Abstract
Epoxy composites filled with nano- and micro-sized silver (Ag) particulate fillers were prepared and characterized based on flexural properties, coefficient of thermal expansion, dynamic mechanical analysis, electrical conductivity, and morphological properties. The influences of these two types of Ag fillers, especially in terms of their sizes and shapes, were investigated. Silver nanoparticles were nano-sized and spherical, while silver flakes were micron-sized and flaky. It was found that the flexural strength of the epoxy composite filled with silver flakes decreased, while the flexural strength of the epoxy composite filled with silver nanoparticles showed an optimum value at 4 vol.% before it subsequently dropped. Both silver composites showed improvement in flexural modulus with increasing filler loads. CTE value indicated significant decrements in filled samples compared to neat epoxy. Results on the electrical conductivity of both systems showed a transition from insulation to conduction at 6 vol.%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Due to increasing concerns over the hazardous effects of tin–lead alloy solders, lead-free solders and electrically conductive adhesives (ECAs) are now being considered as the most promising alternative interconnects to provide necessary conductive paths in the electronic industry. These applications boast high-volume, low-cost, and high-density assembly techniques; small and thin packages; and low power consumption characteristics [1]. In addition, ECAs offer numerous advantages over conventional soldering technology such as environmental friendliness which eliminate the use of lead and flux cleaning, fewer processing step which then reduce the processing cost, low stress on substrates and fine pitch interconnect capability which enabling the miniaturization of electronic devices [2–5].
Electrically conductive adhesives mainly consist of organic or polymeric resins (e.g., epoxy, silicone, and polyimide) and metal fillers (e.g., silver (Ag), gold (Au), nickel (Ni), and copper (Cu)) [1, 5]. The polymeric materials in ECAs provide physical and mechanical properties such as adhesion, mechanical strength, and impact strength, while metal fillers conduct electricity [2, 5]. Silver is unique among all the cost-effective metals by nature of its conductive oxide. Currently, electronic industries use ECAs with micron-sized silver flakes in epoxy resins. However, both academic and industrial interests are now drawn towards investigating the further potentials of nano-sized fillers in replacing micron fillers [5].
Investigations on the potential application of silver nanoparticles in epoxy composites such as ECAs have been previously conducted [6–9]. However, these previous studies were focused only on the conductive properties of these composites. Hence, other properties, such as flexural properties, CTE, and dynamic mechanical properties, need further study. This experimental study was conducted to compare the properties of ECA prepared with silver flakes to that with silver nanoparticles in epoxy composites. Differences in the mechanical, thermal, electrical, and morphological properties of ECAs prepared with these two materials at different filler loadings were observed.
2 Experiment
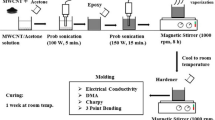
2.1 Materials and sample preparation
The epoxy resin named EPON™ Resin 8281, supplied by Hexion Specialty Chemicals, Inc., was used as a matrix. It is also chemically termed Bisphenol-A-(epichlorohydrin). The curing agent used was Polyetheramine D230 (PEA D230), supplied by BASF Corporation, with a density of 0.946 g/mL at 298 K. The conductive fillers used were silver nanoparticles (average size = 80 nm) and silver flakes (average size = 4–8 μm) with a density of 10.49 g/cm3. The curing agent, PEA D230 (32 part by weight), was added into 100 parts of EPON™ Resin 8281. Conductive fillers (e.g., silver flakes and silver nanoparticles) were added into the resin. The filler loadings were varied from 0 to 8 vol.%. The mixture of epoxy and silver filler was then stirred for approximately 10 min with a homogenizer. The mixture was sonicated for 10 min to facilitate dispersion of the fillers. The mixture was subsequently vacuumed for approximately 0.5 h at 35°C to further remove the bubbles. The curing agent was added, and the mixture was stirred for another 10 min. Finally, the mixture was kept in a vacuum oven for 2 h at room temperature before it was cured in the same oven at 100°C for 1 h. Post-curing followed at 125°C for 3 h.
