Abstract
There are various methods to introduce functional groups into the membrane surface to get oil–water separation performance, but most of them involve complicated reaction conditions and processes. Inspired by click chemistry, we developed a simple method to fabricate click chemistry-modified membranes. Firstly, polyacrylonitrile and γ-mercaptopropyltriethoxysilane were mixed together to prepare nanofiber membrane by electrostatic spinning method. Secondly, 3-[N,N-dimethyl-[2-(2-methylprop-2-enoyloxy)ethyl]ammonium]propane-1-sulfonate inner salt was grafted onto the membrane surface by thiol-ene click chemistry. The obtained modified membrane displays hydrophilicity and underwater oleophobicity. Driven by gravity at 1 kPa, the pure water flux of the membrane reached 787 L/(m2·h). Importantly, it also demonstrates good antifouling properties with a flux recovery rate of 94%, which is superior to the properties of most relative research. Therefore, this work provides a new idea to prepare hydrophilic nanofiber membrane that can be used in the field of oil–water separation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent decades, offshore oil spills, industrial wastewater and domestic sewage discharge have caused serious environmental pollution and even affected people's physical and mental health [1,2,3]. In the face of the increasingly severe wastewater pollution, it is particularly important to explore more efficient wastewater treatment methods. Generally, there are methods contain gravity separation [4], centrifugation [5], adsorption [6], flocculation [7], biodegradation [8], etc. However, these methods have certain limitations, including poor oil–water separation effect and susceptibility to secondary pollution, etc [9, 10]. Hence, the development of a new oil–water separation treatment method with economical preparation, high separation efficiency and stable repetitive utilization performance is an urgent need at present [11].
Aim to the oil–water separation issue, the membrane separation technology has been widely used due to its higher separation efficiency, lower cost and suitability for large scale production [12,13,14]. Many studies have been devoted to preparing oil–water separation membrane with high efficiency [15]. Among them, nanofiber membrane has proven to be beneficial for good oil–water separation with the advantages of large specific surface area, high porosity and small pore size [16]. The application of electrospun nanofiber membranes in the field of oil–water separation has been extremely progressive. However, there is an obvious contamination phenomenon during the use of separation membranes. This is due to the adsorption of pollutants on the membrane surface and in the pores during the separation process, resulting in a gradually reduction of the membrane flux as the use time increases, thus affecting the oil–water separation efficiency of the membrane and reducing the service life of the membrane [17,18,19,20]. Therefore, the exploration of superhydrophilic/underwater superoleophobic materials has attracted people's attention because of their good antifouling properties. For example, Wahid et al. [21] used a method of mixing nanofibers and silica particles, and modified the membrane surface with polydopamine to obtain a superhydrophilic/underwater superoleophobic separation membrane with a separation efficiency of up to 99.9%. Xie et al. [22] used a non-solvent induced phase separation method to graft [2-(methacryloyloxy) ethyl] dimethyl-(3-sulfopropyl) ammonium hydroxide (DMAPS) onto the surface of PVC membrane to improve its fouling resistance. The membrane has excellent membrane performance, and its flux recovery rate was as high as 86.4% after 7 h of fouling test, having an obvious improvement of fouling resistance compared with the conventional membrane. Cao et al. [23] had successfully prepared PA6-rGO nanofiber membranes by electrospinning and electrospray technology. The membrane has good separation performance for emulsions by gravity, and it is the emulsion flux can reach 765.4 L/(m2·h), with an interception rate of 99.6%, which can realize the preliminary separation of heavy oily sewage. To date, a variety of polymers such as PVDF [24], polysulfone [25], polypropylene [26] and polyacrylonitrile [27] has been prepared by electrostatic spinning processes to produce nanofiber membranes for oil–water separation.
Polyacrylonitrile (PAN) is one kind of polymer with acrylonitrile as the repeating unit. PAN fibers have been widely used in the preparation of separation membranes because of their chemical stability, thermal stability and excellent water wettability. Although PAN itself has a certain degree of hydrophilicity, its antifouling performance cannot meet the requirements, especially in the practical applications. Therefore, it is necessary to make further hydrophilic modification to achieve the goal of hydrophilicity and antifouling performance [28]. It is well known that hydrophilic modification can be carried out by physical and chemical methods. The physical methods include surface coating and blending, which has the problems of uneven modification or poor service life. The chemical methods involve hydrophilic grafting to polymers through chemical graft. After the successful modification, the oil–water emulsion can be readily separated since a large number of water molecules will form a hydration layer on the surface of the membrane, which will prevent the adsorption and accumulation of oily substances, thereby improving the antifouling performance of the membrane. Shen et al. [29] used a chemical grafting method to graft hydrophilic short-chain arginine molecules to the surface of polyacrylonitrile/polyacrylonitrile-polyglycidyl methacrylate, and the surface of the modified membrane was hydrophilic. The oil-repellent rate of the membrane is as high as 99.2%, and the flux recovery rate of the membrane still reaches 88.8% after the two-cycle filtration of pure water and oil–water emulsion.
