Abstract
The effects of Cu doping of PrBaCo2O5+δ layered perovskite oxide (PBCCux, x = 0, 0.1, 0.2, and 0.5) were investigated in terms of local atomic structure change and polarization resistance. As the copper doping concentration in PBCCux increased, the peaks in the XRD patterns of the samples shifted to lower angle which indicates lattice expansion by oxygen vacancy formation and larger ion doping. O K-edge XANES was used to determine the change in orbital hybridization with Cu doping, and it was confirmed that the O2− species decreased and the monoxidic species increased with the addition of Cu resulting in covalency increase. As the Cu content increased, the Co–O first shell intensity decreased in the Co K-edge EXAFS spectra because of the formation of oxygen vacancies by Cu doping. The impedance at 600 °C decreased by approximately 73% from 0.91 Ω cm2 (PBCO) to 0.23 Ω cm2 (PBCCu0.5) by the synergetic effect of oxygen vacancy formation and the increase in covalency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Solid oxide fuel cells (SOFC) are promising energy conversion systems that directly convert chemical energy into electrical energy and exhibit a high energy conversion efficiency of over 80%. The high operating temperature of 800–1000 °C induces challenges in selecting surrounding materials and accelerates their deterioration [1, 2]. Research on lowering the operating temperature to 500–800 °C is actively being conducted. Mixed ionic-electronic conductors (MIECs) have received considerable attention as promising cathode materials capable of achieving high performance for intermediate temperature SOFCs (IT-SOFCs, below 800 °C). Perovskite oxides have attracted attention as cathode materials for SOFC/SOECs because of their excellent electrochemical properties and mixed ionic electronic conductivity. Various methods such as transition metal doping, infiltration, and exsolution have been used, and layered perovskite oxides using cation ordering have also been studied to improve the electrocatalytic activity of perovskite oxides [3, 4].
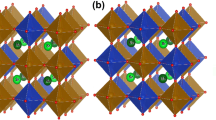
The layered perovskite oxide (AA′B2O5+δ) has a structure in which [BO2]–[AOδ]–[BO2]–[A′O] layers are continuously stacked along the c-axis of the lattice. This layered structure reduces the oxygen-binding force of the [AOδ] layer and effectively improves the diffusion of oxygen ions by forming an oxygen pathway [5]. The electrochemical properties of perovskite oxides are determined by various factors such as B–O covalency, O p-band center, charge transfer energy, and valence band structure resulting from the crystal structure and electronic structure. Lee et al. predicted the ORR catalytic activity of SOFCs by comparing them with measurable indicators, such as the O p-band center, polarization resistance, and surface exchange constant, paying attention to the importance of oxygen conduction [6].
LnBaCo2O5+δ (Ln = Pr, Nd, Sm, etc.) has been reported to exhibit excellent electrical conductivity, fast surface oxygen exchange, and high oxygen-ion conductivity [7]. Kim et al. showed that PrBaCo2O5+d have over 1000 S/cm of electrical conductivity below 600 °C, the order of 10–4 cm2/s bulk diffusion and the order of 10–2 cm/s surface exchange coefficient using ECR and IEDP. These values are the best values among perovskite oxide-based oxides and are the same or better values about 500 °C lower than conventional LSC and LSF. This is the result of the cation ordering and high oxygen vacancy concentration that occur during the formation of PBCO, and the ease of changing the oxidation rate of Co ions [8]. To improve polarization resistance in these PBCOs in various studies, a number of studies on doping with Fe, Cu, Sr, etc., have been conducted. When Cu was doped, the electrical conductivity tended to decrease slightly, but the polarization resistance was reduced by about 30–50%. Studies conducted by Jin et al., Zhao et al. and Suntsov et al. discussed the main reasons for polarization resistance reduction were the increase in Co3+/Co4+, Cu+/Cu2+ reduction pairs, and the increase in oxygen vacancies due to Cu doping. B–O covalency, op-band center, charge transfer energy, and valence band structure suggested above have been discussed in some studies, but research on the interaction between orbitals and the change in band structure according to doping is needed [9,10,11].
