influence on the shape and size of the BaSnO3 was also examined (Fig. 7). For this aim, two samples were prepared at 25 and 90 °C (sample nos. 6 and 7). The shape of the sample no. 6 in Fig. 7a is not uniform sphere-like. The sample no. 7 formed at 90 °C illustrates high agglomerated particle-like structures (Fig. 7b). It can be observed that with the reaction temperature change from 60 to 25 °C, the grain size increases. It is generally accepted that the enhancement of the reaction temperature always causes an enhancement of the rate of the reaction. Thus by performing the reaction at relatively high temperature more BaSnO3 nuclei will obtain before the growth step, which results in the preparation of more particles with smaller sizes. By changing the temperature from 60 to 90 °C (Fig. 7b), the particles agglomeration enhanced and the grain size decreased. According to the FESEM results, the most favorable reaction temperature for uniform spherical nanoparticles preparation is 60 °C temperature.
In continuation, the influence of the surfactant sort on the shape and size was examined (Fig. 8). To study the effect of this factor, two reactions were performed in presence of the SDS and Titriplex III (sample nos. 8 and 9). The sample nos. 8 and 9 illustrate not uniform sphere-like nanostructures and irregular bulk structures, respectively (Fig. 8a, b). The obtained results demonstrated that employing these surfactant types not only is favorable and advantageous to form product with a regular and uniform morphology, but also causes to prepare the inhomogeneous and not uniform products. Maybe owing to employing the Ba(Sal)2, there is no necessity to utilize any other surfactant sort. It seems that separated salicylaldehyde compound with the high steric hindrance influence from Ba(Sal)2 in the reaction solution can act as capping agent to control the shape and size.
To examine the influence of the Ba source on the shape of the BaSnO3, sample no. 10 was prepared with employing Ba(NO3)2 and SnCl2·2H2O in the presence of the TEPA at 60 °C (blank sample). Figure 9a exhibits FESEM image of the sample no. 9. It can be seen that bulk structures were obtained. The Sal ligand with high steric hindrance effect in Ba(Sal)2, can play a capping agent role. It can be clearly observed that employing Ba(Sal)2 as Ba source in presence of TEPA causes to prepare uniform spherical nanoparticles (Fig. 4c). Thus, the privilege of utilizing Ba(Sal)2 is that it results in nanostructured BaSnO3 preparation.
To examine the detailed morphology and size of the prepared BaSnO3 sample in the optimum condition (sample no. 3) TEM analysis was performed. The TEM images (Fig. 9b, c) illustrate that quasi-spherical BaSnO3 nanoparticles with diameter from 12 to 30 nm are sintered together. The high-resolution TEM (HRTEM) images of a single nanoparticle in Fig. 9d, e demonstrate that the nanoparticle is highly crystalline. The marked lattice fringes correspond to (110) plane in cubic phase of BaSnO3 with a d-spacing of 0.291 nm.
It is generally accepted that the band gap (Eg) has a considerable impact on the detecting the characteristics of the nanometer-scale materials employed as photocatalyst and is frequently estimated from the UV–vis diffuse reflectance data. The UV–vis diffuse reflectance spectrum of the as-obtained BaSnO3 nanostructures (sample no. 3) is illustrated in Fig. 10a. In this UV–vis diffuse reflectance spectrum, the absorption peak located at 354 nm. The (Eg) may be calculated based on the UV–vis diffuse reflectance data employing Tauc’s equation [5]. The (Eg) of the BaSnO3 as indirect semiconductor was estimated by extrapolating the linear section of the plot of (αhν)1/2 against hν to the energy axis (Fig. 10b). The Eg value of the as-obtained nanostructured BaSnO3 calculated to be 3.37 eV. According to the obtained Eg value, as-prepared nanostructured BaSnO3 sample can be employed as the photocatalyst.