Compared with other methods, surface grafting is easily conducted to endow the membrane with long-term hydrophilicity and antifouling properties. Surface grafting includes plasma induction, free radical graft polymerization, and atom transfer radical polymerization (ATRP) and click chemistry. Among them, click chemistry is a controllable and efficient synthesis reaction, and it can be realized with high efficiency under common conditions [30, 31]. Thiol-ene click chemistry was discovered by Posner in 1905. In this reaction, the addition reaction of thiol and double bond occurs through photoinitiation or thermal initiation [32, 33]. The reaction conditions are simple, the reaction rate is fast, and the product yield is high. Thus, it has been extensively applied to surface grafting nowadays in biological and medical fields, while in the nanofiber membrane modification is seldom reported. For example, Fang et al. [34] introduced mercaptopropyltriethoxysilane into superhydrophobic fabrics to obtain mercaptans. The fabric was grafted with methacryloxypropyltrimethoxysilane modified silica@ Fe3O4 nanoparticles by UV light-initiated thiol-ene click chemistry, the modified fabric exhibits excellent superhydrophobicity, high separation efficiency, high permeation flux and excellent recyclability. We propose here that the successful utilization of thiol-ene click chemistry in this topic will lead to new ideas for the surface functionalization of nanofiber membranes.
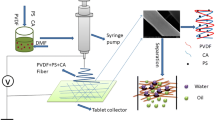
In this work, PAN and γ-mercaptopropyltriethoxysilane (MPTES) are firstly blended and then electrospun into a nanofiber membrane containing sulfhydryl groups, and these embedded seeds will react with 3-[N,N- Dimethyl-[2-(2-methylprop-2-enoyloxy)ethyl]ammonium]propane-1-sulfonic acid inner salt (SPE) in the following step to produce functional nanofiber membrane with robust hydrophilic performance, and the whole preparation process is shown in Fig. 1. The surface morphology of the membrane before and after modification was observed, the surface wettability of the membrane was measured by the contact angle. The experiment was performed by applying a pressure of 1 kPa under the action of gravity to evaluate the separation performance and antifouling performance of the membrane. We hope that this work can provide new ideas and insights for further development in the field of oil–water separation.
Experimental
Materials
DI water was used throughout the experiment. Polyacrylonitrile (PAN, 150000Mw) was provided by China National Pharmaceutical Group Co., Ltd. γ-mercaptopropyltriethoxysilane (KH580) was supplied by China National Pharmaceutical Group Co., Ltd. N, N-Dimethylformamide was purchased from Wendong (Shanghai) Chemical Co., Ltd. Triethylamine was purchased from China National Pharmaceutical Group Co., Ltd. Ethanol was purchased from China National Pharmaceutical Group Co., Ltd. 3-[N, N- Dimethyl-[2-(2-methylprop-2-enoyloxy)ethyl]ammonium]propane-1-sulfonate was purchased from Wendong (Shanghai) Chemical Co., Ltd. Sodium dodecyl sulfate was purchased from Beijing Yinuokai Technology Co., Ltd. All the above chemicals can be used directly without further purification.
Preparation of nanofiber membrane MPTES-PAN
Generally, 1 g of polyacrylonitrile powder was added to 9 g of N, N-dimethylformamide (DMF) solution and then stirred under magnetic stirring for 12 h until the polyacrylonitrile was completely dissolved in the solvent, and then 10 wt% of MPTES were added and stirred for a period of time and then ultrasonically dispersed until MPTES was completely dispersed in the solution. Subsequently, the electrostatic spinning solution was loaded into a syringe with a 19 G stainless steel needle. The solution was advanced at a constant speed of 1 ml/h. The receiving distance was fixed at 18 cm, the working voltage was constant at 15 kV and the constant speed of the rotating collector was 150 rpm. The atmospheric temperature and humidity were about 18 °C and 50%, respectively. PAN membranes were prepared under the same process conditions.
Preparation of treatment solution
First, 5 ml of deionized water and 5 ml of anhydrous ethanol were mixed, and then 10 wt% of 3-[N, N-Dimethyl-[2-(2-methylprop-2-enoyloxy)ethyl]ammonium]propane-1 -sulfonate was added and stirred for 10 min until it was completely dissolved. Next, 2 wt% of triethylamine was added to the solution and was ultrasonically dispersed for 60 min.
Preparation of modified membrane
The MPTES-PAN membrane was immersed in the solution and treated at 70 °C for 40 min. After the reaction, it was washed several times with deionized water until the unreacted materials on the membrane surface were washed away, and then was placed in an oven to dry for later use. The reaction principle is shown in Fig. 1.
