Abstract
A number of synthesis parameters directly influence the degree of reticulation/geopolymerisation of metakaolin exposed to alkaline solutions of sodium hydroxide and/or sodium silicate. In the latter case, a sodium silicate solution can be depolymerised by the introduction of an appropriate amount of NaOH. The effects of the ageing of the activator solution on the reticulation of metakaolin-based geopolymers are quantified for the first time in this work. We studied the anionic species of the sodium silicate solution with the addition of NaOH made just before the preparation of the paste, 24 h or 7 days before. These three ageing periods cause a significant difference in the Si-bearing species in solution, as demonstrated by nuclear magnetic resonance on 29Si. The effect of these anionic species on the reticulation/polymerisation of metakaolin at room temperature was demonstrated by solid-state 27Al and 29Si MAS-NMR, the chemical stability in various solutions (deionised water, HCl, HNO3, H2SO4), and X-ray diffraction on geopolymer powders before and after immersion in acids. Compressive strength before and after the immersion in acidic media was an additional measurement to assess the overall structural stability of the 3D polymerised network of the final dense ceramic-like product. Ageing of the activator solution affected the chemical stability of the hardened geopolymers accompanied by a slight to severe reduction in strength after leaching in HNO3 or HCl and in H2SO4, respectively. The quantitative MAS-NMR description of the Si and Al coordination in the geopolymers was correlated with the chemical stability where the formulations with the higher number of Q4(0Al) and Q4(1Al) for the silicon species were more resistant (lower number of Na+ compensating for Al+3 to be exchanged with H+). The formulations with higher Al content in the structure, i.e. higher number of Q4(3Al) silicon species showed higher mechanical stability. These results show that the timing of the preparation of the alkaline activator is essential for a correct mix design.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In line with recent trends in sustainable binders, the replacement of clinker with other powders with pozzolanic activity sees the use of supplementary cementitious materials, e.g. fly ash from coal-fired power stations and granulated blast furnace slag from the metallurgical industry [1]. Up to 50% clinker replacement has been proved in calcined clay cements [2]. Most of these aluminosilicate powders are capable of setting without the addition of clinker when an alkali-activated route is adopted, the so-called alkali-activated binders or geopolymers, the latter term preferably used for low CaO containing materials [3, 4]. A large number of papers deal with pozzolanic aluminosilicate powders exhibiting alkali reactivity in the production of ‘alkaline cements’ [5], but the simplest starting material adopted as a model aluminosilicate powder to study the alkali activation process is metakaolin, MK (Al2O3·2SiO2), derived from the calcination of kaolin (Al2Si2O5(OH)4) at about 700–800 °C [6]. This calcined mineral presents a disordered polymerised silicon/aluminium network in which Al+3 preferentially occupies four and five-fold structural positions with some residual octahedral coordination, while Si+4 appears in tetrahedral sites as in the earlier kaolinite sheets, but with lower long-range order [7,8,9]. In the case of alkali activation of metakaolin, the definition of “geopolymer” as proposed by Davidovits is widely accepted [10, 11]. In such model MK-based geopolymers, the final network has been described as a poly(sialate) Si–O–Al–O framework structure with alternating (SiO4)4− and (AlO4)5− tetrahedra linked by four bridging oxygen atoms. The substitution of Al3+ (fourfold coordination) for Si4+ results in a negative charge that requires alkali or alkaline earth metal cations such as Na+, K+, Ca2+ or Mg2+ to balance [12]. At room temperature, such a 3D network is easily found in the amorphous state, which makes its study very difficult, but similar to the case of aluminosilicate glasses. The presence of very small crystalline domains, not detectable by X-ray diffraction, as in the case of cryptozeolites, cannot be excluded [13]. In order to obtain a well-balanced 3D aluminosilicate network the mix design of the geopolymer should take into account the most appropriate alkaline activator solution (in terms of type and concentration of alkali content and soluble silicate forms) to react with a given metakaolin [14]. In the alkali activation process, unlike other activation processes where a simple NaOH impregnation is often sufficient to obtain a good catalyst [15], the MK must be partially or totally dissolved in strong alkaline media to obtain a dense and strong ceramic-like material or geopolymer. After such a dissolution step, where tetrahedral monomers of Si(OH)4 and Al(OH)41− are leached from the MK particles, the condensation process at room temperature removes a water molecule and creates the bridging oxygen of the 3D network. Such a process leads to the formation of a gel in 40–60 min at room temperature and continues for a few hours until the reticulation/geopolymerisation process is completed in 1 or 2 days, depending on the amorphousness and impurity level of the starting metakaolin [16].
Over the years of studying 3D aluminosilicate networks in geopolymers, nuclear magnetic resonance (NMR) spectroscopy has emerged as a powerful tool for describing such amorphous structures with atomic and short-range-order molecular level information [5, 17]. NMR spectroscopy has been used to study various aspects of geopolymer behaviour, including the kinetics of the reaction between the alkali activator and the aluminosilicate source material, the formation of the geopolymer network, and the mobility of ions in the network.
Although the alkaline activation of metakaolin has been the subject of recent research [18], the correct preparation of the activator solution has not been studied in detail [19]. While several papers characterise the sodium silicate solutions, few of them go into the correlation with the alkali-activated reticulation [20]. However, it is well known that the degree of polymerisation in sodium silicate solutions is a fundamental process in geopolymerisation technology [21]. In order to fill this knowledge gap, in this work we have chosen to go into more detail on the quantitative measurement of soluble silicate species and to correlate them with the final properties of the geopolymers obtained.
Similarly, it was found that the effect of different anionic Si species of the activator solution on the acid resistance of the metakaolin-based geopolymers has not been investigated by 27Al and 29Si NMR characterisation. In fact, the literature reports that geopolymers have been extensively studied in sulphuric acid [22,23,24], while only a few papers have dealt with nitric acid [25, 26] or hydrochloric acid on cement mortar [27]. The present work aims to evaluate the role of the alkaline activator solution at different ageing times in the 3D network by NMR characterisation of the liquid activator solution (29Si) as well as on the final solid reticulated products (29Si and 27Al). In particular, two liquid activators, i.e. alkaline sodium silicate solutions, with different contents of silica monomers were used to demonstrate the effect of the type of the activator on the cross-linking of the final geopolymer. After 28 days of curing at room temperature, the final compacted products were tested as follows. The ionic conductivity of the leachate solution obtained after immersing the two solid geopolymers in deionised water was determined experimentally in order to demonstrate the mobility of the unreacted activator solutions and the excess of Na+ ions with respect to the fourfold Al units generated during the reticulation process. As a reference material, an MK-based geopolymer was prepared with the same activator solutions as received but without any ageing treatment. The chemical stability was also tested in acidic media: the three MK-based geopolymers were exposed to sulphuric, hydrochloric and nitric acid for 7 days.
