Abstract
The low-density, conductive and magnetic hollow glass microspheres (HGM)/Fe3O4/Ag composites have been successfully synthesized via co-precipitation and chemical plating method. The morphology, composition, microstructure, magnetic and microwave absorbing properties of the composites were investigated based on the analyses of the results using scanning electron microscope, energy dispersive spectroscopy, X-ray diffraction, vibrating sample magnetometer and vector network analyzer. The results showed that the HGM/Fe3O4 composites were successfully prepared, and the coating layers on the surface of HGM are compact and continuous. Moreover, the final composites were completely covered with Ag nanoparticles. With the addition of Ag nanoparticles, the saturation magnetization of the HGM/Fe3O4 composites reduces from 32.08 to 14.77 emu/g, whereas its conductivity increases to 0.48 S/cm. The reflection loss (R) of HGM/Fe3O4/Ag composites is lower than −10 dB at 8.2–8.7, 9.6–10.8 and 11.4–11.9 GHz, and the minimum loss value is −19.1 dB at 9.9 GHz.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Recently, the materials with hollow spherical structure on micro and nano scale have attracted much attention due to their unique structures and outstanding properties [1, 2]. Among these materials, hollow glass microspheres (HGM, the product of fly ash) are now playing a greater role because of their low-density, strong filling capacity, excellent mobility, thermal resistance and chemical inertness [3–5]. In general, the main chemical compositions of HGM are Al2O3 and SiO2, which cannot meet the increasing demand for functional materials, therefore, more and more works have been focused on the preparation of functionalized HGM [6].
Ferrite (Fe3O4) in nanometers is a traditional microwave absorbent owing to its superior magnetic properties and the ease-preparation; therefore, it has been widely applied in the field of magnetic separation, electromagnetic shielding, orientation control, and biological applications, etc. [7–9]. However, the density of Fe3O4 is fairly high and it is also a non-conductive material, which restricts its potentiality in applications requiring lightweight mass and electrical conductivity [10, 11]. Coating Fe3O4 nanoparticles on HGM might be a feasible way to reduce its density; meanwhile, the chemical plating method can usually improve the conductivity of the material [12–14]. In this way, a light-weight and electromagnetic composite can be obtained, such outstanding properties would significantly contribute to fulfill the requirements for next generation magnetic materials. As a consequence, the material might be a promising candidate with low-density and excellent conductive particle in the field of microwave absorbing materials. To our knowledge, this kind of material is seldom reported.
In this work, we reported a versatile approach to prepare high-performance HGM/Fe3O4/Ag composites via two-step method. Firstly, HGM/Fe3O4 composites were synthesized via co-precipitation method to obtain the low-density and magnetic microspheres. Secondly, the HGM/Fe3O4/Ag composites were prepared by chemical plating process. Finally, the structures of HGM, HGM/Fe3O4, and HGM/Fe3O4/Ag were characterized using scanning electron microscope (SEM), energy dispersive spectroscopy (EDS) and X-ray diffraction (XRD), and conductivity, magnetic properties and microwave absorption properties were also measured.
2 Materials and methods
2.1 Materials
Hollow glass microspheres (10–80 μm) were purchased from Qinhuangdao Qinhuang Glass Microsphere Co. Ltd. Ferric chloride (FeCl3·6H2O), silver nitrate (AgNO3), palladium chloride (PdCl2), stannous chloride dihydrate (SnCl2·2H2O) and sodium dodecyl benzene sulfonate (SDBS) were supplied by the Chemical Company of Tianjin. Ferrous sulfate (FeSO4·7H2O), and ammonia (NH3·H2O), 36 %, formaldehyde were purchased from the Chemical Company of Xi’an.