Characterization
The morphology of the membrane samples was performed by scanning electron microscopy (SEM, TM-1000, Hitachi, Japan). The functional groups were analyzed by Fourier-transform infrared spectroscopy (Nicolet 6700, Thermo Fisher, America). Detection of sulfhydryl peaks of samples by Raman tester (inVia-Reflexc). The hydrophilicity of the membrane was measured by contact Angle measuring instrument (Kruss DSA30, Germany). The permeability of the membrane was tested by water management tester (MMT), according to the AATCC test method 195-2009. The thermal properties of the membrane were analyzed by thermogravimetric analyzer (TG209F1). A total organic carbon analyzer (Multi N/C 3100) was used to analyze the oil concentration in the filtrate. An ultra-deep field 3D microscope (VHX-6000) was used to observe the oil droplet distribution of emulsions and filtrates. The particle size and distribution of the oil droplets are tested by a laser particle size analyzer (LS13320, Beckman Coulter). Analysis of the elemental composition of the membrane surface by X-ray photoelectron spectroscopy (XPS, Escalab 250Xi, USA).
Membrane water flux test
The pure water flux was measured by a glass sand core filter device. The membrane was placed on the filter and deionized water was added to the filter until the liquid level reaches 10 cm. At this time, the pressure on the membrane is 1 kPa. The membrane pressure was kept constant during the test. The membrane permeate flux over 5 min was calculated by the following equation:
Among them, J is the permeation flux L/(m2·h), V is the volume of permeated water (L), A is the effective membrane area (m2), and Δt is the measurement time (h).
Separation experiment of oil-in-water emulsion
Petroleum ether, edible oil, n-hexane and toluene were used as contaminants, which were added to deionized water to prepare oil-in-water emulsions with and without surfactant to measure the separation efficiency, and sodium dodecyl sulfate was used as surfactant. In the oil-in-water emulsion, the oil was mixed with deionized water at a ratio of 1:99 (v/v), and the concentration of sodium dodecyl sulfate was about 0.1 mg/ml. The mixture was stirred at a high speed of 10,000 rpm for 1 min with a high-speed disperser internal cutting homogenizer, and then the mixture was dispersed ultrasonically for 5 h until the emulsion was stable. The same preparation process was adopted for the preparation of emulsion without surfactant. A glass sand core filter device is used for oil–water separation. The membrane was placed on a filter with an effective area of 11.95 cm2, the membrane was wetted with deionized water, and then the emulsion was introduced into the filter until the liquid level reached 10 cm, and the liquid level was maintained during the separation process. The amount of water collected in 5 min was calculated as the permeate flux. The separation efficiency was calculated by the following equation:
Among them, the oil retention rate at R, C1 and C0 is the oil concentration of the penetrating emulsion and the original emulsion, respectively. The experiment was carried out at room temperature.
Membrane antifouling test
The membrane was fixed in the filter device, and then pure water was poured into the device. After the water was filtered, the surfactant-free emulsions were poured into the device and filtered. After filtration, the membrane was cleaned by deionized water, and then the pure water flux was measured again under the same pressure, and the water flux recovery rate (FR) was calculated according to the following equation:
where J0 and JX are the pure water fluxes before and after membrane fouling, respectively.
Results and discussion
Membrane morphology and thermal properties
MPTES-PAN nanofiber membranes were successfully prepared by electrospinning PAN/MPTES/N, N-dimethylformamide solution. The addition of MPTES to the spinning solution allows the spinning of PAN fiber membranes containing thiol groups for the subsequent click chemistry reactions. The SEM photograph and fiber diameter distribution of the PAN nanofiber membrane are shown in Fig. 2. It can be clearly observed that the average diameter of PAN fibers in the three pictures does not change significantly, and the fiber diameter is mainly distributed around 300 nm [35]. The fiber diameter is not affected by the addition of the silane coupling agent, but there are beads on the fiber in Fig. 2(b). This is due to the presence of MPTES increases the surface tension of the solvent in the electrospinning jet, which tends to form a spherical shape on the fiber [14, 36]. After clicking the chemical reaction, the beads on the fiber are reduced, and the fiber itself has no effect. Compared with the original PAN membrane, the color and appearance of the modified film did not change significantly.