Compressive strength and changes in the crystalline/ amorphous fraction of all geopolymers before and after acid attack were also investigated. The NMR investigation, extended to both 29Si and 27Al nuclei, was carried out on both the liquid sodium silicate solutions and the hardened geopolymers.
It should be stressed that the decision to test the chemical stability/leaching resistance of the final products in acidic media was inspired by the various works reported in the literature describing the exceptional strength of alkali-activated materials with respect to ordinary Portland cement, OPC [5]. With this study, we intend to provide an additional experimental contribution to the definition of the dissolution mechanism of MK-based geopolymers in acidic media, at least for the corrosive conditions chosen for this work.
Experimental
Materials
The metakaolin used in this study is a commercially available one, i.e. ARGICAL™ M1000 (IMERYS, France). The metakaolin contained SiO2 (55%) and Al2O3 (40%), the remaining 5% being Fe2O3 (1.4%), TiO2 (1.5%), Na2O + K2O (0.8%), CaO + MgO (0.3%) and 1% of minor oxides (Table 1). The Brunauer–Emmett–Teller (BET) surface area (Gemini-V instrument (Micromeritics) with N2 as the probe gas) of the metakaolin was 17.1 m2/g, and the 50% of the particles had a size smaller than 8.2 μm (i.e. d50 = 8.2 μm).
The sodium silicate solution used has a molar ratio SiO2/Na2O = 3.16; (SiO2 = 27.09 wt%, Na2O = 8.85 wt%) and pH = 11.7 with a density of 1.373 g/cm3 at 20 °C. The sodium silicate solution was supplied by Ingessil, Verona, Italy.
The sodium hydroxide solution was prepared by dissolving NaOH pellets (96 wt%, Sigma-Aldrich, Italy) in deionised/Milli-Q® water, keeping all containers sealed wherever possible to minimise contamination by atmospheric carbonation. The chosen molarity of the sodium hydroxide solution was 8 M.
The activating solution was prepared by adding 38 g of 8 M sodium hydroxide solution to 40 g of sodium silicate solution in a 1:1 volume ratio. The other two activating solutions were prepared using the same procedure but with different ageing times, i.e. Activating Solution 1 (AS1) was stored for 24 h and Activating Solution 2 (AS2) was prepared similarly to AS1 and stored for 7 days before use. Both the activating solutions were stored in tightly sealed bottles to prevent absorption of carbon dioxide from the atmosphere.
The resulting activator solution has a SiO2:Na2O ratio of 6.2, corresponding to a weight ratio of 6.0. The total molar composition of the well mixed solutions was 1 Na2O:1 SiO2:18 H2O. Such a solution was used once mixed (in the GP0 formulation), aged for 24 h (in the GP-AS1 formulation) and 7 days (in the GP-AS2 formulation).
Geopolymer preparation
In order to investigate the ageing effect of the alkaline precursors, the formulation of the geopolymers prepared in this study was kept constant in terms of the total alkaline activators‐to‐binder ratio (a/b), the binder being the fresh paste obtained by mixing the metakaolin and the activator solutions. This ratio is of particular interest as it can be correlated with the mechanical performance of the final hardened product [28].
The reference geopolymer paste, or binder, GP0 was prepared by adding freshly mixed (not stored) 38 g of NaOH 8 M solution and 40 g sodium silicate solution to 100 g of metakaolin (MK), as optimised in a previous paper [29].
The other two geopolymers were prepared by adding Activating Solution 1 (AS1) and Activating Solution 2 (AS2) to MK and were labelled GP-AS1 and GP-AS2, respectively, so that to maintain the total alkaline activators‐to‐binder ratio.
The water content in all the sample preparations was kept constant with a nominal molar ratio of H2O/Na2O = 20.6. The same consideration could be extended to the Al2O3/Na2O molar ratio, which was about 5.9, while SiO2/Na2O was 16.2, and Si/Al was 1.36. The calculations took into account the total number of moles of silica contained in the metakaolin, including the α-quartz, SiO2, phase (see the XRD section). The water content used in the calculations took into account the amount of water contained in the alkaline activators, i.e. it was the sum of the water from the NaOH solution and the water from the sodium silicate solution.
Different batches of fresh paste were carefully prepared by mixing powders and liquids in a planetary mixer (Aucma 1400W, China). The fresh paste was then delicately poured into precise cubic silicone moulds, each measuring 25 mm × 25 mm × 25 mm. Any trapped air bubbles were expertly removed using a vibrating table, a process that took 5 min. Once this crucial step had been completed, the moulds were carefully sealed and left to cure naturally at room temperature with 100% relative humidity. At the end of the one day curing period, the moulds were opened to allow the samples to acclimatise to the ambient conditions of the laboratory atmosphere until the time of testing. Each formulation resulted in a set of twelve samples.
Sample characterisation techniques
We have selected a number of characterisation techniques that have proven their success in identifying the information at the atomic and short-range order molecular level information (MAS-NMR, [5, 17]) to have a direct information on the anionic species of Si in the solutions as well as in the hardened geopolymer product. The evaluation of the chemical resistance in different solutions, including water, is an indirect investigation technique to measure the reticulation/polymerization of the aluminosilicate structure by measuring the ions weakly bound to the 3D aluminosilicate network [30]. Measurements of the ionic conductivity of the water after the immersion of the hardened geopolymers give a good indication of the amount of ions released. If the leach solution is a strong acid, the optimum measurement is the weight loss of a bulky sample after immersion. Again, in the case of acid tests, we have added an assessment of the mineralogical phases remaining in the geopolymer after immersion. To conclude, the measurement of compressive strength should be considered as an additional, albeit less precise, and indirect evaluation of the three-dimensional nature of the aluminosilicate network [31]. The retention of such mechanical strength after acid attack is a measure of the chemical stability of the newly formed Si–O–Al bonds in the geopolymer binder.
NMR characterisation
For liquid-state NMR, all samples (0.75 mL) were transferred into 5-mm tubes together with a capillary filled with deuterium oxide (D2O) in order to lock the field frequency. Spectra were recorded at 300 K using a Bruker Avance II 400 spectrometer operating at 400.15 and 79.49 MHz for the 1H and 29Si nuclides, respectively, and equipped with an inverse broadband (BBI) probe. 29Si spectra were acquired using a 7.5 µs pulse, 10 s delay time and 8000 scans.