In a previous study, we observed the effect of changes in the oxidation number and valence band structure of PrBaCo2−xCuxO5+δ (PBCCux) layered perovskite samples according to Cu doping on the reduction of polarization resistance [12]. Herein, the changes in the local atomic structure and orbital hybridization with Cu doping were analyzed. Co K-edge EXAFS was used to analyze the crystal structure and consequent local structural changes, and O K-edge XANES was used to analyze changes in orbital hybridization.
Materials and methods
Powder synthesis
PBCO (PrBaCo2O5+δ) and PBCCux (PrBaCo2−xCuxO5+δ, x = 0.1, 0.2, 0.5) powders were synthesized using an EDTA-citrate complexing process. Pr(NO3)3·6H2O (99.9%, Sigma-Aldrich), Co(NO3)3·6H2O, Cu(NO3)2·2.5H2O (98%, Sigma-Aldrich), and Ba(NO3)2 (99%+, Alfa Aesar) metal precursors were dissolved in deionized water. EDTA (99.5%, Alfa Aesar) was added to 1 N NH4OH (Junsei Chemical Co.) solution to obtain an NH3-EDTA buffer solution. The NH3-EDTA solution and crystallized citric acid (99.5%, Samchun Chemical) powders were added to the metal precursor solution to make a sol with a total metal ion/EDTA/citric acid molar ratio of 1:1:2. The solution was heated to 75 °C while adjusting the pH to 9 using NH4OH, and the solvent was evaporated to obtain clear gels. These gels were pre-calcined at 450 °C and calcined in air at 950 °C.
Symmetric cell preparation
Symmetric cells (PBCCux|SDC|PBCCux) were prepared to investigate the electrochemical properties of the PBCCu. The SDC pellets were sintered at 1400 °C for 4 h using SDC powder (SDC20-HP, Fuelcellmaterials). The PBCCu powders were mixed with a binder prepared from α-terpineol and ethylcellulose to form PBCCu pastes, which were screen-printed onto both sides of the SDC pellets. After drying, the symmetric cells were calcined at 950 °C for 2 h in air.
Characterization
To examine the crystal structures of the calcined and sintered powders, powder X-ray diffraction (XRD, PANalytical X’pert-Pro MPD PW3040/60) was performed at room temperature using a step scan procedure (0.02°/2θ step, time per step 0.5 s) in the 2θ range of 10°–90°. O K edge NEXAFS and Co K edge XAS analysis was performed at the PLS-II 4D beamline (base pressure 3.0 × 10–10 Torr) and 10C beamline in the Pohang Accelerator Laboratory (PAL, Republic of Korea). XAS analysis was performed using the IFEFFIT interactive software package (with ATHENA graphical interfaces). Background subtraction was processed by fitting linear polynomials to the pre-edge and the post-edge region of an absorption spectrum, respectively. The v(E) data were converted into k-space, k-weighted, and Fourier transformed using a Hanning window, into r-space.
Impedance measurements were conducted using an IviumStat instrument (Ivium, the Netherlands) over the frequency range of 106–0.01 Hz with an excitation voltage of 10 mV at an operating temperature of 600–700 °C under open-circuit conditions in air. The electrochemical impedance spectroscopy results were multiplied by 0.5 to account for the two electrodes. The impedance spectra data were further fitting by an EC-lab software. Distribution of relaxation time (DRT) method was applied to analyze the EIS data.
Results and discussion
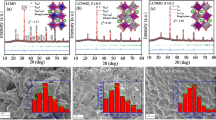
Figure 1a shows the XRD pattern of Cu-doped PBCO. All compositions showed an orthorhombic structure, and secondary phases, including CuO, were not observed in PBCCux. Each peak of PrBaCo2O5+δ (PBCO), which is the basic composition, was indexed according to the P4/mmm structure. The peak position shifted to a lower angle according to the Cu content as shown in Fig. 1b. This is a chemical expansion caused by the expansion of the lattice by replacing Co3+ ions (61 pm) with Cu2+ (73 pm). Oxygen vacancy concentration is increased due to the B site oxidation state reduction from 3.26 (PBCO) to 2.90 (PBCCu0.5) as shown in Figure S1 and Table S1. The lattice free volume increases due to the synergetic effect of lattice expansion by chemical expansion and oxygen vacancy formation and oxygen ion mobility increases accordingly [12,13,14].