a–c Scanning electron microscopy images of the polyacrylonitrile membrane before and after modification at 5000× magnification. The insets in a–c show the fiber diameter distribution of polyacrylonitrile fibers before and after modification. d TG curves of polyacrylonitrile membranes before and after modification
The thermal stability of the polyacrylonitrile fiber membrane before and after modification was analyzed by TG test [37, 38], the heating rate was 10 °C/min, the test was carried out in an air environment, and the temperature rose to 600 °C. As shown in Fig. 2d. The first weight loss stage of polyacrylonitrile occurs at 250–330 °C. There is only a small mass loss at this stage, which is mainly low molecular weight toxic gas released by the cleavage and decomposition of polyacrylonitrile side groups and end groups. The weight loss rate of PAN fiber membrane before and after modification is not much different. With the increase of temperature, the second weight loss phase was entered. As can be seen, the weight loss of MPTES-PAN was much higher than that of PAN and SPE-PAN, it is due to the early degradation of MPTES resulting in a lower initial degradation temperature of MPTES-PAN fiber membrane than that of PAN. Compared with MPTES-PAN membrane, the weight loss of SPE-PAN membrane was reduced, but still higher than PAN membrane due to the sulfonic acid group on SPE decomposes with increasing temperature to produce acid, which inhibits the thermal decomposition of PAN membrane. In summary, the thermal stability of the blended modified PAN membrane is lower than that of the original PAN membrane, but it still has good thermal stability, and the thermal stability below 200 °C is similar, indicating there will be no significant impact on daily use.
Chemical structure of membranes
In order to further prove the existence of sulfhydryl groups, Fourier infrared spectroscopy was used for analysis. In Fig. 3a, the characteristic peak of -SH was not displayed. The reason is the content of sulfhydryl groups on the membrane is too small to be observed. In order to characterize it, we measured it with a Raman tester. It can be seen from Fig. 3b that there are sulfhydryl groups on the blended MPTES-PAN membrane, but there are no sulfhydryl groups on the modified SPE-PAN membrane. At the same time, the membrane was tested with Elman's reagent. As shown in Fig. 3b1-2, the MPTES-PAN membrane has an obvious yellow color, and the SPE-PAN membrane has no yellow color, which proves that the sulfhydryl group and PAN blend successfully.
To further demonstrate the completion of the reaction, the SPE-PAN membrane was characterized by XPS and the corresponding plots are shown in Fig. 3c, d. Figure 3c shows that the SPE-PAN membrane contains S, Si and other elements. The presence of C–S/C–O bond at 286.4 eV and the presence of C=O bond at 288 eV can be seen in Fig. 3d. Because of the presence of ester groups on the SPE molecule, the thiol-ene click chemistry proved to be successful.
Wettability of membrane surface
The wettability of the membrane surface is a key factor for separating oil–water emulsions. The oleophobicity and separation efficiency of the membrane mainly depend on the wettability of the membrane surface [39, 40]. The number of hydrophilic groups on the surface of the fiber affects the wetting performance of the fiber. With the increase of the hydrophilic groups, the rate of wetting of the fiber on the surface of the membrane also increases. Since SPE is a molecule containing zwitterions and can be dissolved in water, when grafted onto the surface of the fiber, the hydrophilicity of the fiber has a dramatically improvement, and the droplets can wet the fiber faster at the same time. The wettability of the PAN membrane before and after the modification was evaluated by the water contact angle [41]. PAN itself has low hydrophilic, with a water contact angle (WAC) of less than 90° and only 78° at room temperature, as can be seen in Fig. 4a, the water droplets start with a droplet-like appearance on the membrane surface, followed by complete wetting within 7 s. The blending of PAN and MPTES improved the hydrophilicity of PAN membrane to some extent, and the water contact angle of the blended PAN membrane was reduced to 39°, water droplets completely wetted the MPTES-PAN film within 3 s (Fig. 4a). Then the zwitterions are grafted by click chemistry method to greatly improve the hydrophilicity of the SPE-PAN membrane. As shown in Fig. 4c, the contact angle could reach 23°, and the time for the membrane to be wetted was reduced to within 1 s. This means that the modified membrane has better hydrophilicity and can better separate oil–water emulsions.
The underwater oleophobicity of the membrane is also an important factor in measuring its oil–water separation performance. Figure 4b shows the underwater oleophobicity of the membrane, where the oil droplets do not spread out on the membrane surface and do not wet the membrane surface. The underwater oil contact angles (UOCAs) measured over a period of time are shown in Fig. 4d. The UOCAs measured on the fiber membranes were all above 130°, and the underwater oil repellency of the SPE-PAN membranes was further demonstrated.
Permeability of membranes
The permeability of the membrane was evaluated by using the moisture absorption level of the membrane, in which the dynamic response of liquid moisture within 2 min was recorded [42]. The MMT results in Table 1 summarize the differences in the performance of the samples in terms of wetting time (WT), absorption rate (AR), spreading speed (SS), maximum wetting radius (MWR) and unidirectional transport capacity (OWTC) on the top and bottom surfaces of the membranes. Comparing the data in the table, it can be seen that the wettability of the blended MPTES-PAN membrane has a large improvement compared with the original PAN membrane, and the membrane can be directly wetted within 0.324 s. In addition, the modified SPE-PAN membrane has improved unidirectional transport ability compared with MPTES-PAN, and the wetting time is the same with it, this can be more intuitively seen through Fig. 5a. This shows that compared with the pristine PAN membrane, MPTES-PAN and SPE-PAN have a great improvement in the wetting speed, and the wetting time is shortened, and the liquid moisture can penetrate from one side to the other side in the shortest time, which indicates that the wetting property of the blended and modified PAN membrane is greatly enhanced. The above MMT test results show that the modified PAN membrane has good water management performance and meets the requirements in oil–water separation.