2.2 Synthesis of HGM/Fe3O4 composites
HGM was first pretreated with NaOH solution (0.5 mol/L) for 30 min to improve their surface activity, and then added to a flask. Some amounts of FeSO4·7H2O and FeCl3·6H2O (mole ratio 1:2) were dissolved into 200 mL deionized water and then also added in the flask. When the solution heated to 30 °C, a mixture of NH3·H2O and SDBS was added dropwise into the mixture until the pH value reached 10. The reaction was carried out at 90 °C for 3 h. When the reaction was complete, the crude products were washed, then dried under vacuum at 80 °C for 24 h, and finally calcined at 600 °C for 2 h under argon gas protection.
2.3 Synthesis of HGM/Fe3O4/Ag composites
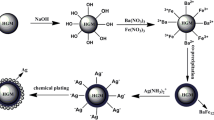
HGM/Fe3O4 composites were first sensitized with SnCl2·2H2O for 30 min. Then the sensitized HGM/Fe3O4 composites were activated by PdCl2 to improve the adhesion between HGM/Fe3O4 composites and silver coating. After that, the treated HGM/Fe3O4 composites were immersed in Ag(NH3)2OH (0.5 mol/L, 100 mL) solution while HCHO (2.15 mL) was added dropwise, the solution was then stirred for 0.5 h under ultrasonic treatment. After washed and dried, the HGM/Fe3O4/Ag composites were obtained [15, 16]. The process is shown as Fig. 1.
2.4 Characterization
The morphology of composites was characterized using SEM (JSM-6390, HITACHI, Japan), the composition of composites was analyzed via EDS analyzer (JED-2200 Series) and the crystal structure of composites was measured by XRD (PANalytical, Holland).The magnetic property was measured by Lake Shore7307 vibrating sample magnetometer (VSM). The electromagnetic parameters of the composites were also analyzed using a HP8753D vector network analyzer, and the samples had a dimension of 22.86 mm × 10.16 mm × 2 mm.
3 Results and discussion
Figure 2 presents the SEM images of the samples at different magnifications. As shown in Fig. 2a, b, the diameter of the HGM is in the range of 10–80 μm, and the microspheres display very smooth surfaces. The panoramic images of Fig. 2c, d demonstrate that a coating is grown on the surface of HGM after the co-precipitation reaction, which encapsulated completely and uniformly. Figure 2e, f show the appearance of HGM/Fe3O4/Ag composites, it is obvious that HGM/Fe3O4 composites are fully covered with Ag particles.
The EDS patterns of HGM/Fe3O4 composites and HGM/Fe3O4/Ag composites are presented in Fig. 3. As shown in Fig. 3a, it appears that Fe exists in the coating, which suggests that Fe3O4 was co-precipitated on the surface of the HGM. The elements of Al and Si are attributed to the HGM itself. Figure 3b shows the presence of Ag in the HGM/Fe3O4/Ag composites, with a high mass content in the composites. In addition, the conductivity increases as Ag content increases.
Figure 4a is the X-ray patterns of pristine HGM, a broad diffraction peak is appeared at 2θ = 20°–35° indicating the amorphous structure of HGM. In Fig. 4b, peaks of Fe3O4 appear at 2θ = 30.10°, 35.40°, 44.08°, 53.64°, 57.96° and 62.80° ascribing to (220), (311), (400), (422), (511) and (440) (JCPDS No. 03-0863) [17], indicated the formation of Fe3O4. Figure 4c is the X-ray patterns of HGM/Fe3O4/Ag composites. The typical peaks of silver (111), (200), (220) and (311) at 2θ = 38.14°, 44.28°,64.46° and 77.68° (JCPDS, File No. 04-0783) [18] appear in the curve, and the peaks of Fe3O4 can be observed as well. These results indicate that the HGM/Fe3O4/Ag composites were successfully obtained, which can also verify the results of SEM and EDS analysis.
The strength of HGM/Fe3O4/Ag composites peaks is weaker than those of HGM and HGM/Fe3O4 composites. The reason could be attributed to that the surface of composites is completely covered with silver particles, and the strength of Ag peaks is very strong, other peaks will appear to be relatively weak in the same coordinate.