Solid-state NMR experiments were performed with the spectrometer described above using a 2.5 mm F/H–X CPMAS probe at 79.49 and 104.26 MHz for the nuclides 29Si and 27Al, respectively. 29Si MAS spectra were acquired with a MAS spin rate of 15 kHz, 2048 scans, a delay time of 60 s and a pulse of 4.5 µs. 27Al MAS spectra were acquired with a MAS spinning speed of 15 kHz, 128 scans, a delay time of 2 s and a pulse of 1 µs.
Leaching resistance in deionised water
The chemical resistance of the hardened pastes was firstly tested by leaching resistance in water, i.e. hydrolytic stability. After 28 days of ageing, which corresponds to the typical period adopted for ordinary Portland cement, all three geopolymers were immersed in deionised water (0.29 mS/m at 25 °C), used as a leachant, with a ratio of liquid volume to geopolymer sample surface area (L/S) of 10. The monolithic geopolymer sample was completely submerged in water, and the solution was not stirred. Measurements of the ionic conductivity, IC, of the leachate were made every 2 h for the first 10 h, then at 24 h.
Leaching resistance in acidic media
The performance of the consolidated geopolymers in inorganic acids was investigated in terms of: (1) change in specimen weight, (2) change in specimen appearance, (3) change in the test medium appearance, and (4) change in specimen compressive strength (detailed in the next paragraph) according to the procedures of ASTM C267 [32] with a modification in specimen conditioning. Conditioning prior to immersion of the specimen in acidic media was carried out at the prescribed temperature (23 ± 2 °C) for 28 days instead of 7 days, plus chemical dehydration with acetone to remove physisorbed water from its intrinsic mesoporosity [33, 34]. After 28 days of curing, the samples were immersed in an acid solution for 7 days, at room temperature in a static condition [35]. The choice of the three acids, H2SO4, HCl and HNO3, was inspired by a number of previous published works [22,23,24,25,26,27, 36], and in particular we kept the concentration medium to high but we reduced to 7 days the immersion time. Our objective was to measure the chemical stability in an acidic environment to compare the geopolymerisation degree of the three hardened products, rather than to test their durability [30].
The normality of the three acids used in this study was N = 2.5 (corresponding to: 15 wt% for nitric acid; 8 wt% for hydrochloric acid; 12 wt% for sulphuric acid), and for all acids, the tests were performed five times to obtain more reliable results. All the solutions were prepared by adding concentrated acid to distilled water. The ratio between the surface’s area of the samples and mL of solution is equal to 8.
Contrary to what has been suggested in some publications [37, 38], the shape of the sample was not a ground geopolymer but a cubic dense monolith, so that the compression tests could be carried out after dissolution.
Compressive strength
As mentioned in the “Geopolymer preparation” section, the mechanical performance of a hardened geopolymer product is somehow dependent on the total alkaline activators‐to‐binder ratio. Therefore, expecting different anionic silica forms in the three alkaline solutions, we decided to check their indirect influence on the three-dimensional reticulated matrix present in the hardened geopolymer.
Compression tests (Instron 5567 Universal Testing Machine, Norwood, MA, USA) were performed to evaluate the mechanical performance of all geopolymers aged 1, 7 and 28 days prior to acid attack. For sake of comparison, the test was repeated after the acid attack on the 28 day aged geopolymers.
Cubic specimens of 25 mm × 25 mm × 25 mm were used for the tests. A controlled load, with a limit of 30 kN, was applied at a displacement rate of 1 mm/min. The tests were carried out in displacement control mode, with a constant loading rate and no preload. The test procedure was terminated when ten valid tests had been obtained for each different geopolymer composition, ensuring thorough and reliable results. Compressive strength values were calculated as the arithmetic mean derived from the ten tests, accompanied by the mean absolute deviation.
Mineralogical characterisation
Crystalline phases of all geopolymers cured for 28 days before and after acid attack were identified on powdered samples by X-ray diffraction (XRD) (X’Pert PRO, Malvern Panalyical Ltd., Malvern, UK). The diffractometer was operated at 40 kV and 40 mA using Cu-Kα radiation (Ni-filtered). Diffraction patterns were collected by the X’Celerator detector from 5 to 70° 2θ with a step size of 0.02° 2θ and a counting time of 3 s.
The powdered samples (average grain size less than 20 m) were loaded laterally on the Al sample holder to reduce the likelihood of preferential orientation and ensure the accuracy and reliability of the XRD analysis.
Results
No apparent aesthetic or mechanical difference among the three hardened geopolymers obtained from the three different activators could be detected by handling the samples. The three materials are cohesive and hard solids of the same colour.
The study of the materials prepared for this investigation started with the characterisation of the species in the three activator solutions by liquid-phase NMR; then, the three geopolymers were identified by direct determination of the 3D aluminosilicate structure by MAS-NMR, followed by indirect characterisation by chemical resistance in water and acids and XRD analysis.
NMR characterisation of alkaline activators
The activator solution was prepared by mixing NaOH 8 M with sodium silicate solution and used once mixed (in formulation GP0), aged for 24 h (in formulation GP-AS1) and 7 days (in formulation GP-AS2). The NMR speciation of the silica soluble forms present in the solutions was performed shortly after the ageing period (24 h and 7 days). The 29Si NMR spectra of the as-received commercial sodium silicate solution (R3) and of the two mixed activator solutions (AS1 and AS2) are shown in Fig. 1.
The spectrum of the sodium silicate starting solution (R3 silicate) shows four broad signals. The very small peak at about − 72 ppm is due to the silicate ion (Q0 units) (Fig. 1). The other three peaks at around − 80, − 88 and − 97 ppm are due to the silicon units Q1, Q2 and Q3, respectively. The broadness of these signals indicates the presence of large aggregates.
In solutions AS1 and AS2, as reported in the experimental part, the modulus R (i.e. the SiO2/Na2O ratio) is reduced by the addition of NaOH. The decrease in modulus R causes the broad signals to be split into sharper signals due to the formation of smaller aggregates as a result of the depolymerisation process (Fig. 1). In particular, in both AS1 and AS2 spectra, an intense signal at − 71 ppm is to silicate species, Si(OH)4 Q0 [39]. It is worth noting that all the signals are shifted lowfield due to the deprotonation of the Si–OH groups, while the Q2 and Q3 signals are split into groups of resonances (− 81, − 87 and − 89, − 95 ppm, respectively) which are due to the presence of different species including monomers, dimers, trimers and cyclic species containing up to eight silicon atoms [40, 41].