Water flux analysis
After five cycles of measurement, the pure water flux results of the membranes before and after modification are shown in Fig. 5b. It can be seen that the water flux of the modified SPE-PAN membrane is significantly increased compared with the MPTES-PAN membrane and the PAN membrane. Because of the hydrophilicity of the membrane, water can quickly penetrate the membrane under the drive of gravity. It can be clearly seen that the water flux of the blended MPTES-PAN membrane is only 542.6 L/(m2·h). The low flux may also be caused by the reduced pore size of the MPTES-PAN membrane. The pore size of the membrane is one of the factors affecting the membrane performance [43, 44]. However, the pore size of the membrane did not change much before and after the modification as measured by capillary flow porosimetry. This is because the pore size of the fiber membrane has not changed, but the introduction of hydrophilic groups greatly increases the hydrophilic properties of the membrane, so water molecules can penetrate the membrane faster.
Durability test
The durability of the membrane was characterized by subjecting it to a harsh environment for 24 h and measuring the change in its underwater oleophobic angle. The membranes were immersed in hydrochloric acid (pH = 1), sodium hydroxide solution (pH = 14) and toluene organic solution for 24 h, respectively. Subsequently, its underwater oil contact angle was measured, and it can be seen from Fig. 5d that the UOCAs of the membrane were not affected and remained at a high level even under the harsh environment, which shows that the membrane has good durability.
Considering the problem of clogging of the filter during use, the durability of the filter was also tested. Before the penetration test, the amount of pure water passing through the filter within 1 min was 91.6 ml. Subsequently, the membrane was used for the emulsion separation test. After each separation, the filter was cleaned. After five cycles, the amount of pure water passing through the filter in 1 min was measured to be 90.1 ml. It can be seen that the filter also has good durability.
Separation performance of oil-in-water emulsion
SPE-PAN membrane has extremely strong hydrophilic properties and great application prospects in the field of oil–water separation. As shown in Fig. 5c, water can pass through the SPE-PAN membrane, while the oil stays on the surface of the membrane, and the oil (dyed with oil red) on the membrane still cannot penetrate down even if the pressure increases. Eight different oil-in-water emulsions were prepared by using petroleum ether, edible oil, n-hexane and toluene, respectively, while classified into surfactant-stabilized and surfactant-free classes. The filtrates obtained by separation are shown in Fig. 6. Taking the surfactant-free emulsions as an example, their particle size and distribution are displayed in Fig. 6. We can see that the particle size distribution of edible oil emulsion is mainly around 1 micron, while the particle size of organic solvent emulsion is relatively larger. The droplets before and after the separation can also be observed through a super-depth of field three-dimensional microscope. It can be seen that there are a large number of oil droplets in the four emulsions, and there are only a small amount of oil droplets in the filtrate, which shows that the modified membrane has a good separation effect.
The separation of the above various emulsions yielded the following results, as shown in Fig. 7a. It can be seen that the separation of emulsions surfactant-free has a good permeate flux, which is due to the presence of surfactants that adsorb oil droplets on the hydrated layer, which leads to a decrease in permeate flux. Moreover, after several cycles of separation tests, the SPE-PAN membrane still has good separation efficiency, as shown in Fig. 7b. The carbon content of the emulsions and filtrates were tested by TOC, and it can be seen from Fig. 7c that the separation efficiency of edible oil emulsion is the lowest. This is because the particle size of edible oil emulsion is mainly distributed below 1 micron compared with other emulsions, and more oil droplets permeate out through the membrane pores.
Antifouling performance of SPE-PAN membrane
Compared with the traditional membrane separation process, the gravity-driven microfiltration membrane not only saves energy, but also reduces the membrane fouling caused by the clogging of the membrane pores [45]. During the filtration process, the surface of the SPE-PAN membrane exhibits strong hydrophilicity, which can capture water molecules, form a hydration layer on the membrane surface, while repelling the oil droplets on the membrane surface, and therefore, have a good oil–water separation performance. Due to the phenomenon of electrostatic adsorption during gravity driving, a small amount of oil droplets will pass through the porous membrane, which will affect the efficiency of oil–water separation [46].
