Abstract
UV-to-red downshifting phosphors such as BaAl2O4:Eu3+ find broad range of application in sensors, displays, and in solid-state lighting, yet new synthetic routes to improve their luminescence are envisaged. In this regard, herein, it is introduced two new methods to synthesize this environmentally friendly BaAl2O4:Eu3+, by an adapted sol–gel route and a modified Pechini synthesis. Additionally, a systematic study was carried out about the Eu3+ doping concentration and charge compensation effects on the structural, morphological and spectroscopic features. Both routes enabled high-crystalline and nanostructured phosphors displaying optic bandgap near to 4.4 eV, although the sol–gel route also led to low amounts of BaCO3 spurious phase. Upon UV (250 nm) excitation, all Eu3+-doped samples emit red light displaying high emission color purity, characteristic of the 5D0 → 7F0-4 electronic transitions of Eu3+. The Pechini method led to the highest intrinsic emission quantum yield (85% for the 3%-doped sample). Eu3+ replaces Ba2+ within the BaAl2O4 lattice, but in the sol–gel-derived samples, the dopant may also replace Ba2+ into the BaCO3 spurious phase, confirming that the Pechini route is the best one to optimize the luminescence and structure of the phosphor.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
UV-to-red downshifting emitting phosphors find a broad range of applications [1] in televisions [2, 3], solid-state lighting [4, 5], multifunctional light-emitting diodes [6, 7], field emission displays [2, 3], photonics, plasma display panels (PDPs) [2, 3], fiber amplifiers [8], optoelectronics for image storage [9], and detectors of energy radiation [9]. Although the main commercially available red-emitting phosphors, i.e., Zn1-xCdxS:Ag+ or Y2O2S:Eu3+, feature high overall emission quantum yield, some drawbacks must still be overcome, especially that related to their composition based on heavy metals and sulfur atom that is released over time of usage [3, 10]. In this regard, new strategies toward the synthesis of environmentally friendly red-emitting phosphors displaying intense luminescence are of large societal relevance.
Among several red-emitting systems, BaAl2O4:Eu3+ stands out due to its environmentally friendly properties, chemical, thermal, and photostability, relatively low cost of production, simplicity of the synthesis methods, and similarity between the ionic radius of Ba2+ and Eu3+, altogether making BaAl2O4 a desirable host. [11] Moreover, Eu3+ is a well-known luminescent activator characterized by narrow emission bands within the red spectral region that arise from intraconfigurational 4f → 4f electronic transitions [12]. Due to these features, several studies have shown the potential application of BaAl2O4:Eu3+ in solid-state lighting, scintillation, or dosimetry [13, 14]. Yet, some issues need to be addressed concerning the development of new synthetic strategies toward the enhancement of the Eu3+ luminescence and the charge imbalance between Eu3+ and Ba2+, which leads to structural defects that often quench the radiative process arising from the 5D0 emitting state of Eu3+. [15]
Some routes for the obtention of BaAl2O4:Eu3+ are reported so far, such as methods based on combustion [13, 16], conventional Pechini [11, 15], hydrothermal [17], floating zone [13], and protein [18,19,20,21] or polyvinyl alcohol [22]-assisted sol–gel. Moreover, methods applied for the synthesis of other Eu3+-doped aluminates are also reported, such as the combustion method used in the ZnAl2O4:Eu3+:Tb3+ synthesis [23] or the urea-assisted combustion approach for obtaining CaAl2O4:Eu3+,Er3+ [24]. Although some of these routes enable a quite good particle shape and size control, there is not a consensus on which synthesis is the best regarding the enhancement of the Eu3+ luminescence. In this regard, we introduce two new routes for the phosphor obtention based on a sol–gel route adapted from the Ba2SiO4:Eu3+ synthesis [25] and a modified Pechini method using sorbitol instead of the classic ethylene glycol as polymerizing agent, which enhances the Eu3+ luminescence according to our previous study for the Y2O3:Eu3+,Er3+,Yb3+ phosphor [26]. The justifications behind these selections lie in the relatively low cost, simplicity, achievement of high level of purity, well-crystallized phosphor, and decrease in the annealing temperature compared to solid-state synthesis [27, 28].
Therefore, in this study, BaAl2O4:Eu3+ red-emitting phosphors were synthesized by two adapted routes, the sol–gel and the Pechini method, by applying a charge compensation mechanism in the Eu3+ doping within the BaAl2O4 lattice. Furthermore, structural, morphological, and spectroscopic studies were performed to evaluate Eu3+ site occupancy, influence of concentration on structure and luminescence as well as the mechanism behind energy transfer among Eu3+ ions, which is responsible for luminescence quenching by concentration.
Materials and methods
Acetic acid (CH3COOH, Cinética, 99.7%), citric acid (C6H8O7, Fluka analytical, 99.5%), nitric acid (HNO3, FMaia, 65%), barium acetate (Ba(CH3COO)2, Vetek, 99%) aluminum nitrate (Al(NO3)3.9H2O, Across, 98%), D-sorbitol (C6H14O6, Aldrich, 99%), aluminum isopropoxide (C9H21O3Al, Across, 99%), and europium oxide (Eu2O3, Aldrich, 99.99%) were used as starting reactants without any further purification.