Figure 2 shows the entire O K-edge XAS spectra of the PBCCux powder to investigate the electronic structure change, which is affected by the addition of Cu. The peaks in the 527–532 eV region originate from the interaction between the unoccupied state of the Co/Cu 3d orbital; the peak in the 535 eV region is a peak formed by reflection by oxygen vacancies; the peak formed in the 537.5 eV region is attributed to the hybridization of the Pr/Ba 4d orbital and O 2p orbital; the peaks in the 540–545 eV region are formed by the hybridization of Co/Cu 4sp and O 2p orbitals; the 541 eV region is due to O2− during the hybridization of Co/Cu 4sp and O 2p orbitals; and the peaks in the 544 eV region are Co/Cu 4sp and O 2p orbitals. It has been reported that the hybridization of Cu 4sp and O 2p orbitals is caused by monoxidic species [15,16,17,18,19,20,21,22].
The pre-edge peaks are 529.5 529.3 529.2 and 529.0 eV for PBCO, PBCCu0.1, PBCCu0.2, PBCCu0.5, respectively. It has been reported that the shift of the pre-edge peak to a lower energy is due to the increased covalency of Co–O. The O 2p ligand holes were split into two peaks, indicating the eg and t2g orbitals, by crystal field splitting. With the addition of Cu, the intensity ratio of the two orbitals formed by the crystal field splitting (\(I_{{t_{2g} }} /I_{{e_{{\text{g}}} }}\)) gradually increased. This is because as the Cu content increased, Co3+ with a 3d6 electron configuration was replaced with Cu2+ with a 3d9 electron configuration, and the number of electrons in the t2g orbital gradually increased as shown in Fig. 3. The peak due to Pr/Ba 4d–O 2p hybridization did not shift at 535 eV because the hybridization of the A-site cation and O anion did not change. The O2− peak gradually decreases, and the monoxidic species peak gradually increases in the peak due to Co/Cu 4sp–O 2p hybridization. This corresponds to the fact that the covalency increased, as in the previous pre-edge peak shift [23].
Figure 4a shows the Co K-edge XAS spectrum, and (b) shows the pre-edge structure of the Co K-edge. As Cu was added to the pre-edge structure, the ratio of the t2g orbital increased. This is because the number of electrons corresponding to the t2g orbital is increased by Cu doping, as previously confirmed by O K-edge XANES.
Figure 4c, d shows the EXAFS k3χ spectrum and Fourier transformed EXAFS spectra (R space) of the Co K-edge of PBCO and PBCCux. The first peak is formed by the nearest neighbor, and the second peak in the 2.8 Å is formed by Co–Co/Cu bonding. The first shell intensity gradually decreased with increasing Cu content, which corresponds to a decrease in the Co–O coordination shell due to the formation of oxygen vacancies according to Cu doping [24, 25]. The peak around 3.0 Å was formed by the adjacent Pr/Ba. The second and third peaks are due to the Co-Pr/Ba shell and corner shared CoO6 octahedron, respectively, and similar to the fingerprint of the perovskite structure [26].
Figure 5a shows the electrochemical impedance spectra (EIS) measured at 600–700 °C for the symmetric cell of the PBCCux electrode. The polarization resistance was calculated by measuring the distance between the intercepts of Z′ when Z″ is equal to 0. The polarization resistances of PBCCux were 0.91 Ω cm2 (PBCO), 0.60 Ω cm2 (PBCCu0.1), 0.47 Ω cm2 (PBCCu0.2) and 0.23 Ω cm2 (PBCCu0.5) and gradually decreased as Cu was doped, so that PBCCu0.5 showed a 74.72% smaller value compared to that of PBCO. In addition, the activation energy was calculated at 600–700 °C and is shown in Table 1. Arrhenius dependence was observed in all compositions, and the activation energy decreased gradually as the Cu doping concentration increased, with the smallest value being observed in PBCCu0.5.