Emulsions using sodium dodecyl sulfate as a surfactant was used to study the antifouling performance of SPE-PAN membrane. Since the hydration layer formed by zwitterions adsorbing water molecules on the surface of the fiber membrane will prevent the contaminants from adhering to the fiber, the SPE-PAN membrane has achieved antipollution effect [47]. As a result, the oil droplets accumulated on the membrane surface are easily washed away by water. Therefore, the flux recovery rate can reach up to 94%, due to the low separation efficiency of the edible oil emulsion, the flux recovery rate is also relatively low, as shown in Fig. 7d. The result indicates that the PAN electrospun membrane prepared by thiol-ene click chemistry has an excellent separation efficiency with good cycling stability.
Conclusions
In summary, we have proposed a simple and efficient method to prepare hydrophilic modified PAN membranes. In this study, by blending PAN and MPTES, the electrospun microfiber membrane is rich in sulfhydryl groups, so that SPE can be grafted by click chemistry to give the fiber membrane excellent hydrophilicity. The hydrophilic performance of the modified PAN membrane is significantly improved, with a lower water contact angle and a higher underwater oil contact angle, and the pure water flux is 787.2 L/(m2·h). At the same time, the membrane shows good separation efficiency and good antifouling performance, which is the bottleneck problem of most hydrophilic membrane. Therefore, we can see the introduction of thiol-ene click chemistry can effectively solve this problem and provide a new idea for the design of efficient oil–water separation membranes.
References
Jia YD, Guan KC, Zhang L et al (2021) Enabling polyketone membrane with underwater superoleophobicity via a hydrogel-based modification for high-efficiency oil-in-water emulsion separation. J Membr Sci 618:10. https://doi.org/10.1016/j.memsci.2020.118705
Zhang J, Liu L, Si Y, Yu J, Ding B (2021) Rational design of electrospun nanofibrous materials for oil/water emulsion separation. Mater Chem Front 5:97. https://doi.org/10.1039/d0qm00436g
Bolto B, Zhang JH, Wu X, Xie ZL (2020) A review on current development of membranes for oil removal from wastewaters. Membranes 10:18. https://doi.org/10.3390/membranes10040065
Maggay IV, Chang Y, Venault A, Dizon GV, Wu CJ (2021) Functionalized porous filtration media for gravity-driven filtration: Reviewing a new emerging approach for oil and water emulsions separation. Sep Purif Technol 259:17. https://doi.org/10.1016/j.seppur.2020.117983
Niu HF, Qiang Z, Ren J (2021) Durable, magnetic-responsive melamine sponge composite for high efficiency, in situ oil-water separation. Nanotechnology 32:11. https://doi.org/10.1088/1361-6528/abef2e
Karki HP, Kafle L, Ojha DP, Song JH, Kim HJ (2018) Three-dimensional nanoporous polyacrylonitrile-based carbon scaffold for effective separation of oil from oil/water emulsion. Polymer 153:597. https://doi.org/10.1016/j.polymer.2018.08.069
Putatunda S, Bhattacharya S, Sen D, Bhattacharjee C (2019) A review on the application of different treatment processes for emulsified oily wastewater. Int J Environ Sci Technol 16:2525. https://doi.org/10.1007/s13762-018-2055-6
Ao CH, Zhao JQ, Li QY et al (2020) Biodegradable all-cellulose composite membranes for simultaneous oil/water separation and dye removal from water. Carbohydr Polym 250:11. https://doi.org/10.1016/j.carbpol.2020.116872
Zhang Y, Ye L, Zhang B et al (2019) Characteristics and performance of PVDF membrane prepared by using NaCl coagulation bath: relationship between membrane polymorphous structure and organic fouling. J Membr Sci 579:22. https://doi.org/10.1016/j.memsci.2019.02.054
Zhao Y, Yang X, Yan L et al (2021) Biomimetic nanoparticle-engineered superwettable membranes for efficient oil/water separation. J Membr Sci 618:118525. https://doi.org/10.1016/j.memsci.2020.118525
Zhang Y, Wang H, Wang X, Liu B, Wei Y (2021) An anti-oil-fouling and robust superhydrophilic MnCo2O4 coated stainless steel mesh for ultrafast oil/water mixtures separation. Sep Purif Technol 264:118435. https://doi.org/10.1016/j.seppur.2021.118435
Shen K, Cheng C, Zhang T, Wang X (2019) High performance polyamide composite nanofiltration membranes via reverse interfacial polymerization with the synergistic interaction of gelatin interlayer and trimesoyl chloride. J Membr Sci 588:117192. https://doi.org/10.1016/j.memsci.2019.117192