NMR characterisation of hardened geopolymers
27Al MAS-NMR spectra of the as-received metakaolin and the GP0, GP-AS1 and GP-AS2 materials aged 28 days are shown in Fig. 2. All spectra show two well-defined resonances at 7.5 and 63 ppm which are due to Al species in octahedral and tetrahedral coordination, respectively. No signals from five-coordinated Al are present in the spectra of the alkali-activated geopolymers. From the analysis of the signal integrals, the amount of Al in tetrahedral coordination is 82, 84 and 78% for GP0, GP-AS1 and GP-AS2 samples, respectively (Fig. 2). This is consistent with the occurrence of polymerisation to a degree which is typical of amorphous geopolymers. It is worth noting that the GP-AS2 sample shows the lowest amount of tetrahedral coordinated aluminium. This may indicate a lower degree of polymerisation [42].
The 29Si MAS-NMR spectra of the materials GP0, GP-AS1 and GP-AS2 are shown in Fig. 3. All spectra show a very broad signal which is due to the convolution of resonances belonging to all the tetrahedral coordinated silicon species Q4(nAl) where n ranges from 0 to 4. This notation was first used by Engelhardt et al. [43] to indicate the number of O–Al groups bonded to silicon atoms in tetrahedral coordination in many aluminosilicate materials. This broad signal is centred at around 90 ppm, which is typical of sodium-based geopolymers [5] (Fig. 3). In order to quantify all the Q4(nAl) species, a deconvolution process was performed using five Gaussian-shaped component bands.
The results of the deconvolution are shown in Table 2. The chemical shift values of the five Gaussians were taken from the literature [12, 44, 45] together with the variable parameter used in the fitting procedure. The comparison of the data for the three samples shows that those obtained with the AS1 and AS2 activating solutions have a lower amount of Q4(2Al) species and a higher amount of Q4(1Al) and Q4(4Al) (Table 2). In particular, the GP-AS2 sample shows a dramatic decrease in Q4(2Al) species, thus confirming the 27Al results describing a geopolymer with a lower degree of polymerisation.
In order to check the reliability of the deconvolution process, Eq. 1 is used to calculate the Si/Al ratios, as reported by Duxon et al. [12]:
In this equation, ISi(mAl) is the intensity of the signals obtained by deconvolution of each species in the 29Si spectra. The Si/Al ratio (mol/mol) values obtained for the GP0, GP-AS1 and GP-AS2 samples were 1.76, 1.75 and 1.78, respectively. These values, which are slightly higher than the nominal values (1.37), can be explained by taking into account the Al–O–Al species that may be present and are not involved in the geopolymer structure. The presence of these units therefore leads to an overestimation of the Si/Al ratio as obtained by the deconvolution procedure.
The 27Al and 29Si MAS-NMR spectra of the geopolymers after three months of ageing are shown in Figs. 4 and 5, where no differences in either the chemical shifts or the shape of the signals can be seen with respect to those collected after 28 days. Also in this case, the integration of the two signals [Al(IV) and Al(VI)] in the 27Al spectrum was performed, and the amounts of Al in tetrahedral coordination were 83, 85 and 85% for the GP0, GP0-AS1 and GP0-AS2 aged samples, respectively (Fig. 4). It is interesting to note that the GP0-AS2 sample shows an increase in 4-coordinated Al with the ageing process, whereas the other two formulations reached this level in shorter times. The absence of an increase in the Al(VI) signal, at around − 8 ppm, can be interpreted as a low availability of Na+···OH− species in the pore solution [46].
The results of the deconvolution of the 29Si MAS-NMR spectra are shown in Fig. 5 and are compared with the results obtained after 28 days of ageing. It is noticeable the absence of Q0, Q1, Q2, Q3 signals, indicating a well reticulated aluminosilicate network [47]. Over time, the environment around the Si atoms remains almost unchanged, with a slight increase in the Q4(3Al) species for the GP0-AS1 and Q4(1Al) species for the GP0-AS2 after 3 months of ageing. When looking to the small differences among the three samples, the signal Q4(2Al) of GP0 seems to transform toward higher interconnected species, Q4(1Al) and Q4(0Al), where purer SiO2 regions are present. These results, in agreement with literature data [10, 11], indicate that the geopolymerisation in metakaolin is very rapid and stable during the first 28 days of ageing, with very limited modification during the following 3 months.
For the GP0, GP0-AS1 and GP0-AS2 aged samples, the Si/Al values obtained from the deconvolution process are 1.84, 1.76 and 1.77, respectively. These values indicate a small increase in reactive Si with respect to Al.
Leaching resistance in water
In order to measure the influence of the type of the alkali silicate solution on the cross-linking of the geopolymer network, the consolidated samples GP0, GP-AS1 and GP-AS2 were leached in water, and the resulting eluates were characterised by ionic conductivity, IC, measurements.
The ionic conductivity of the eluate from geopolymers immersed in deionised water is influenced by a number of factors [48]. These include the composition of the aluminosilicate network, the presence of mobile ions originating from the unreacted solution, and the arrangement of the aluminosilicate structure in terms of the number of the fourfold Al and corresponding balancing ions. Within geopolymer networks, the primary source of ionic conductivity is in the presence of alkali ions such as Na+ or K+. These ions are generated during the reaction between the alkali activator solution and the aluminosilicate powder and are ionically bonded to Al in fourfold coordination. Upon immersion in water, the H+ or H3O+ is exchanged with the monovalent sodium/potassium ions of the geopolymer network [23] while the unreacted activator solution is released, resulting in an overall increase in the alkalinity of the eluate. A high interconnectivity of the pores where the unreacted solution has been deposited increases the mobility of soluble species. In the case studied, Fig. 6 shows that after 28 days of ageing, the ionic conductivity of the eluates after immersion of samples GP0 and GP-AS1 appear comparable, with a rapid increase in the first two hours, followed by a slight increase over the next 20 h or so. The aqueous eluate after immersion of sample GP-AS2 shows a much higher conductivity (Fig. 6). This discrepancy is probably due to differences in the reactivity of the activating solutions with the MK powder, demonstrating the profound effect of solution composition on the resulting reticulation of the geopolymer. In the case of the AS2 solution, its lower reactivity is measured as an increased ionic conductivity of the eluate, which appears to be a very sensitive and quantitative technique, at least when used as a comparison [48, 49].