EIS fitting using equivalent circuit and distribution of relaxation time (DRT) analysis conducted for better understanding of the ORR process. The impedance spectra were fitted using series of two ZARC element as shown in Fig. 5b and two distinctive peaks in DRT were observed in around 105 Hz, 103 Hz and 10 Hz in Fig. 5c. Both peaks shifted to lower frequency with Cu content increasing, while 10 Hz peak remains same position. According to the literature, the high frequency (> 103 Hz), intermediate frequency (1–103 Hz) and low frequency (10–2 to 1 Hz) characteristic peaks are related to oxygen ion charge transfer from electrolyte to cathode at TPB, surface exchange or ion transfer at cathode and gas diffusion process, respectively. As Cu was doped, it was found that the oxygen ion transfer and surface electron exchange in TPB were accelerated, and the impedance decreased. The doping of Cu accelerated oxygen ion transfer and surface electron exchange at TPB, which decreased the impedance [27].
The doping of Cu in PBCO improves the electrochemical properties. This is because the doping of Cu increases the number of oxygen vacancies in the lattice and the free volume of the lattice, which improves the mobility of oxygen ions. In addition, the improvement of B–O bond covalency due to the change in B–O bond structure and orbital hybridization accelerates surface electron exchange and oxygen ion transfer at TPB.
Conclusion
We investigated the orbital hybridization and the polarization resistance changes of PrBaCo2O5+δ doped with Cu (PBCCux, x = 0.1, 0.2, and 0.5). O K-edge XANES analysis confirmed that the lower energy shift of the pre-edge peak and the covalency of the Co–O bond increased by decreasing the O2− species and increasing the monoxidic species in the lower energy shift of the pre-edge peak and the Co/Cu 4sp–O 2p hybridization. Co K-edge EXAFS analysis indicated that the Co–O first shell intensity decreased as the Cu content increased, which was due to the formation of oxygen vacancies by Cu doping. With an increase in the Cu content, the impedance decreased by approximately 73% from 0.91 Ω cm2 (PBCO) to 0.23 Ω cm2 (PBCCu0.5) at 600 °C due to the synergetic effect of the increase in oxygen vacancies in the lattice along with the increase in the covalency of B–O bonds.
Data availability
Not applicable.
References
Jiang L, Wei T, Zeng R, Zhang WX, Huang YH (2013) Thermal and electrochemical properties of PrBa0. 5Sr0. 5Co2−xFexO5+δ (x = 0.5, 1.0, 1.5) cathode materials for solid-oxide fuel cells. J Power Sources 232:279–285. https://doi.org/10.1016/j.jpowsour.2013.01.064
Zhu C, Liu X, Yi C, Yan D, Su W (2008) Electrochemical performance of PrBaCo2O5+δ layered perovskite as an intermediate-temperature solid oxide fuel cell cathode. J Power Sources 185(1):193–196. https://doi.org/10.1016/j.jpowsour.2008.06.075
Pelosato R, Cordaro G, Stucchi D, Cristiani C, Dotelli G (2015) Cobalt based layered perovskites as cathode material for intermediate temperature solid oxide fuel cells: a brief review. J Power Sources 298:46–67. https://doi.org/10.1016/j.jpowsour.2015.08.034
Wang B, Long G, Ji Y, Pang M, Meng X (2014) Layered perovskite PrBa0.5Sr0.5CoCuO5+δ as a cathode for intermediate-temperature solid oxide fuel cells. J Alloys Compd 606:92–96. https://doi.org/10.1016/j.jallcom.2014.03.138