Baggio A, Doan HN, Vo PP et al (2021) Chitosan-functionalized recycled polyethylene terephthalate nanofibrous membrane for sustainable on-demand oil-water separation. Glob Chall 5:10. https://doi.org/10.1002/gch2.202000107
Dehkordi TF, Shirin-Abadi AR, Karimipour K, Mahdavian AR (2021) CO2-, electric potential-, and photo-switchable-hydrophilicity membrane (x-SHM) as an efficient color-changeable tool for oil/water separation. Polymer 212:9. https://doi.org/10.1016/j.polymer.2020.123250
Zhang J, Liu L, Si Y, Yu J, Ding B (2020) Electrospun nanofibrous membranes: an effective arsenal for the purification of emulsified oily wastewater. Adv Funct Mater 30:2002192. https://doi.org/10.1002/adfm.202002192
Hou J, Park C, Jang W, Byun H (2021) Facile fabrication and characterization of aliphatic polyketone (PK) micro/nano fiber membranes via electrospinning and a post treatment process. RSC Adv 11:678. https://doi.org/10.1039/d0ra08119a
Gou X, Zhang Y, Long L et al (2020) Superhydrophilic and underwater superoleophobic cement-coated mesh for oil/water separation by gravity. Colloids Surf Physicochem Eng Aspects 605:125338. https://doi.org/10.1016/j.colsurfa.2020.125338
Helali N, Rastgar M, Farhad Ismail M, Sadrzadeh M (2020) Development of underwater superoleophobic polyamide-imide (PAI) microfiltration membranes for oil/water emulsion separation. Sep Purif Technol 238:116451. https://doi.org/10.1016/j.seppur.2019.116451
Liu M, Wang S, Wei Z, Song Y, Jiang L (2009) Bioinspired design of a superoleophobic and low adhesive water/solid interface. Adv Mater 21:665. https://doi.org/10.1002/adma.200801782
Waghmare PR, Gunda NSK, Mitra SK (2015) Under-water superoleophobicity of fish scales. Sci Rep 4:7454. https://doi.org/10.1038/srep07454
Wahid F, Zhao XJ, Duan YX, Zhao XQ, Jia SR, Zhong C (2021) Designing of bacterial cellulose-based superhydrophilic/underwater superoleophobic membrane for oil/water separation. Carbohydr Polym 257:117611. https://doi.org/10.1016/j.carbpol.2020.117611
Xie W, Tiraferri A, Ji X et al (2021) Green and sustainable method of manufacturing anti-fouling zwitterionic polymers-modified poly(vinyl chloride) ultrafiltration membranes. J Colloid Interface Sci 591:343. https://doi.org/10.1016/j.jcis.2021.01.107
Cao M, Chen YB, Huang XJ et al (2021) Construction of PA6-rGO nanofiber membrane via electrospraying combining electrospinning processes for emulsified oily sewage purification. J Taiwan Inst Chem Eng 118:232. https://doi.org/10.1016/j.jtice.2021.01.019
Obaid M, Mohamed HO, Yasin AS et al (2017) Under-oil superhydrophilic wetted PVDF electrospun modified membrane for continuous gravitational oil/water separation with outstanding flux. Water Res 123:524. https://doi.org/10.1016/j.watres.2017.06.079
Obaid M, Yang E, Kang D-H, Yoon M-H, Kim IS (2018) Underwater superoleophobic modified polysulfone electrospun membrane with efficient antifouling for ultrafast gravitational oil-water separation. Sep Purif Technol 200:284. https://doi.org/10.1016/j.seppur.2018.02.043
Hao J, Fan Z, Xiao C, Zhao J, Liu H, Chen L (2017) Effect of stretching on continuous oil/water separation performance of polypropylene hollow fiber membrane. Iran Polym J 26:941. https://doi.org/10.1007/s13726-017-0566-5
Makaremi M, De Silva RT, Pasbakhsh P (2015) Electrospun nanofibrous membranes of polyacrylonitrile/halloysite with superior water filtration ability. J Phys Chem C 119:7949. https://doi.org/10.1021/acs.jpcc.5b00662
Shen X, Liu P, He CX et al (2021) Surface PEGylation of polyacrylonitrile membrane via thiol-ene click chemistry for efficient separation of oil-in-water emulsions. Sep Purif Technol 255:14. https://doi.org/10.1016/j.seppur.2020.117418
Shen X, Liu P, Xu J et al (2018) Covalent immobilization of arginine onto polyacrylonitrile-based membrane for the effective separation of oil/water emulsion. Macromol Res 26:1241. https://doi.org/10.1007/s13233-019-7012-9
Xue CH, Guo XJ, Zhang MM, Ma JZ, Jia ST (2015) Fabrication of robust superhydrophobic surfaces by modification of chemically roughened fibers via thiol–ene click chemistry. J Mater Chem A 3:21797. https://doi.org/10.1039/c5ta04802h