Leaching resistance in acids
The performance of cements and binders is adversely affected by acid corrosion caused by contact with acid rain or acidic groundwater. The resistance of the hardened binders to chemical attack by acids such as sulphuric, nitric and hydrochloric can also be used as an indirect structural analysis to assess the degree of reticulation of the 3D geopolymer network, as this chemical attack dissolves the aluminosilicate gel formed during alkaline activation of the metakaolin [50]. However, acid attack has little effect on the unreacted MK particles.
To study the chemical modifications of the aluminosilicate framework during the corrosion process, the weight loss of the three geopolymers cured 28 days after the acid attack was determined on samples exposed to highly concentrated acids for 7 days (Fig. 7). As can be seen, the reference geopolymer GP0 always shows a greater loss than GP-AS1 and GP-AS2, followed by GP-AS2 and then GP-AS1.
Comparing the weight loss data with the chemical stability (Fig. 6), it can be concluded that sample GP0 has the highest proportion of weight loss due to newly formed gel. For sample GP-AS2, it cannot be said that the weight loss corresponds entirely to gels formed as a result of geopolymerisation, but since the release in deionised water is high, it can be assumed that some of the sodium silicate has remained unreacted.
Compressive strength
The mechanical integrity of the cured cubes used for the compression test was initially assessed by visual inspection. The specimens were removed from the mould after only 24 h of ageing and placed directly into unrestrained shrinkage conditions (20 °C—50% relative humidity). These conditions are harsh for early age samples, especially as there is no aggregate to limit shrinkage. Initially, all samples contained exactly the same amount of water, approximately 18% by weight, from the alkaline activator solution. The water was partially evaporated during the ageing period without leaving any defects or cracks.
Figure 8 shows the increase in compressive strength with time, from 1 to 28 days of ageing for the three samples. Sample GP-AS1 shows the highest values of compressive strength, indicating a 3D resistant aluminosilicate network. Sample GP0 follows with slightly lower values. The lowest values of sample GP-AS2 confirm that the weight loss in acidic media (Fig. 7) is not entirely related to the dissolution of the newly formed 3D geopolymer network, but to partially reticulated aluminosilicate chains.
Figure 9 compares the compressive strength values of the three geopolymers aged 28 days before and after immersion in acids. Before acid attack, it can be seen that the GP-AS1 sample has a better compressive strength. The difference with the GP0 reference sample is due to the difference in the Si/Al ratio, as demonstrated in other studies [51, 52].
The low value of GP-AS2 compared to the other two samples reflects what is measured with the ionic conductivity (compare Fig. 9 with Fig. 6). In fact, the ionic conductivity of GP-AS2 is much higher than that of GP0 and GP-AS1 conductivity (Fig. 6). On the other hand, after immersion in acids instead the GP0 values are better than those of GP-AS1, although the difference is small. The resistance of GP-AS2 drops dramatically after immersion in acids compared to before immersion. Compared to GP0 and GP-AS1, GP-AS2 values are very low after each acid attack.
Mineralogical characterisation
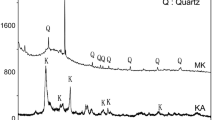
As can be seen from the X-ray diffraction patterns of the three geopolymers after 28 days of ageing in Fig. 10, only the crystalline phases of the as-received metakaolin are visible: alpha-quartz (SiO2, JCPDS no. 46-1045; RRUFF ID R040031), anatase (TiO2, JCPDS no. 21-1272; RRUFF ID R060277), together with some traces of kaolinite planes unaffected by dihydroxylation at about 20° in 2θ (Al2O3·2SiO2, JCPDS file no. 06-0221; RRUFF ID R140004). The three patterns are very similar (Fig. 10). The presence of quartz justifies the presence of the NMR signal Q4(0Al) corresponding to the pure SiO2 regions around 8% present in all the geopolymers (Table 2).
For the pure MK, the characteristic broad hump in the 2θ range from 15 to 30° indicates the presence of the typical lack of long-range order of the amorphous silicates obtained by the dihydroxylation process of kaolinite [53]. Such an XRD feature of MK varies with NaOH solution decreasing its intensity towards the high 2θ values. Nevertheless, the XRD traces of the samples GP0, GP-AS1 and GP-AS2 show a slight shift of the amorphous hump towards higher values (from ~ 23° to ~ 28° in 2) typical of the geopolymers with an X-ray amorphous network richer in Al [54].
The multiple peaks around 20° in 2θ typical of MK disappear in the geopolymer XRD patterns leaving a single anatase peak. It has recently been proved that crystalline TiO2 is not soluble in alkaline media and therefore does not participate in the geopolymerisation reaction [53].
After the immersion in acid, all the geopolymer series show a decrease in the intensity of the amorphous hump around 20°–30° in 2θ, indicating the destruction of the newly formed disordered lattice, regardless of the type of acid tested.
Discussion
The structure of sodium silicate solutions at pH > 11 (in our case pH is 11.7 for the commercial solution R = 3) depends on the SiO2:Na2O ratio, presenting small colloidal particles in the tens of nm range above the ratio of 2.0–2.5 [55]. For values below 2.0–2.5 it is proposed that the silicate solutions mainly contain low molecular weight species such as mono- and dimeric species [56]. In addition, a relevant variable for silica speciation in sodium silicate solutions is the concentration of the solution. In the case studied, the concentration of SiO2 is 27 wt% in the commercial silicate with a SiO2:Na2O weight ratio of 3.0, similar to those studied by Marsmann [57]. In particular, for a solution of 27.2 wt% SiO2 and a SiO2:Na2O ratio of 3.3, he demonstrated that 35% of the silica is fully polymerised. These literature data indicate that the addition of the NaOH solution to the sodium silicate solution with R = 3 and high concentration, as presented in this study, induces depolymerisation. The experimental show polymerised Q2, Q3 and Q4 species in the 29Si spectra of the commercial sodium silicate solution that was added together with NaOH solution directly to metakaolin in the GP0 formulation (Fig. 1). Solutions AS1 and AS2 are depolymerised and show a high amount of monomeric Q0 species. The AS2 solution already at 7 days of ageing presents an increase in Q3 signal indicating an incipient polymerisation. The AS2 solution already shows an increase in the Q3 signal after 7 days of ageing, indicating the start of polymerisation. These results suggest that in order to have a solution rich in monomeric, and therefore more reactive, Si species in solution, it is advisable to prepare the activator mixture no more than 24 h before use.