Photodegradation of eriochrome black T (anionic dye) as water contaminant under UV light illumination was employed to evaluate the properties of the as-synthesized BaSnO3 (sample no. 3). Figure 11 exhibits the obtained result. It has been reported that the Ba ions in the Ba containing ternary oxides play a key role for the photon polarization of the anionic subsystem [28]. No eriochrome black T was practically broken down after 120 min without employing UV light illumination or as-prepared nanostructured BaSnO3. This observation illustrated that the contribution of self-degradation was insignificant. The proposed mechanism of the photocatalytic degradation of the eriochrome black T can be assumed as:
Utilizing photocatalytic calculations by Eq. (1), the eriochrome black T degradation was about 84 % after 120 min illumination of UV light. This obtained result demonstrates that as-prepared BaSnO3 nanostructures have high potential to be applied as favorable and appropriate material for photocatalytic applications under illumination of UV light. The heterogeneous photocatalytic processes have diffusion, adsorption and reaction steps. It has been shown that the desirable distribution of the pore has effective and important impact on the diffusion of the reactants and products, and therefore effects on the photocatalytic activity. It seems that the enhanced photocatalytic activity of the as-obtained nanostructured BaSnO3 can be owing to desirable and appropriate distribution of the pore, high hydroxyl amount and high separation rate of charge carriers [29–35] (Scheme 3).
4 Conclusions
A novel, reliable, facile and surfactant-free coprecipitation-calcination process has been developed to prepare pure barium stannate (BaSnO3) nanostructures by employing Ba(Sal)2 (Sal = salicylidene) and SnCl2·2H2O as precursors in presence of TEPA at 60 °C. To the best of our knowledge, it is the first time that Ba(Sal)2 is employed as Ba source for the synthesis of the BaSnO3 nanostructures. This investigation reveals that the Ba(Sal)2 in presence of TEPA is an extremely good selection for uniform spherical BaSnO3 nanostructures preparation without employing any surfactants. By changing the reaction temperature, precipitator and surfactant, we could obtain BaSnO3 micro/nanostructures with various shapes and particle sizes. The as-obtained BaSnO3 nanostructures can be employed as favorable and appropriate material for photocatalytic applications under illumination of UV light such as removal of eriochrome black T as anionic dye, since the eriochrome black T photodegradation percentage was found to be 84 within 120 min. FT-IR, XRD, and EDS analyses confirmed high purity of the as-prepared BaSnO3.
References
M. Sabet, M. Salavati-Niasari, Omid Amiri. Electrochim. Acta 117, 504 (2014)
S. Mortazavi-Derazkola, S. Zinatloo-Ajabshir, M. Salavati-Niasari, Ceram. Int. 41, 9593 (2015)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, Int. J. Appl. Ceram. Technol. 11, 654 (2014)
F. Beshkar, S. Zinatloo-Ajabshir, M. Salavati-Niasari, J. Mater. Sci.: Mater. Electron. 26, 5043 (2015)