2.2 Measurement
2.2.1 Electrical resistivity
Resistivity was tested using an LCR meter. A 2 cm × 2 cm specimen was prepared. The test was carried out at a constant potential source of 1 V. The value of volume resistivity is defined as the electrical resistance through a cube of material and is determined according to Eq. 1 (Ohm’s Law) and Eq. 2.
where \( \rho_{v} \) is volume resistivity, \( R_{v} \) is volume resistance, A is the area of the conductor, and t is the thickness of the sample pieces.
2.2.2 Three-point bending test
Three-point bending test was performed based on the standard test method ASTM D 790-98 for samples less than 1.6 mm in thickness. The specimens used were 50.8 mm × 12.7 mm bars of rectangular cross-section. The test was carried out using Instron 3366 at room temperature with crosshead speed set at 5 mm/min. The span length to sample’s thickness ratio was maintained at 16:1.
2.2.3 Coefficient of thermal expansion analysis
The coefficients of thermal expansion of the composites were measured using LINSEIS Dilatometer L75/1550. The specimens were prepared in dimensions of 20 mm × 4 mm × thickness of the samples and were tested at 30–230°C at a heating rate of 3°C/min under ambient conditions.
2.2.4 Dynamic mechanical analysis
Dynamic Mechanical Analysis (DMA) was carried out using Mettler Toledo DMA 861 analyzer. Dual cantilever mode was selected for the present study because it is particularly suitable for hard samples (e.g., reinforced thermoset composites, metals, and alloys). All specimens were in solid 50 mm × 10 mm × thickness rectangular bars. The temperature was increased from room temperature to 150°C at a heating rate of 5°C/min. The oscillatory sine-wave stress was applied at a frequency of 1 Hz. The force amplitude was decided at 1 N, and the displacement amplitude was set at 25 μm. The system was cooled down with liquid nitrogen.
3 Results and discussion
3.1 Electrical resistivity
A comparison of the electrical resistivity of the epoxy composites filled with silver flakes and silver nanoparticles is shown in Fig. 1. Theoretically, the resistivity of the composite will slowly decrease as silver content increases, and sharply decrease as silver content exceeds the critical volume (V c ). When the percolation threshold is reached, the adhesive conductive channels begin to form three-dimensional network conductive loops. However, as observed in the experiment, the continuing increase in silver content above percolation threshold did not obviously affect the resistivity of the composites. According to previous studies [10], above the percolation threshold, multiple electron paths already exist in polymer composites; hence, the electrical conductivity of the composites reaches a saturation value.
Both epoxy composites filled with silver nanoparticles and flakes showed similar resistivity trends (see Fig. 1). However, the electrical resistivity of the silver nanoparticles/epoxy composite started to decrease at 2 vol.%, as compared to the silver flakes/epoxy composite where electrical conductivity started to decrease at 4 vol.%. Below the critical volume, some of the conductive fillers might form isolated islands and not contact well everywhere, where there are narrow gap separating them and prevent the continuous linkage hence decrease the current carrier throughout the composite [7]. In this study, a different observation could be seen at higher filler loadings (V f ≥ 6 vol.%) where the resistivity of the silver nanoparticles surpassed the resistivity of the silver flakes at a given filler loading. This further resulted in the slightly lower conductivity of the silver nanoparticles/epoxy composite compared to the silver flakes/epoxy composite. Ye et al. [7] reported a similar phenomenon, that is, the addition of nanoparticles brings about a negative effect on electrical conductivity. They reported two types of contact resistance that could affect the electrical conductivity of composites: restriction resistance due to small contact area and tunneling resistance when nanoparticles are included in the system. As reported by previous studies [11–14], the critical volume (V c ), which corresponds to percolation threshold, may vary with filler types, sizes, shapes, and on the orientations of fillers in the polymer matrix. The low performance of nanoparticles incorporated in the ECA is perhaps due to the agglomeration of nanoparticles in the ECA. The agglomeration in the composite system can be observed in the scanning electron microscopy (SEM) image (see Fig. 2a, b). Nanoparticles are 1,000 times smaller; hence, they have higher surface to volume of particles aspect ratio as compared to micron-sized fillers. As a result, there are higher possibilities for the particles to exist adjacently and to attract on another, rather than repel one another, due to surface energy attraction. Agglomeration induces the formation of various distributions of the filler in the matrix, affecting the thermal, mechanical, and electrical properties of ECAs [15, 16].