Once mixed with the MK, these three solutions produce dense and well-reticulated geopolymers in which the coordination of Al was found to be predominantly in the tetrahedral geometry, indicating an almost complete dissolution of the six and the complete dissolution of the five sites typical of the metakaolinite product (Fig. 2) [8]. The presence of unreacted kaolin in geopolymers has been reported elsewhere in the literature and it is statistically acceptable [58]. The presence of low Q4 species in GP-AS2 (Fig. 2) can be explained by two concomitant features: the lower number of monomers in the AS2 solution compared to the AS1 solution and the absence of the high number of OH− groups as in the fresh NaOH solution used in GP0. The absence of the resonance of 27Al at 80 ppm (Fig. 2), assigned to the signal of aqueous Al(OH)4 [59], indicates that the species released during the dissolution of the metakaolin react completely during the geopolymerisation and do not remain in the pores of the hardened material [47]. Apart from these details, we can assess from the NMR that the amorphous MK powder characterised by a disordered polymerised silicon and aluminium network has been almost completely reacted to produce a 3D aluminosilicate network (Fig. 3, Table 2) by dissolution and condensation in all the three activator solutions used for this study. The fraction of Al atoms in tetrahedral coordination (Fig. 4) reaches a maximum of about 82–85% of all the samples after 28 days (GP0 and GP-AS1) or 3 months (GP-AS2), indicating that this value is the maximum possible. Such a result can be correlated with a fraction of the as-received kaolinite that was never disordered by the thermal treatment or, on the contrary, with a fraction of mullite [60] that was generated during the thermal treatment. The latter crystalline phase was not found in the XRD spectra (Figs. 10, 11, 12, 13), whereas the kaolinite was. For the sake of comparison, we must report recent evidence of a few percentage points of non-framework Al(VI) species acting in a charge-balancing role [61]. The absence of efflorescence on the surface of the bulky hardened geopolymer paste indicates that a correct formulation has been adopted, where Na+ ions do not migrate to the surface to react with the atmospheric CO2 to form carbonated species but remain bound to balance the missing charge in the Al+3 in fourfold coordination replacing Si in the bridging tetrahedra [62]. The chemical stability of this structure was first investigated in deionised water (Fig. 6) and then in acid media (Fig. 7). In deionised water, the stability of GP-AS2 is poor compared to the other two compositions (Fig. 6), as the released ions increase the ionic conductivity of the eluate. Presumably, the OH− have not fully reacted with the metakaolin, which is already partially polymerised after 7 days in the AS2 solution, as indicated by the NMR analysis (compare Fig. 2 with Fig. 4). The higher degree of reticulation of GP0 and GP-AS1 is also reflected in the resistance to acid (Fig. 7) and to compression (Fig. 8) or a combination of the two (Fig. 9). The chemical stability and the mechanical resistance appear to be very sensitive to the degree of depolymerisation of the Na− silicate solution, although the 29Si NMR signal does not reflect such a large difference. A slight preference towards the pure SiO2 region with Q4(1Al) or Q4(0Al) of GP0 with respect to the other two hardened geopolymers was observed with 3 months ageing (Fig. 5), accompanied by a reduced number of exchangeable Na+ ions.
XRD patterns of the GP0 sample at 28 days curing time after immersion in the three different acidic solutions compared with the pristine geopolymer. Enlarged area shows the amorphous halo (peaks identification: see Fig. 10).
The reduced number of Na+, compensating chargers for tetrahedral Al bound to tetrahedral Si, justifies the chemical stability in acid for the GP0 formulation with respect to the other two.
The XRD investigation proved to be sensitive enough to detect the change in the amorphous structure of the three hardened geopolymers as a shift of the amorphous halo to higher 2θ angles as one moves from metakaolin to geopolymer (Figs. 10, 11, 12, 13). The amorphous halo is then shifted to lower 2θ values after the acid attack indicating the loss of Al from the aluminosilicate geopolymer gel. Very similar behaviour has been demonstrated by FT-IR analysis combined with NMR observations by Allahverdi and Škvára [63]. The ejection of tetrahedral aluminium from the aluminosilicate framework of the geopolymer in the process of acid corrosion and due to the electrophilic attack of acid, protons on polymeric Si–O–Al bonds shift the position of the stretching modes to a higher frequency.
Conclusions
This paper presents new quantitative NMR results on the presence of different anionic silicate species in the activator solution aged for 0, 24 h and 7 days, used for the preparation of geopolymer pastes.
A commercial metakaolin was reticulated by the addition of an activator solution based on a commercial sodium silicate. The procedure and timing adopted for the preparation of the three different activator solutions starting from NaOH and sodium silicate mixtures, strongly affect the Al and Si species in the 3D aluminosilicate network of the final products.
The detailed 27Al and 29Si NMR investigation of the alkali activator solutions used to prepare geopolymers starting from disordered metakaolin proved to be crucial for the interpretation of the chemical behaviour in deionised water and acid solutions of the hardened materials.
It has been demonstrated that the synthesis procedure can be directed towards a preferred chemical stability, high fraction of Q4(1Al) and Q4(0Al), or towards a mechanical stability, high number of Al bound to tetrahedral Si. As a practical indication, the short ageing time of the NaOH + Na-silicate activator solution is more suitable for rapid reticulation of the SiO2-rich regions of the aluminosilicate 3D network in acid-resistant hardened products. Mixing of the two solutions with 24 h prior to the paste preparation seems to result in mechanically resistant geopolymers.
The evolution of the geopolymer 3D aluminosilicate network and its improvements with time has been assessed in terms of the 27Al signal (tetrahedral coordination increase) and the 29Si broad resonance at − 93 ppm combined with the absence of Q0, Q1, Q2, Q3 signals.
Data availability
All data are made available if requested.