S. Zinatloo-Ajabshir, M. Salavati-Niasari, New J. Chem. 39, 3948 (2015)
V.G. Wagner, H. Binder, Z. Anorg. Allg. Chem. 298, 12 (1959)
Z. Zhigang, Z. Gang, Ferroelectrics 101, 43 (1990)
P.H. Borse, J.S. Lee, H.G. Kim, J. Appl. Phys. 100, 124915 (2006)
W. Wang, Sh Liang, K. Ding, J. Bi, J.C. Yu, P. Keung, L. Wong, J. Wu, J. Mater. Sci. 49, 1893 (2014)
S.S. Shin, J.S. Kim, J.H. Suk, K.D. Lee, D.W. Kim, J.H. Park, I.S. Cho, K.S. Hong, J.Y. Kim, ACS Nano 7, 1027 (2013)
J. Cerda, J. Arbiol, G. Dezanneau, R. Díaz, J.R. Morante, Sensors Actuators B 84, 21 (2002)
T. Huang, T. Nakamura, M. Itoh, Y. Inaguma, O. Ishiyama, J. Mater. Sci. 30, 1556 (1995)
W. Zhang, J. Tang, J. Ye, J. Mater. Res. 22, 1859 (2007)
H. Mizoguchi, H.W. Eng, P.M. Woodward, Inorg. Chem. 43, 1667 (2004)
V. Vorgelegt, L. Wensheng, Synthesis of nanosized BaSnO3 powders. Doctoral thesis in Engineering of Natural Sciences, Faculty of Engineering, University of Saarlandes, Saarbrücken—Germany, 2002, pp. 1–2
S. Upadhyay, O. Parkash, D. Kumar, Mater. Lett. 49, 251 (2001)
A.S. Deep, S. Vidya, P.C. Manu, S. Solomon, A. John, J.K. Thomas, J. Alloys Compd. 509, 1830 (2011)
W. Lu, H. Schmidt, J. Sol-Gel. Sci. Technol. 42, 55 (2007)
J. Ahmed, C.K. Blakely, S.R. Bruno, V.V. Poltavets, Mater. Res. Bull. 47, 2282 (2012)
Y.H.O. Muñoz, M. Ponce, J. E. R. Páez Powder Technol. 279, 86 (2015)
F. Beshkar, S. Zinatloo-Ajabshir, M. Salavati-Niasari, Chem. Eng. J. (2015). doi:10.1016/j.cej.2015.05.076
S. Zinatloo-Ajabshir, M. Salavati-Niasari, M. Hamadanian, RSC Adv. 5, 33792 (2015)
S. Mortazavi-Derazkola, S. Zinatloo-Ajabshir, M. Salavati-Niasari, RSC Adv. 5, 56666 (2015)
M. Shakouri-Arania, M. Salavati-Niasari, New J. Chem. 38, 1179 (2014)
M. Ghaed-Amini, M. Bazarganipour, M. Salavati-Niasari, J. Ind. Eng. Chem. 21, 1089 (2015)
G. Pfaff, V.D. Hildenbrand, H. Fuess, J. Mater. Sci. Lett. 17, 1983 (1998)
S. Taoa, F. Gao, X. Liu, O. Toft Sùrensen. Sensors Actuators B 71, 223 (2000)
B. Andriyevsky, A. Patryn, K. Dorywalski, Ch. Cobet, M. Piasecki, I. Kityk, N. Esser, T. Łukasiewicz, J. Dec, Ferroelectrics 426, 194 (2012)
J. Zhong, J. Li, F. Feng, Y. Lu, J. Zeng, W. Hu, Z. Tang, J. Mol. Catal. A: Chem. 357, 101 (2012)
D. Ghanbari, M. Salavati-Niasari, S. Karimzadeh, S. Gholamrezaei, J. NanoStruct. 4, 227 (2014)
G. Nabiyouni, S. Sharifi, D. Ghanbari, M. Salavati-Niasari, J. NanoStruct. 4, 317 (2014)
M. Panahi-Kalamuei, M. Mousavi-Kamazani, M. Salavati-Niasari, J. NanoStruct. 4, 459 (2014)
F. Beshkar, M. Salavati-Niasari, J. NanoStruct. 5, 17 (2015)
M. Goudarzi, D. Ghanbari, M. Salavati-Niasari, J. NanoStruct. 5, 110 (2015)
S. Moshtaghi, M. Salavati-Niasari, D. Ghanbari, J. NanoStruct. 5, 169 (2015)
Acknowledgments
The authors are grateful to University of Kashan for supporting this work by Grant No. 159271/20.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moshtaghi, S., Zinatloo-Ajabshir, S. & Salavati-Niasari, M. Preparation and characterization of BaSnO3 nanostructures via a new simple surfactant-free route. J Mater Sci: Mater Electron 27, 425–435 (2016). https://doi.org/10.1007/s10854-015-3770-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-015-3770-0