Furthermore, it has been reported that interfacial resistance between fillers influences the electrical resistivity of composites [11]. Interfacial resistance is resistance forming at a surface boundary between adjacent regions; in this case, between one particle and another particle. Fan et al. [11] showed that a silver nanoparticle having an average size of 25 nm has a higher interfacial resistance than a silver flake. This is caused by the nature of nano-sized fillers. Nanoparticles tend to form an aggregate and affect the distribution of the filler throughout the matrix as compared to micron-sized filler. According to a previous work [16], only conductive fillers, which have good distribution over the matrix, can ensure the efficient conduction of electricity across the ECA. Poor distribution only increases surface resistance and reduces the conductivity of the ECA.
Table 1 shows a comparison of the different conductive systems and their corresponding electrical conductivity at critical volumes based on previous works [3, 7, 14, 16]. In the present study, electrical resistivity was found to range from 101 to 102, which is within the conductive range (if referring to Fig. 3). As known, electrical conductivity of the conductive polymer composites are governed by many factors such as processing method, filler loading, curing condition, purity of the conductive fillers, and the conductivity of the matrix itself. According to Wu et al. [18] and Dasari et al. [19], the filler volume fraction at which a conductive network formed in conductive composites are greatly influenced by the filler content and also dispersion of the filler throughout the matrix. By referring to Table 1, the critical volume in this present study is approximately 33 wt% which is obviously lower than other composite systems which exhibit at least 63–70 wt% to reach the percolation threshold.
Electrical resistivity ranges for plastics and adhesives used in electrical applications [17]
A morphological study on the fracture surfaces of neat epoxy and of the composites filled with silver flakes and silver nanoparticles was conducted. The results are shown in Fig. 4. Pure epoxy is transparent; however, the addition of silver fillers turned the composite opaque. SEM has the advantage of giving a broad overview of the micro-scale dispersion of fillers in composites. The fracture surface morphology of pure epoxy showed radial striations. As filler loading increased, the fracture surface became almost entirely featureless. Filler agglomerations were observed, dominating the morphology of the composites. From the figure, the agglomeration of the filler can be clearly seen as the filler loading increased from 2 to 8 vol.%.
Fracture surface of: a neat epoxy at ×500, b 2 vol.% (silver flakes)/epoxy at ×500, c 2 vol.% (silver nanoparticles)/epoxy at ×500, d 4 vol.% (silver flakes)/epoxy at ×500, e 4 vol.% (silver nanoparticles)/epoxy at ×500, f 6 vol.% (silver flakes)/epoxy at ×500, g 6 vol.% (silver nanoparticles)/epoxy at ×500, h 8 vol.% (silver flakes)/epoxy at ×500, i 8 vol.% (silver nanoparticles)/epoxy at ×500
By referring to the conductivity properties of the epoxy composite filled with silver in Fig. 1, it is clear that the silver filler at 2 and 4 vol.% were dispersed in the epoxy system with significant separation by the insulating matrix (Fig. 4b–e). An insufficient filler amount is the primary reason for the much lesser formation of physical contact between fillers. The absence of particle networks and physical contact among fillers led to the low electrical conductivity behavior of the samples at 2 and at 4 vol.%. However, when the silver filler content was increased up to the critical filler loading (6 vol.%) (Fig. 4f, g), the separation between lumps of Ag filler by the insulating matrix was significantly reduced, leading to the formation of continuous linkages. These continuous linkages increase current flow and electrical conductivity.
