Abstract
Colloidal spherical nanoparticles (NPs) of cadmium selenide (CdSe) have been prepared by laser ablation of cadmium target in methanol and toluene solutions. The properties of CdSe nanoparticles ablated in methanol and toluene were investigated and compared. The morphology and structure of synthesised CdSe NPs were analyzed by X-ray diffraction (XRD), scanning electron microscopy and transmission electron microscope (TEM). XRD investigation revealed that the nanoparticles are crystalline and have hexagonal structure. Optical absorption showed that the value of optical energy gap of ablated CdSe nanoparticles depends on the solution type. TEM measurements showed that CdSe NPs with diameters ranging from 25 to 35 nm were synthesised in methanol while, the nanoparticles ablated in toluene have diameters in the range of (40–50) nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Nanomaterials have drawn attentions due to their excellent properties compared to the bulk materials. Cadmium selenide (CdSe) has received considerable attentions as opto-electronic material [1]. CdSe is n-type semiconducting material which has an optical band gap of 1.74 eV. Extensive studies were devoted to CdSe colloidal nanoparticles due to their superior optical and electrical properties. Many methods were adopted to synthesis CdSe nanoparticles such as inverse Micelle technique [2], chemical bath deposition [3], electrodeposition [4], laser ablation in liquid [5], successive ionic layer adsorption and reaction (SILAR) [6], and atomic layer deposition (ALD) [7]. Pulsed laser ablation in liquid (LAL) has attracted much attention in production of nanoparticles. This technique is simple, low-cost, require minimum amount of chemical species, high control on ablation atmosphere, and do not needs catalyst [8]. Furthermore, this technique is useful for high purity nanoparticles. The reported results have indicated that the solution type in laser ablation method plays important role in controlling of properties of ablated nanoparticles [9]. In this paper, CdSe nanoparticles are synthesised using laser ablation in liquid, and the effect of solution type on the properties of CdSe NPs is reported.
2 Experimental work
Cadmium selenide nanoparticles were produced by laser ablation of CdSe pressed pellet having diameter of 1 cm2 with purity of 99.99 % provided from Poch Company in methanol (CH4O) or toluene (C7H8) at room temperature. The CdSe target was placed in the bottom of quartz vessel filed with 5 ml of solution above the target. The colloidal solutions were synthesised by irradiating of CdSe pellet with pulsed Nd:YAG laser operated at λ = 1064 nm(type HUAFEI),7 ns pulse width and 10 Hz repetition. The laser energy used for ablation was fixed at 200 mJ/pulse and the ablation time was 6 min.The laser beam was focused on the target surface by using converging lens of 10 cm focal length. The spot size of laser beam on pellet was measured and found to be 2.3 mm.
The laser energy reached the surface was measured with Joule meter. The schematic diagram of laser ablation system used in this study is shown in Fig. 1. The structural properties were studied by means of X-ray diffraction (XRD) (XRD-6000, Shimadzu X-ray diffractometer) using CuKα X-ray source. The shape and size of CdSe nanoparticles were examined using field emission scanning electron microscopy (FE-SEM Image Library) and transmission electron microscopy (type CM10 pw6020, Philips-Germany). The optical absorption of nanoparticles was studied with the aid of UV–Vis spectrophotometer.
3 Results and discussion
3.1 XRD studies
The XRD diffraction patterns of synthesised CdSe nanoparticles films ablated in toluene and methanol are shown in Fig. 2. The XRD patterns of CdSe ablated in toluene contain ten main peaks at diffraction angle of 23.67°, 25.35°, 27.08°, 35.19°, 41.96°, 45.78°, 48.84°, 49.66°, 50.67° and 55.84° corresponds to (100), (002), (101), (102), (110), (103), (200), (112), (201) and (202) planes. The XRD pattern of CdSe nanoparticles prepared in methanol showed presence of additional three peaks at diffraction angle 35°, 45.3° and 49.4° corresponds to Miller indices (102), (103) and (201). These additional peaks can be ascribed to formation of other phases. All the diffractions peaks are indexed to the hexagonal structure and there is no trace of cubic face which were well matched with standard peaks (JCPDS No. 77-2307) [10].