Jun A, Kim J, Shin J, Kim G (2016) Perovskite as a cathode material: a review of its role in solid-oxide fuel cell technology. ChemElectroChem 3(4):511–530. https://doi.org/10.1002/celc.201500382
Lee YL, Kleis J, Rossmeisl J, Shao-Horn Y, Morgan D (2011) Prediction of solid oxide fuel cell cathode activity with first-principles descriptors. Energy Environ Sci 4(10):3966–3970. https://doi.org/10.1039/C1EE02032C
Verduzco LE, Garcia-Díaz R, Martinez AI, Salgado RA, Méndez-Arriaga F, Lozano-Morales SA, Avendano-Alejo M, Padmasree KP (2020) Degradation efficiency of methyl orange dye by La0.5Sr0.5CoO3 perovskite oxide under dark and UV irradiated conditions. Dyes Pigm 183:108743. https://doi.org/10.1016/j.dyepig.2020.108743
Kim G, Wang S, Jacobson AJ, Reimus L, Brodersen P, Mims CA (2007) Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered A cations. J Mater Chem 17(24):2500–2505. https://doi.org/10.1039/B618345J
Jin F, Shen Y, Wang R, He T (2013) Double-perovskite PrBaCo2/3Fe2/3Cu2/3O5+δ as cathode material for intermediate-temperature solid-oxide fuel cells. J Power Sources 234:244–251. https://doi.org/10.1016/j.jpowsour.2013.01.172
Zhao L, Nian Q, He B, Lin B, Ding H, Wang S et al (2010) Novel layered perovskite oxide PrBaCuCoO5+δ as a potential cathode for intermediate-temperature solid oxide fuel cells. J Power Sources 195(2):453–456. https://doi.org/10.1016/j.jpowsour.2009.08.009
Suntsov AY, Leonidov IA, Markov AA, Patrakeev MV, Blinovskov YN, Kozhevnikov VL (2009) Oxygen nonstoichiometry and the thermodynamic and structural properties of double perovskites PrBaCo2–xCuxO5+δ. Russ J Phys Chem A 83:832–838. https://doi.org/10.1134/S0036024409050264
Jo K, Kim T, Ryu J, Noh T, Lee H (2020) Valence band structure and oxygen reduction reaction of Cu doped PrBaCo2O5+δ. Mater Lett 277:128399. https://doi.org/10.1016/j.matlet.2020.128399
Chen H, Lim C, Zhou M, He Z, Sun X, Li X, Ye Y, Tan T, Zhang H, Yang C, Han J, Chen Y (2021) Activating lattice oxygen in perovskite oxide by B-site cation doping for modulated stability and activity at elevated temperatures. Adv Sci 8(22):2102713. https://doi.org/10.1002/advs.202102713
Li Z, Li M, Zhu Z (2022) Perovskite cathode materials for low-temperature solid oxide fuel cells: fundamentals to optimization. Electrochem Energy Rev 5(2):263–311. https://doi.org/10.1007/s41918-021-00098-3
Suntsov AY, Leonidov IA, Patrakeev MV, Kozhevnikov VL (2015) Defect formation in double perovskites PrBaCo2−xCuxO5+δ at elevated temperatures. Solid State Ion 274:17–23. https://doi.org/10.1016/j.ssi.2015.02.004
Wang X, Huang K, Yuan L, Xi S, Yan W, Geng Z, Cong Y, Sun Y, Tan H, Wu X, Li L, Feng S (2018) Activation of surface oxygen sites in a cobalt-based perovskite model catalyst for CO oxidation. J Phys Chem Lett 9(15):4146–4154. https://doi.org/10.1021/acs.jpclett.8b01623
Tafaroji S, Farbod M, Kazeminezhad I, Kheirmand M (2019) Effect of pre-sintering temperature and ball-milling on the conductivity of Ba0.5Sr0.5Co0.8Fe0.2O3-δ as a cathode for solid oxide fuel cells prepared by sol–gel thermolysis method. Mater Res Express 6(9):095522. https://doi.org/10.1088/2053-1591/ab3265
Mueller DN, Machala ML, Bluhm H, Chueh WC (2015) Redox activity of surface oxygen anions in oxygen-deficient perovskite oxides during electrochemical reactions. Nat Commun 6(1):1–8. https://doi.org/10.1038/ncomms7097