3.2 Three-point bending test
In electronic packaging, ECAs are subjected to mechanical loading because they carry the load of the chipset on top of them [16]. Hence, tests, such as three-point bending, have been conducted to determine the mechanical properties of composites and ensure the suitability of the application of ECAs to electronic packaging. Figure 5 shows the flexural strengths and flexural modulus of the composites with different silver flakes and silver nanoparticles loadings. In composites filled with silver flakes, flexural strength decreased with increasing filler loading. However, the composite filled with nanoparticles showed an optimum value at 4 vol.% before it subsequently dropped. Nanoparticles impart a high portion of interface to the matrix [16]. The superior properties possessed by nanoparticle fillers are only beneficial if the interface between the filler and matrix is well established. It can be suggested that at 4 vol.%, less particle-to-particle contact or agglomeration occurs in the systems, resulting in good stress transfer and elastic deformation from the matrix to the filler. However, it is believed that at high filler loadings, the insufficiency of epoxy resins to coat the fillers results in low filler-matrix interactions. This result is in good agreement with previous studies [5, 15], which showed that the incorporation of fillers, especially metal fillers, normally enhances the flexural strength only up to certain amount of filler loading before it deteriorates the flexural strength of the composite [5, 15]. In addition, high filler content also leads to an increase in viscosity, which in turn reduces ease of processing. This subsequently increases void content and reduces the strength properties of the composite [20–22].
The flexural modulus of the epoxy composite filled with silver flakes and nanoparticles showed increasing trends with additional filler loadings. The addition of fillers, which are usually stiffer than the matrix, generally leads to an increase in the Young’s modulus and a reduction in the ultimate elongation of the matrix. Therefore, in preparing composite materials, it is important to minimize the conductive filler loading while optimizing filler properties. The increment in flexural modulus as filler loading increases is also in good agreement with the rule of mixture. Theoretically, the rule of mixture suggests increments to the composite modulus with increasing filler loading.
3.3 Coefficient of thermal expansion analysis
CTE is vital in the application of ECAs in electronic packaging. Heat released during the service can cause ECAs to expand and can induce warping to die and cause it to mismatch [16]. Low CTE is often desirable in achieving dimensional stability. This can be achieved by the incorporation of a rigid and low-CTE filler material in the ECAs. The effects of fillers on the CTE of polymeric composites have been widely studied, both experimentally and theoretically [23]. However, specific experimental data regarding composites are still scarce, almost inexistent, especially on CTE in both glassy and rubbery states.
For the experimental reasons given below, two cycles of heating were completed. Figure 6 shows an example of silver a composite behavior when subjected to temperature for first and second heating cycles. During the first cycle, shrinkage of the sample was detected, followed by a change in the slope of dL/L o versus T plot. This shrinkage, in which the final length of the samples was less than the initial length, could be attributed to the relaxation of the residual stress–strain existing in the samples around the glass transition temperature [23]. It is important to note that samples cured at high temperatures which in this present study, 125°C could develop strong stresses during the curing process. The same observation was reported by Case et al. [24]. Upon heating the samples in the dilatometer, the polymer chains acquire mobility on the experimental time scale within the vicinity of the glass transition temperature. As the polymer chains relax, stresses that created from curing are released. This leads to shrinkage of the samples. However, it is important to note that in the second heating cycle, the traditional behavior of polymers is observed as the thermal history of the polymer has been removed during the first cycle. Here, CTE values were measured based on dL/L o temperature curves. The results are presented in Table 2.
CTE is one of the properties that changes at T g . CTE of polymers are generally higher than that of metals and ceramics. Polymer materials generally have two regions with different characteristics of thermal expansion: glassy and rubbery regions. Results in Table 2 show that the CTE before and after the T g decreased with increasing filler loading. The trend was expected because silver is rigid and has lower CTE value (18.9 ppm/K) compared to the CTE of unfilled epoxy before and after T g (73.5 ppm/K and 213 ppm/K, respectively). Compared to filled samples, the unfilled sample showed the highest CTE value, before and after T g . This is due to an increase in the free volume content at any temperature, which generates more fluctuations in molecular structure and leads to an increase in the rate of expansion upon heating. However, as reported by previous works [5, 24, 25], increasing filler content decreases the free volume content. This reduces the space for expansion of the polymer and further reduces the CTE of composites. Thermal expansion is directly related to the bonding forces between atoms in a solid material. Thus, composites with higher filler contents have much lower CTE than polymer matrices due to higher bonding forces present in the rigid fillers.