References
Lothenbach B, Scrivener K, Hooton RD (2011) Supplementary cementitious materials. Cem Concr Res 41:1244–1256
Scrivener K, Martirena F, Bishnoi S, Maity S (2018) Calcined clay limestone cements (LC3). Cem Concr Res 114:49–56
Pacheco-Torgal F, Castro-Gomes J, Jalali S (2008) Alkali-activated binders: a review: part 1. Historical background terminology reaction mechanisms and hydration products. Constr Build Mater 22(7):1305–1314
Duxson P, Provis JL, Lukey GC, Mallicoat SW, Kriven WM, van Deventer JSJ (2005) Understanding the relationship between geopolymer composition, microstructure and mechanical properties. Colloids Surf A 269(1–3):47–58
Fernández-Jimenez A, de la Torre AG, Palomo A, López-Olmo G, Alonso MM, Aranda MAG (2006) Quantitative determination of phases in the alkali activation of fly ash. Part I. Potential ash reactivity. Fuel 85:625–634
Garg N, White CE (2017) Mechanism of zinc oxide retardation in alkali-activated materials: an in situ X-ray pair distribution function investigation. Mater Chem A 5:11794–11804
Rocha J, Klinowski J (1990) 29Si and 27Al magic-angle-spinning NMR studies of the thermal transformation of kaolinite. Phys Chem Miner 17(2):179–186
Massiot D, Dion P, Alcover JF, Bergaya F (1995) 27Al and 29Si MAS NMR study of kaolinite thermal decomposition by controlled rate thermal analysis. J Am Ceram Soc 78(11):2940–2944
Autef A, Joussein E, Poulesquen A, Gasgnier G, Pronier S, Sobrados I, Sanz J, Rossignol S (2013) Role of metakaolin dehydroxylation in geopolymer synthesis. Powder Technol 250:33–39
Davidovits J, Davidovits M (1988) Geopolymer: room temperature ceramic matrix for composites. Ceram Eng Sci Proc 9(7–8):835–842
Davidovits J, Comrie DC, Paterson JH, Ritcey DJ (1990) Geopolymeric concretes for environmental protection. Concr Int 12(7):30–40
Duxson P, Provis JL, Lukey GC, Mallicoat SW, Kriven WN, van Deventer JSJ (2005) 29Si NMR study of structural ordering in aluminosilicate geopolymer gels. Langmuir 21:3028–3036
Duxson P, Mallicoat SW, Lukey GC, Kriven WM, van Deventer JSJ (2007) The effect of alkali and Si/Al ratio on the development of mechanical properties of metakaolin-based geopolymers. Colloids Surf A 292(1):8–20
Lizcano M, Kim HS, Basu S et al (2012) Mechanical properties of sodium and potassium activated metakaolin-based geopolymers. J Mater Sci 47:2607–2616
Hindryawati N, Maniam GP (2015) Novel utilization of waste marine sponge (demospongiae) as a catalyst in ultrasound-assisted transesterification of waste cooking oil. Ultrason Sonochem 22:454–462
Gao K, Lin K-L, Wang D, Hwang C-L, Shiu H-S, Chang Y-M, Cheng T-W (2014) Effects SiO2/Na2O molar ratio on mechanical properties and the microstructure of nano-SiO2 metakaolin-based geopolymers. Constr Build Mater 53:503–510
Gharzouni A, Joussein E, Samet B, Baklouti S, Pronier S, Sobrados I, Sanz J, Rossignol S (2015) The effect of an activation solution with siliceous species on the chemical reactivity and mechanical properties of geopolymers. J Sol Gel Sci Technol 73(1):250–259
Khaled Z, Mohsen A, Soltan A, Kohail M (2023) Optimization of kaolin into metakaolin calcination conditions, mix design and curing temperature to develop alkali activated binder. Ain Shams Eng J 14(6):102142
Phair JW, Van Deventer JSJ (2002) Effect of the silicate activator pH on the microstructural characteristics of waste-based geopolymers. Int J Miner Process 66(1–4):121–143
Nordström J, Nilsson E, Jarvol P, Nayeri M, Palmqvist A, Bergenholtz J, Matic A (2011) Concentration- and pH-dependence of highly alkaline sodium silicate solutions. J Colloid Interface Sci 356(1):37–45
Dimas D, Giannopoulou I, Panias D (2009) Polymerization in sodium silicate solutions: a fundamental process in geopolymerization technology. J Mater Sci 44:3719–3730
Rostami H, Brendley W (2003) Alkali ash material: a novel fly ash-based cement. Environ Sci Technol 37(15):3454–3457
Allahverdi A, Skvara F (2005) Sulphuric acid attack on hardened paste of geopolymer cements part I: mechanism of corrosion at relatively high concentrations. Ceram Silik 49(4):225–229
Bakharev T (2005) Resistance of geopolymer materials to acid attack. Cem Concr Res 35(4):658–670
Pavlik V (1994) Corrosion of hardened cement paste in acetic and nitric acids part II: formation and chemical composition of the corrosion products layer. Cem Concr Res 24(8):1495–1508
Provis JL, Van Deventer JS (2009) Geopolymers Structure, processing, properties and industrial applications. Woodhead Publishing Limited and CRC Press LLC, Sawston, pp 1–6
Chandra S (1988) Hydrochloric acid attack on cement mortar—an analytical study. Cem Concr Res 18(2):193–203
Xie T, Visintin P, Zhao X, Gravina R (2020) Mix design and mechanical properties of geopolymer and alkali activated concrete: review of the state-of-the-art and the development of a new unified approach. Constr Build Mater 256:119380
Dal Poggetto G, D’Angelo A, Catauro M, Barbieri L, Leonelli C (2022) Recycling of waste corundum abrasive powder in MK-based geopolymers. Polymers (Swis) 14(11):2173
Ruiz-Santaquiteria C, Fernández-Jiménez A, Skibsted J, Palomo A (2013) Clay reactivity: production of alkali activated cements. Appl Clay Sci 73(1):11–16
Rossi L, de Miranda Lima L, Sun Y et al (2022) Future perspectives for alkali-activated materials: from existing standards to structural applications. RILEM Tech Lett 7:159–177
https://www.civilnode.com/download-standard/10634596867172/C267-Standard-Test-Methods-for-Chemical-Resistance-of-Mortars-Grouts-and-Monolithic-Surfacings-and-Polymer-Concretes. Accessed April 2024
Ettahiri Y, Bouargane B, Fritah K, Akhsassi B, Pérez-Villarejo L, Aziz A, Bouna L, Benlhachemi A, Novais RM (2023) A state-of-the-art review of recent advances in porous geopolymer: applications in adsorption of inorganic and organic contaminants in water. Constr Build Matls 395:132269
Li CJ, Zhang YJ, Chen H, He PY, Meng Q (2022) Development of porous and reusable geopolymer adsorbents for dye wastewater treatment. J Clean Prod 348:131278
Wallah SE, Hardjito D, Sumajouw DMJ, Rangan BV (2005) Sulphate and acid resistance of fly ash based geopolymer concrete. In: Presented at the Australian structural engineering conference, Newcastle, Australia, September 2005.