Padilla-Pantoja J, Herrero-Martín J, Gargiani P, Valvidares SM, Cuartero V, Kummer K, Watson O, Brookes NB, García-Muñoz JL (2014) Stability of the cationic oxidation states in Pr0.50Sr0.50CoO3 across the magnetostructural transition by X-ray absorption spectroscopy. Inorg Chem 53(17):8854–8858. https://doi.org/10.1021/ic403117j
Suntivich J, Hong WT, Lee YL, Rondinelli JM, Yang W, Goodenough JB, Dabrowski B, Freeland WJ, Shao-Horn Y (2014) Estimating hybridization of transition metal and oxygen states in perovskites from Ok-edge X-ray absorption spectroscopy. J Phys Chem C 118(4):1856–1863. https://doi.org/10.1021/jp410644j
Li X, Sun Y, Ren F, Bai Y, Cheng Z (2021) Smart oxygen vacancy engineering to enhance water oxidation efficiency by separating the different effects of bulk and surface vacancies. Mater Today Energy 19:100619. https://doi.org/10.1016/j.mtener.2020.100619
Zhao J, Liu C, Li J, Wu R, Wang J, Qian H, Guo H, Li J, Ibrahim K (2019) Oxygen vacancy induced electronic structure variation in the La0.2Sr0.8MnO3 thin film. AIP Adv 9(5):055208. https://doi.org/10.1063/1.5088738
Karvonen L, Valkeapaa M, Liu RS, Chen JM, Yamauchi H, Karppinen M (2010) O-K and Co-L XANES study on oxygen intercalation in perovskite SrCoO3-δ. Chem Mater 22(1):70–76. https://doi.org/10.1021/cm9021563
Luo Y, Zheng Y, Feng X, Lin D, Qian Q, Wang X, Zhang Y, Chen Q, Zhang X (2020) Controllable P doping of the LaCoO3 catalyst for efficient propane oxidation: optimized surface Co distribution and enhanced oxygen vacancies. ACS Appl Mater Interfaces 12(21):23789–23799. https://doi.org/10.1021/acsami.0c01599
Kriventsov VV, Kochubey DI, Ismagilov ZR, Podyacheva OY, Nemudry AP (2005) EXAFS study of Nb doped Sr(Co/Fe)O3–x perovskites. Phys Scr T115:740. https://doi.org/10.1238/Physica.Topical.115a00740
Risch M, Grimaud A, May KJ, Stoerzinger KA, Chen TJ, Mansour AN, Shao-Horn Y (2013) Structural changes of cobalt-based perovskites upon water oxidation investigated by EXAFS. J Phys Chem C 117(17):8628–8635. https://doi.org/10.1021/jp3126768
Yang Q, Tian D, Liu R, Wu H, Chen Y, Ding Y et al (2021) Exploiting rare-earth-abundant layered perovskite cathodes of LnBa0.5Sr0.5Co1.5Fe0.5O5+δ (Ln = La and Nd) for SOFCs. Int J Hydrog Energy 46(7):5630–5641. https://doi.org/10.1016/j.ijhydene.2020.11.031
Acknowledgements
This work was supported by Korea Institute for Advancement of Technology grant funded by the Korea Government (MOTIE) (P0008335, The Competency Development Program for Industry specialist).
Author information
Authors and Affiliations
Contributions
Kanghee Jo was involved in conceptualization, Writing—original draft; Jiseung Ryu contributed to investigation, writing—original draft; Ilguk Jo was involved in writing—review and editing; Heesoo Lee contributed to supervision, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical approval
No experiments involving human tissue were conducted in current study.
Additional information
Handling Editor: Till Froemling.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jo, K., Ryu, J., Jo, I. et al. Orbital hybridization and polarization resistance of Cu-doped PrBaCo2O5+δ. J Mater Sci 58, 10677–10685 (2023). https://doi.org/10.1007/s10853-023-08699-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-023-08699-7