The d-values of nanocrystalline CdSe are given in Table 1. The average grain size D for a knowing X-ray wavelength λ at the diffraction angle θ of CdSe nanoparticles was calculated by using Scherrer formula [11, 12] (the peak widths of the strong diffraction planes have been taken for calculation) and listed in Table 1.
where the β is the full width at the half maximum of the characteristic spectrum in units of radians and K is the Scherrer constant (1 > K > 0.89).
The average grain size of CdSe particles ablated in (toluene and methanol) was found to be around (45 and 33.3 nm) respectively from XRD analysis. This difference in grain size can be ascribed to the difference in the density of toluene and methanol (ρ = 0.87 g/ml for toluene and ρ = 0.79 g/ml for methanol).
The strong and narrow peaks may be ascribed to the preferential growth along (110) plane of CdSe crystallites.
The strain value ‘η’ and the dislocation density ‘δ’ can be evaluated by using the following relations [13, 14], see Table 1:
Table 1 shows the strain and dislocation density of CdSe nanoparticles films ablated in toluene and methanol, the strain of the films varies from (5.40 to10.72) × 10−4 lin−2 m−4 and (9.18 to 10.64) × 10−4 lin−2 m−4 respectively. The dislocation density of the same films varies from (2.22 to 8.77) × 10−14 lin m−2 and (6.43 to 8.65) × 10−14 lin m−2. The results revealed that the strain and dislocation density are decreased with the increasing of the grain size.
3.2 SEM studies
Scanning electron microscopy (SEM) is a convenient technique to study microstructure of thin films. Figure 3 shows the SEM images of the CdSe nanoparticles ablated in toluene and methanol which show these films are polycrystalline. The CdSe particles ablated in methanol have approximately spherical shape (see Fig. 3a), while in the case of toluene the particles are agglomerated and have multi-twin crystals. SEM images are clearly show that particle sizes of CdSe ablated in toluene (Fig 3b) are bigger than those ablated in methanol due to high density of toluene. We think that the lacking of oxygen atoms in toluene affecting the morphology, size and crystalinity quality of CdSe nanoparticles.
3.3 TEM investigation
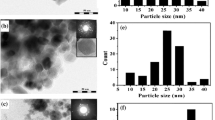
The TEM images for CdSe nanoparticles is shown in Fig. 4, The TEM micrographs confirm the formation of well-defined CdSe nanoparticles having a diameter of approximately 50 nm ablated in toluene and about 34 nm for those ablated in methanol. The particle size appeared in SEM images are much greater than that of particle size measured by TEM analysis due to agglomeration effect.
The size distribution histogram is found from TEM analysis and plotted in linear scale. Figure 5 shows the size distribution of CdSe nanoparticles ablated in toluene and methanol solutions. It is clear from Fig. 5 that the distribution is nearly Gaussian type.
3.4 Optical properties
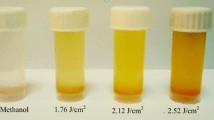
Figure 6 displays UV–Vis spectra of methanol and toluene solutions containing CdSe nanoparticles produced by laser ablation. The spectra exhibited no plasmon resonance peak. The absorption curve decreases sharply up to 400 nm and after this wavelength tends to saturate. The optical absorption of toluene solution containing CdSe nanoparticles is higher than that of CdSe nanoparticles dissolved in methanol solution.
The optical reflectance plots for methanol and toluene solutions containing CdSe nanoparticles are given in Fig. 7a. It is clearly shown that the reflectance of CdSe produced in toluene solution is higher than that of CdSe nanoparticles ablated in methanol solution due the scattering arise from the irregular shapes of particles ablated in toluene. From reflectance data, the refractive index was calculated and plotted as function of wavelength in Fig. 7b. The values of refractive index of CdSe nanoparticles are close to those reported in ref [14].
The band gap of CdSe NPs was estimated by extrapolating of linear part of (αhν)2 versus hν plot to photon energy axis [15–17]. The toluene solution containing CdSe nanoparticles has direct band gap of 1.92 eV and around 2.5 eV for CdSe nanoparticles ablated in methanol (see Fig. 8), which means a ‘blue shift’ of 0.18 and 0.76 eV respectively from standard bulk band gap (Eg = 1.74 eV) [2]. The blue shift might be caused by nanosized effect and structural defects of nanocrystals.