Yuan T, Meng J, Hao T, Zhang Y, Xu M (2014) Polysulfone membranes clicked with poly (ethylene glycol) of high density and uniformity for oil/water emulsion purification: effects of tethered hydrogel microstructure. J Membr Sci 470:112. https://doi.org/10.1016/j.memsci.2014.07.013
Wang B, Gao C, Huang Y et al (2021) Preparation of superhydrophobic nylon-56/cotton-interwoven fabric with dopamine-assisted use of thiol–ene click chemistry. RSC Adv 11:10699. https://doi.org/10.1039/d1ra00410g
Fattah TA, Saeed A, Albericio F (2018) Recent advances towards sulfur (VI) fluoride exchange (SuFEx) click chemistry. J Fluorine Chem 213:87. https://doi.org/10.1016/j.jfluchem.2018.07.008
Fang Y, Liu C, Li M et al (2020) Facile generation of durable superhydrophobic fabrics toward oil/water separation via thiol-ene click chemistry. Ind Eng Chem Res 59:6130. https://doi.org/10.1021/acs.iecr.9b06761
Jun I, Han HS, Edwards JR, Jeon H (2018) Electrospun fibrous scaffolds for tissue engineering: viewpoints on architecture and fabrication. Int J Mol Sci 19:14. https://doi.org/10.3390/ijms19030745
Tiwari SK, Venkatraman SS (2012) Importance of viscosity parameters in electrospinning: of monolithic and core-shell fibers. Mater Sci Eng C 32:1037. https://doi.org/10.1016/j.msec.2012.02.019
Surianarayanan M, Vijayaraghavan R, Raghavan KV (1998) Spectroscopic investigations of polyacrylonitrile thermal degradation. J Appl Polym Sci 36:2503. https://doi.org/10.1002/(sici)1099-0518(199810)36:14%3c2503::Aid-pola9%3e3.3.Co;2-I
Martin SC, Liggat JJ, Snape CE (2001) In situ NMR investigation into the thermal degradation and stabilisation of PAN. Polym Degrad Stab 74:407. https://doi.org/10.1016/s0141-3910(01)00173-2
Wang K, Zhang TC, Wei BB, Chen SX, Liang Y, Yuan SJ (2021) Durable CNTs reinforced porous electrospun superhydrophobic membrane for efficient gravity driven oil/water separation. Colloids Surf Physicochem Eng Aspects 608:9. https://doi.org/10.1016/j.colsurfa.2020.125342
Zhu YZ, Wang D, Jiang L, Jin J (2014) Recent progress in developing advanced membranes for emulsified oil/water separation. NPG Asia Mater 6:11. https://doi.org/10.1038/am.2014.23
Ao CH, Zhao JQ, Xia T et al (2021) Multifunctional La(OH)3@cellulose nanofibrous membranes for efficient oil/water separation and selective removal of dyes. Sep Purif Technol 254:10. https://doi.org/10.1016/j.seppur.2020.117603
Zhao K, Wang Y, Wang W, Yu D (2018) Moisture absorption, perspiration and thermal conductive polyester fabric prepared by thiol-ene click chemistry with reduced graphene oxide finishing agent. J Mater Sci 53:14262. https://doi.org/10.1007/s10853-018-2671-z
Shakiba M, Nabavi SR, Emadi H, Faraji M (2021) Development of a superhydrophilic nanofiber membrane for oil/water emulsion separation via modification of polyacrylonitrile/polyaniline composite. Polym Adv Technol 32:1301. https://doi.org/10.1002/pat.5178
Zang LL, Zheng SX, Wang LB, Ma J, Sun LG (2020) Zwitterionic nanogels modified nanofibrous membrane for efficient oil/water separation. J Membr Sci 612:8. https://doi.org/10.1016/j.memsci.2020.118379
Zhang TH, Zhang CY, Zhao GQ et al (2020) Electrospun composite membrane with superhydrophobic-superoleophilic for efficient water-in-oil emulsion separation and oil adsorption. Colloids Surf Physicochem Eng Aspects 602:11. https://doi.org/10.1016/j.colsurfa.2020.125158
Guo JW, Wang CF, Chen SH, Lai JY, Lu CH, Chen JK (2020) Highly efficient self-cleaning of heavy polyelectrolyte coated electrospun polyacrylonitrile nanofibrous membrane for separation of oil/water emulsions with intermittent pressure. Sep Purif Technol 234:15. https://doi.org/10.1016/j.seppur.2019.116106
Ying T, Su JF, Jiang YJ, Ke QF, Xu H (2020) A pre-wetting induced superhydrophilic/superlipophilic micro-patterned electrospun membrane with self-cleaning property for on-demand emulsified oily wastewater separation. J Hazard Mater 384:11. https://doi.org/10.1016/j.jhazmat.2019.121475
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Gregory Rutledge.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shen, B., Du, C., Wang, W. et al. Hydrophilic SPE/MPTES-PAN electrospun membrane prepared via click chemistry for high efficiency oil–water separation. J Mater Sci 57, 1474–1488 (2022). https://doi.org/10.1007/s10853-021-06688-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06688-2