Sata V, Sathonsaowaphak A, Chindaprasirt P (2012) Resistance of lignite bottom ash geopolymer mortar to sulfate and sulfuric acid attack. Cem Concr Compos 34:700–708
de la Fernández-Jimenez A, Torre AGA, Palomo G, López-Olmo MM, Alonso MAGA (2006) Quantitative determination of phases in the alkali activation of fly ash. Part II: degree of reaction. Fuel 85(14–15):1960–1969
Longhi MA, Walkley B, Rodríguez ED, Kirchheim AP, Zhang Z, Wang H (2019) New selective dissolution process to quantify reaction extent and product stability in metakaolin-based geopolymers. Compos Part B Eng 176:107172
Wijnen PWJG, Beelen TPM, de Haan JW, Rummens CPJ, van de Ven LJM, van Santen RA (1989) Silica gel dissolution in aqueous alkali metal hydroxides studied by 29Si NMR. J Non Cryst Solids 109(1):85–94
Jansson H, Bernin D, Ramser K (2015) Silicate species of water glass and insights for alkali activated green cement. AIP Adv 5:067167
Cho H, Felmy AR, Craciun R, Keenum JP, Shah N, Dixon DA (2006) Solution state structure determination of silicate oligomers by 29Si NMR spectroscopy and molecular modelling. J Am Chem Soc 128(7):2324–2335
Brus J, Abbrent S, Kobera L, Urbanova M, Cuba P (2016) Advances in 27Al MAS NMR studies of geopolymers. Annu Rep NMR Spectrosc 88:79–147
Engelhardt G, Hoebbel D, Tarmak M, Samoson A, Lippmaa E (1982) 29Si-NMR-untersuchungen zur anionenstruktur von kristallinen tetramethylammoniumalumosilicaten und -alumosilicatlösungen. Z Anorg Allg Chem 484:22
Wan Q, Rao F, Song S, Zhang Y (2019) Immobilization forms of ZnO in the solidification/stabilization (S/S) of a zinc mine tailing through geopolymerization. J Mater Res Technol 8(6):5728–5735
Engelhardt G, Michel D (1988) High-resolution solid-state NMR of silicates and zeolites. Appl Catal 42:187–188
Singh PS, Trigg M, Burgar I, Bastow T (2005) Geopolymer formation processes at room temperature studied by 29Si and 27Al MAS-NMR. Mater Sci Eng 396(1–2):392–402
Davidovits J (1994) Geopolymers: man-made rock geosynthesis and the resulting development of very early high strength cement. J Mater Educ 16:91–137
Dal Poggetto G, Kittisayarm P, Pintasiri S, Chiyasak P, Leonelli C, Chaysuwan D (2022) Chemical and mechanical properties of metakaolin-based geopolymers with waste corundum powder resulting from erosion testing. Polymers 14:5091
Ponomar V, Ohenoja K, Illikainen M (2023) Optimizing activating solution and environmental leaching characteristics of Fe-rich alkali-activated Zn slag. J Hazard Mater 445:130575
Fernández-Jiménez A, García-Lodeiro I, Palomo A (2007) Durability of alkali-activated fly ash cementitious materials. J Mater Sci 42(9):3055–3065
Vafaei M, Allahverdi A, Dong P, Bassim N (2018) Acid attack on geopolymer cement mortar based on waste-glass powder and calcium aluminate cement at mild concentration. Constr Build Mater 193:363–372
Qu F, Li W, Wang K, Zhang S, Sheng D (2021) Performance deterioration of fly ash/slag-based geopolymer composites subjected to coupled cyclic preloading and sulfuric acid attack. J Clean Prod 321:128942
Scherb S, Köberl M, Beuntner N, Thienel K-C, Neubauer J (2020) Reactivity of metakaolin in alkaline environment: correlation of results from dissolution experiments with XRD quantifications. Matls (MDPI) 13(10):2214
Nasab GM, Golestanifard F, MacKenzie KJD (2014) The effect of the SiO2/Na2O ratio in the structural modification of metakaolin-based geopolymers studied by XRD, FTIR and MAS-NMR. J Ceram Sci Technol 5(3):185–191
Böschel D, Janich M, Roggendorf H (2003) Size distribution of colloidal silica in sodium silicate solutions investigated by dynamic light scattering and viscosity measurements. J Colloid Interface Sci 267:360–368
Engelhardt LG, Zeigan D, Jancke H, Wieker W, Hoebbel D (1975) 29Si-NMR-spektroskopie an silicatlösungen II. Zur abhängigkeit der struktur der silicatanionen in wäßrigen natriumsilicatlösungen vom Na Si-verhältnis. Anorg Allg Chem 418:17–28
Marsmann HC (1974) 29Si NMR-studies on silicate solutions in water. Z Naturforsch B 29:495
Provis JL, Duxson P, Lukey GC, van Deventer JSJ (2005) Statistical thermodynamic model for Si/Al ordering in amorphous aluminosilicates. Chem Mater 17(11):2976–2986
Zibouche F, Kerdjoudj H, de Lacaillerie J-B, Van Damme H (2009) Geopolymers from Algerian metakaolin. Influence of secondary minerals. Appl Clay Sci 43(3–4):453–458
Merwin LH, Sebald A, Rager H, Schneider H (1991) 29Si and 27Al MAS NMR spectroscopy of mullite. Phys Chem Miner 18:47–52
Walkley B, Rees GJ, San Nicolas R, Van Deventer JSJ, Hanna JV, Provis JL (2018) New structural model of hydrous sodium aluminosilicate gels and the role of charge-balancing extra-framework Al. J Phys Chem C 122(10):5673
Baral K, Li A, Ching W-Y (2019) Ab initio molecular dynamics simulation of Na-doped aluminosilicate glasses and glass-water interaction. AIP Adv 9(7):075218
Allahverdi A, Škvára F (2001) Nitric acid attack on hardened paste of geopolymeric cements—part 2. Ceram Silik 45(4):143–149
Acknowledgements
Authors are greatly thankful to Mirko Braga and Pasquale Pansini, Ingessil, Verona, Italy, for supplying the sodium silicate solution and to Maria Paola Morsiani, Imerys Ceramics Italy, Modena, Italy, and Sophie Maraninchi Product Manager Refractory, Abrasives and Constructions Business Area, Imerys, France, for supplying the metakaolin. UNIMORE is acknowledged for administrative support.
Funding
Open access funding provided by Università degli Studi di Modena e Reggio Emilia within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
G.D.P. involved in investigation, data curation, writing—original draft, and review and editing. C.L. involved in conceptualisation, data curation, funding acquisition, and writing—review and editing. A.S. involved in investigation, methodology, supervision, data curation, formal analysis, and writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest exist.
Additional information
Handling Editor: Annela M. Seddon.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Poggetto, G.D., Leonelli, C. & Spinella, A. Influence of anionic silica forms in clear sodium silicate precursors on metakaolin geopolymerisation via 29Si and 27Al MAS-NMR and microstructural studies. J Mater Sci (2024). https://doi.org/10.1007/s10853-024-09869-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10853-024-09869-x