4 Conclusion
The reported study demonstrates the effect of solution type on structural, optical and morphological properties of CdSe nanoparticles synthesised by laser ablation. To the best of our knowledge, the production of CdSe nanoparticles in toluene by laser ablation is reported for the first time. The produced nanoparticles were polycrystalline in nature with hexagonal (wurtzite) phase. SEM observations on CdSe nanoparticles ablated in toluene showed the formation of large grains (multi-twin crystals) due to agglomeration effect. The optical properties data revealed that the direct optical band gap of CdSe ablated in toluene and methanol were 1.92 and 2.5 eV respectively.
References
K.D. Patel, M.S. Jani, V.M. Pathak, R. Srivastava, Deposition of CdSe thin films by thermal evaporation and their structural and optical properties. Chalcogenide Lett. 6(6), 279–286 (2009)
D. Patidar, K.S. Rathore, N.S. Saxena, K. Sharma, T.P. Sharma, Energy band gap and conductivity measurement of CdSe thin films. Chalcogenide Lett. 5(2), 21–25 (2008)
O. Toma, S. Iftimie, C. Besleaga, T.L. Mitran, V. Gghenescu, O. Porumb, A. Toderas, M. Radu, L. Ion, S. Antohe, New investigations on cadmium sulfide thin films for photovoltaic applications. Chalcogenide Lett. 8(12), 747–756 (2011)
S. Vadivel, M. Vimalan, J. Madhavan, A. Cyrac Peter, Electrical and photoelectrochemical (PEC) properties of slurry coated CdSxSe1-xFilms. Der Chemica Sinica 2(1), 118–124 (2011)
N.J. Suthan, J. Suthagar, B. Saravana, T. Balasubramaniamand, K. Perumal, Effect of substrate temperature on the structural and optical properties of nanocrystalline cadmium selenide thin films prepared by electron beam evaporation technique. Acta. Phys. Pol. A 118(4), 623–628 (2010)
S.A. Mahmoud, A. Ashour, E.A. Badawi, Processing parameters and transport properties of vacuum evaporated CdSe thin films. Appl. Surf. Sci. 253, 2969–2972 (2006)
K.R. Murali, C. Kannan, P.K. Subramanian, Photo-electrochemical properties of flash evaporation cadmium sulphide films. Chalcogenide Lett. 5(9), 195–199 (2008)
R.S. Singh, S. Bhushan, Structural and optical studies of chemically deposited CdS–Se films. J. Non Oxide Glass. 2(3), 135–141 (2010)
R.S. Singh, S. Bhushan, Structural and optical studies of chemically deposited Cd(S-Se): CdCl2, Sm films. Bull. Mater. Sci. 32(2), 125–133 (2009)
JCPDS-International centre for diffraction data (1998) USA card No. 77-2307
K.R. Murali, P. Elango, P. Andavan, K. Venkatachalam, Preparation of CdSxSe1−x films by brush plating technique and their characteristics. J. Mater. Sci. Mater. Electron. 19(3), 289–293 (2008)
R.B. Kale, C.D. Lokhande, Semicond. Sci. Technol. 20, 1 (2005)
P.A. Chate, D.J. Sathe, P.P. Hankare, Electrical and crystallographic properties of nanocrystalline CdSe0.5S0.5 composite thin films deposited by dip method. J. Mater. Sci. Mater. Electron. 22(2), 111–115 (2011)
A.A. Yadav, E.U. Masmdar, Photoelectrochemical performances of n-CdS1−xSex thin films prepared by spray pyrolysis technique. Sol. Energy 84, 1445–1452 (2010)
H. Sharma, S.N. Sharma, G. Singh, S.M. Shivaprasad, Physica E 31, 180 (2006)
K.N. Shreekanthan, B.V. Rajendra, V.B. Kasturi, G.K. Shivakumar, Cryst. Res. Technol. 38, 30 (2003)
P.D. Pathinettam, A. Marikani, R.K. Murli, Proc. SSP Symp. 42, 691 (1990)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abd, A.N., Habubi, N.F. & Ismail, R.A. Preparation of colloidal cadmium selenide nanoparticles by pulsed laser ablation in methanol and toluene. J Mater Sci: Mater Electron 25, 3190–3194 (2014). https://doi.org/10.1007/s10854-014-2002-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-014-2002-3