Conductivities of HGM/Fe3O4, Ag and HGM/Fe3O4/Ag are shown in Table 1. The conductivity of HGM/Fe3O4 is only 6.89 × 10−7 S/cm, indicated that HGM/Fe3O4 is a non-conductive material, whereas the conductivity of Ag can reach to 8.75 × 105 S/cm. After coating Ag nanoparticles on the surface of HGM/Fe3O4, the conductivity of composites increases to 0.48 S/cm, which is higher than HGM/Fe3O4 but lower than Ag. With the addition of Ag particles, the conductivity of HGM/Fe3O4 can enhance prominently.
To characterize the magnetic properties, the M–H hysteresis loops of the HGM/Fe3O4 and HGM/Fe3O4/Ag composites were measured with VSM. Figure 5 shows that the curves are typical of soft-magnetic materials. The saturation magnetizations (Ms) of HGM/Fe3O4 composites are 32.08 emu/g, while the Ms of HGM/Fe3O4/Ag composites are 14.77 emu/g. From the VSM results, we can see that both HGM/Fe3O4 and HGM/Fe3O4/Ag composites possessed some magnetic property to a certain extent. However, Ms of the HGM/Fe3O4/Ag composites decrease, mainly attributing to the contribution of the volumes of the non-magnetic silver to the total sample volume [19].
The mechanism of microwave energy loss in a material is the result of its magnetic and electronic properties, which are related to the complex permittivity (ε* = ε′ − jε″), complex permeability (μ* = μ′ − jμ″), dielectric loss (tan δe = ε″/ε′) and magnetic loss (tan δm = μ″/μ′). Figure 6 shows the electromagnetic parameters of Fe3O4, HGM/Fe3O4 and HGM/Fe3O4/Ag composites. In the X band, the ε′ of HGM/Fe3O4/Ag is much higher than those of Fe3O4 and HGM/Fe3O4, confirmed that the HGM/Fe3O4/Ag composites display a higher conductivity, whereas the ε″, μ′, μ″, of HGM/Fe3O4/Ag and HGM/Fe3O4 composites are similar. The tan δe and tan δm of HGM/Fe3O4/Ag and HGM/Fe3O4 composites are higher than those of Fe3O4, indicated that the composites may have better microwave absorption properties.
The microwave absorbing properties of materials can be calculated based on the measured magnetic parameters; the equation is as follows [20]:
where R (dB) is the reflection loss, Z 0 = (μ 0 ε 0)1/2 = 377 Ω and Z in can be described as:
where t is the thickness of the absorber in millimeter, f is the microwave frequency in hertz; c is the velocity of light e, and μ r and ε r is relative complex permeability and permittivity, respectively.
The reflection losses of Fe3O4, HGM/Fe3O4 and HGM/Fe3O4/Ag are shown in Fig. 7. It is obvious that the reflection loss of Fe3O4 is about −6.5 dB, this result suggests that a single Fe3O4 is not a good microwave absorbing materials; Fig. 7b shows that after coating Fe3O4 on the surface of HGM, the microwave absorbing properties significantly improve, the reflection loss of HGM/Fe3O4 is below −10 dB (90 % absorption) at 8.6–10.9 and 11.5–11.9 GHz, and the minimum loss value is −13.7 dB at 9.2 GHz. This reason is mainly about that after coating Fe3O4 on the HGM surface, a cavity was formed in the composite, and electromagnetic waves attenuate gradually after multiple reflection in the cavity, thus the absorbing properties of composites significantly improve; Fig. 7c shows that HGM/Fe3O4/Ag composites have excellent microwave absorbing properties in a certain frequency, the reflection loss is below −10 dB at 8.2–8.7, 9.6–10 .8 and 11.4–11.9 GHz, and the minimum loss value occurs at 9.9 GHz, reaching to −19.1 dB, which is nearly 99 % absorption. This result show that conductive particles can improve the microwave absorbing properties of composites in a certain frequency, the reason is mainly about that the good electrical conductivity makes it easier for electromagnetic wave to enter the material, so the microwave absorbing property is higher. From these results, we can conclude that HGM/Fe3O4/Ag composites are excellent candidates for application as a microwave absorber.