In this study, an anomalous trend was observed for samples F8 vol.% and N8 vol.%, where the addition of fillers did not result in the decrease of CTE values. This can be explained with regard to the inevitable aggregation of silver at higher concentrations, especially for silver nanoparticles, which have higher surface energy than silver flakes. As reported by Chan et al. [16], compared to composites that have lower degrees of filler dispersion, fillers with better dispersion can absorb more energy across the epoxy matrix and can better constrict the expansion of the epoxy matrix when heat is imposed. A similar behavior was also reported in a previous work [26], where graphite/epoxy composite with 5 wt% exhibited higher CTE compared to graphite/epoxy composite at 2.5 wt%. However, the effect of shapes and sizes on the CTE of the composites did not seem to be too critical. This observation is supported by McGrath et al. [27]. They showed that the decrease in CTE before and after T g is independent of the average particle size, size distribution, and shape, rather than of filler loading.
3.4 Dynamic mechanical analysis (DMA)
The storage modulus (E′)–temperature profiles are shown in Fig. 7a for epoxy composites filled with silver flakes and silver nanoparticles as function of filler loading. As shown in Fig. 7a, the storage modulus (E′), in both glassy and rubbery regions, increased with increasing filler loading. However, the epoxy composites filled with silver nanoparticles did not reveal any trend. At 2 vol.%, the samples showed lower storage modulus in comparison to neat epoxy. Among all the samples depicted in Fig. 7a, it is evident that the highest storage modulus corresponds to the sample at 8 vol.% of silver nanoparticles. This sample also showed appreciable changes from glassy region to rubbery region, which later exhibited the lowest T g compared to other samples (Fig. 7b).
Glass transition temperature (T g ) was more clearly identified as a maximum in the curves of the tan δ, as shown in Fig. 7b. The effect of the incorporation of fillers into a polymer matrix on the T g has been intensively researched [4]. For the epoxy composite system filled with silver flakes, the T g value increases with increasing silver loading, as compared to the neat epoxy. The maximum value in tan δ, which is associated with α-relaxation of the epoxy composite filled with silver flakes at 8 vol.%, decreases and it shifts to a higher temperature, indicating that T g is increased compared to the neat epoxy. For example, in the composite at 8 vol.% of silver flakes loading, the glass transition temperature increased approximately 6.3°C compared to the neat epoxy. The increase in glass transition temperature (T g) is attributable to particle-polymer interaction and to the immobilizing action induced by silver flakes at high temperatures [4]. However, the decrement in T g as filler loading increased for the epoxy composite filled with silver nanoparticles might be related to the free surface areas and reduced cross-linking density of the samples [4]. These reports illustrate that the nature of the interface affects the properties of polymers in its vicinity [27].
4 Conclusions
In this study, the flexural strength of the composite filled with silver flakes decreased with increasing filler loading, while the flexural strength of the composite filled with silver nanoparticles showed an optimum at 4 vol.% before it subsequently dropped. Both composite systems showed improvements in flexural modulus as filler loadings were increased. From the results, it can be concluded that the size and shape of fillers do not have critical effects on the CTE of composites. The epoxy composite filled with silver nanoparticles showed lower conductivity compared to the epoxy composite filled with silver flakes, which might correspond to higher resistance between nanoparticles themselves, as well as to the poor dispersion throughout the matrix. The distribution of fillers in the epoxy matrix, which contribute to the properties of the composite, has been revealed by SEM micrographs.