4 Conclusions
In this contribution, the low-density, magnetic and conductive HGM/Fe3O4/Ag composites have been successfully synthesized via co-precipitation and chemical plating methods. The obtained composites exhibit smooth, compact and continuous Fe3O4 coating on the surface of the HGM, and HGM/Fe3O4 composites are fully covered with silver particles. The HGM/Fe3O4/Ag composites are soft-magnetic materials, and with the addition of the non-magnetic particles of silver, the saturation Ms of the composite reduce to 14.77 emu/g, whereas its conductivity increases to 0.48 S/cm. The HGM/Fe3O4/Ag composites have excellent microwave absorbing properties in a certain frequency, the reflection loss is below −10 dB at 8.2–8.7, 9.6–10.8 and 11.4–11.9 GHz, and the minimum loss value is −19.1 dB at 9.9 GHz.
References
Y. Zhao, L. Jiang, Adv. Mater. 21, 3621 (2009)
C.L. Yuan, Y.S. Hong, J. Mater. Sci. 45, 3470 (2010)
S.J. Park, F.L. Jin, C. Lee, Mate. Sci. Eng. A 402, 335 (2005)
X. Duan, R. Gao, Y. Zhang, Z. Jian, Mater. Lett. 65, 3625 (2011)
L. Sun, Q. Li, W. Wang, J. Pang, J. Zhai, Appl. Surf. Sci. 257, 10218 (2011)
Z.G. An, J.J. Zhang, Mater. Lett. 85, 95 (2012)
R.Y. Hong, J.H. Li, H.Z. Li, J. Ding, Y. Zheng, D.G. Wei, J. Magn. Magn. Mater. 320, 1605 (2008)
K. Hatakeyama, T. Inui, IEEE Trans. Magn. 20, 1261 (1984)
G. Zhou, D.W. Wang, F. Li, L. Zhang, N. Li, Z.S. Wu, H.M. Cheng, Chem. Mater. 22, 5306 (2010)
Q. Liu, Z. Zi, M. Zhang, P. Zhang, A. Pang, J. Dai, Y. Sun, J. Mater. Sci. 48, 6048 (2013)
W.Y. Fu, S.K. Liu, W.H. Fan, J. Magn. Magn. Mater. 316, 54 (2007)
W. Liu, X.Q. Shen, D.H. Li, Powder Technol. 186, 273 (2008)
K. Zhong, Y. Mao, X. Sun, C. Liang, P. Liu, Y. Tong, J. Electrochem. Soc. 159, 161 (2012)
I. Nedkov, T. Merodiiska, L. Slavov, R.E. Vandenberghe, Y. Kusano, J. Takada, J. Magn. Magn. Mater. 300, 358 (2006)
Y. Zhang, S. Qi, X. Wu, G. Duan, Synth. Met. 161, 516 (2011)
Y. Yang, S. Qi, X.X. Zhang, Mater. Lett. 66, 229 (2012)
Y. Yang, S. Qi, J. Magn. Magn. Mater. 324, 2380 (2012)
F. Liu, Mater. Lett. 59, 1458 (2005)
X. Pang, W. Fu, H. Yang, H. Zhu, J. Xu, X. Li, G. Zou, Mater. Res. Bull. 44, 360 (2009)
Y. Yang, S. Qi, J. Wang, J. Alloys Compd. 520, 114 (2012)
Acknowledgments
The work is supported by the Graduate Starting Seed Funds of Northwestern Polytechnical University (Z2014071).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
He, Z., Qi, S., Zhong, X. et al. Preparation and microwave absorbing properties of hollow glass microspheres/Fe3O4/Ag composites with core–shell structure. J Mater Sci: Mater Electron 25, 3455–3460 (2014). https://doi.org/10.1007/s10854-014-2038-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2038-4