References
Y. Li, C.P. Wong, Mat. Sci. Eng. R 51, 1–35 (2006)
H. Jiang, K. Moon, C.P. Wong, in Proceeding of the 56th Electronic Components and Technology Conference, 2006
H.P. Wu, X.J. Wu, G.Q. Zhang, Y.W. Wang, J.Z. Jiang, Compos. Sci. Technol. 67, 1116–1120 (2007)
C.F. Goh, H. Yu, S.S. Yong, S.G. Mhaisalkar, F.Y.C. Boey, P.S. Teo, Mater. Sci. Eng. B 117, 153–158 (2005)
T.D. Inn, M. Mariatti, A. Azizan, C.H. See, K.F. Chong, Compos. Sci. Technol. 67, 2584–2591 (2007)
S. Kotthaus, B.H. Günther, R. Haug, H. Schäfer, IEEE Trans. Comp. Packag. Manuf. Technol. A 20, 15–20 (1997)
L. Ye, Z. Lai, J. Liu, A. Thölén, IEEE Trans. Electron Packag. Manuf. 22, 299–302 (1999)
C.P. Wong, L. Daoqiang, L. Meyers, in Proceedings of the 1st IEEE International Symposium on Polymeric Electronics Packaging, 1997
P. Mach, R. Radev, A. Pietrikova, 2nd Electronics System Integration Technology Conference, Greenwich UK, 2008
H. Wei, H. Eilers, Thin Solid Films 517, 575–581 (2008)
L. Fan, B. Su, J. Qu, C.P. Wong, in 9th International Symposium on Advanced Packaging Materials, 2004
Y. Shimada, L. Daoqiang, C.P Wong, in International Symposium of Advanced Packaging Materials, 2000
L. Zhihong, H. Ying, G. Licong, in 8th International Conference on Solid-State and Integrated Circuit Technology, 2006
H. Zhao, T. Liang, B. Liu, Int. J. Adhes. Adhes. 27, 429–433 (2007)
H. Frank, W. Bernd, in Polymer Composites From Nano- to Macro- Scale, ed. by K. Friedrich, S. Fakirov, Z. Zhang (Springer, New York, 2005), pp. 47–53
K.L. Chan, M. Mariatti, Z. Lockman, L.C Sim, J. Mater. Sci. Mater. Electron (2009). doi:10.1007/s10854-009-9991-3
M.P. Edward, Adhesion and bonding: Methods for improving electrically and thermally conductive adhesive. http://www.metalfinishing.com. Accessed 22 August 2009
L. Daoqiang, C.P. Wong, Int. J. Adhes. Adhes. 20, 189–193 (2000)
A. Dasari, Z.-Z. Yu, Y.-W. Mai, Polymer 50, 1577–1587 (2009)
T.D. Inn, M.Sc Thesis, Universiti Sains Malaysia, 2006
J. Móczó, B. Pukánszky, J. Ind. Eng. Chem. 14, 535–563 (2008)
J. Sandler, M.S.P. Shaffer, T. Prasse, W. Bauhofer, K. Schulte, A.H. Windle, Polymer 40, 5967–5971 (1999)
S. Tognana, W. Salgueiro, A. Somoza, J.A. Pomarico, Mater. Sci. Eng. B 157, 26–31 (2009)
S.L. Case, E.P. O’Brien, T.C. Ward, Polymer 46, 10831–10840 (2005)
I. Glavhev, Kr. Petrova, M. Ivanova, Polym. Test. 21, 177–179 (2002)
A. Yasmin, I.M. Daniel, Polymer 45, 8211–8219 (2004)
L.M. McGrath, R.S. Parnas, R.S. King, S.H. King, J.L. Schroeder, D.A. Fischer, J.L. Lenhart, Polymer 49, 999–1014 (2008)
Acknowledgments
The authors gratefully acknowledge the support from the Research University (Grant 814055) and USM Fellowship. We also thank the School of Materials and Mineral Resources, USM, and its staff for their useful discussions and their help during the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suriati, G., Mariatti, M. & Azizan, A. Effects of filler shape and size on the properties of silver filled epoxy composite for electronic applications. J Mater Sci: Mater Electron 22, 56–63 (2011). https://doi.org/10.1007/s10854-010-0082-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10854-010-0082-2