Modified Pechini synthesis. Stoichiometric amount of Ba(CH3COO)2 aiming to prepare 0.5000 g of the final product was dissolved in acetic acid (6 mL) under agitation and heating (50 °C), followed by the addition of H2O (25 mL). After 10 min, stoichiometric amounts of Al(NO3)3, Eu(NO3)3 (prepared from the dissolution of Eu2O3 in concentrated nitric acid, in the case of the doped samples, and titrated by using EDTA as complexing agent and xylenol orange as indicator), and citric acid (3 mols of citric acid:1 mol of metal) were added, enabling the metal-citrate complex formation. The temperature of the system was kept at ~ 85 °C/1 h until halving the initial volume, and then, sorbitol was added (1 mol of sorbitol:2 mols of metal). The system was kept under stirring and heating until the formation of a clear foamy polymer resin, which was pre-calcined (300 °C) for 2 h in static air. A spongy-like agglomerate, also called “puff,” was then obtained [26]. The “puff” precursor was de-agglomerated and sieved at 325 mesh sieves, and annealed at 1000 °C (heating ramp of 5 °C/min) for 2 h in a circulating air atmosphere.
Sol–gel synthesis. Stoichiometric amount of Ba(CH3COO)2 aiming to prepare 0.5000 g of the final product was dissolved in acetic acid (1.5 mL) under agitation and heating (50 °C), followed by the addition of H2O (10 mL). After 10 min, stoichiometric quantities of Eu(CH3COO)3 solution (previously prepared from the dissolution of Eu2O3 in CH3COOH) was added. In the sequence, C9H21O3Al in stoichiometric proportions was added under vigorous stirring and heating (80 °C) until complete dissolution and gel formation. This gel was dried in an oven for 1 h to yield the xerogel precursor that was thermally treated at 300 °C in a static air atmosphere (heating ramp of 5 °C/min for 2 h) [25]. Finally, the precursor was de-agglomerated in a mortar and annealed at 1,000 °C in a circulating air atmosphere (heating ramp of 5 °C/min) for 2 h.
The Eu3+ doping concentrations were isoelectronically varied from 1 to 7% (Table 1), i.e., keeping the same overall charge in the BaAl2O4 matrix, according to the Ba2+, Eu3+ and \(Al_{2} O_{4}^{2 - }\) charges (\(3n_{{Eu^{3 + } }} + 2n_{{Ba^{2 + } }} = + 2\), where \(n_{{Ba^{2 + } }}\) and \(n_{{Eu^{3 + } }}\) are the mol number of Ba2+ and Eu3+, respectively, in the BanBaEunEuAl2O4 formula). Samples will be hereafter labeled as PCX or SGX, where SG = sol–gel-derived samples, PC = Pechini-derived samples, and X = Eu3+-doping percentage.
Characterization. All samples were characterized, from the structural point of view by X-ray diffraction (XRD) using a SHIMADZU diffractometer model XDR-6000, Cu(Kα) radiation (λ = 1.5406 Å), interval of 10o—80º (2θ), increment of 0.02º and integration time of 0.6 s. The crystallite size of samples was evaluated via Scherrer method, according to Eq. 1 [29], where βp is the width at half maximum in radians of a given (h k l) reflection peak due to the grain size (ε) in the corresponding (h k l) direction, and c is a constant that depends on the reflection symmetry which usually is given as 1. To calculate the crystallite size, the three most intense peaks related to (2 0 0), (2 0 2), and (2 2 0) plans at 19.7°, 28.2° and 34.2° were considered, respectively:
Thermogravimetry of precursors doped with 1% was measured by using an SDT-Q600TA instrument with a heating rate of 10 °C/min from 25 °C to 1,400 °C in air flow (100 mL/min). SEM measurements were carried out using a scanning electron microscope CARLS ZEISS, model EVO LS15 with a secondary electron detector (SE), 25.00 KX of magnification, EHT of 30.00 kV. For that, an ethanolic suspension containing particles was deposited on a glass cover plate, which was fixed with double carbon facing tape on a stub and then metalized with gold using a Quorum sputtering, model Q 150R ES.
UV–Vis diffuse reflectance was measured in a PERKIN ELMER LAMBDA 1050 spectrophotometer. From the diffuse reflectance spectrum, the optical bandgap was estimated through a graphic of (αhν)n versus the energy of the incident photon (eV), Figure S1 (Supplementary Material), where n is equal to 2 for direct electronic transitions or 0.5 for indirect transitions between the valence and conduction bands [30]. α is the ratio of the scattering (K) and absorption (S) coefficients determined from the Kubelka–Munk method [31], Eq. 2, where R is the reflectance observed for different incident energies.
Photoluminescence measurements at 300 K or at 77 K were carried out in a Horiba Jobin Yvon spectrometer Fluorolog-3 with a Xe lamp (450 W) source with double excitation monochromator. The slits were placed at 1.1 and 0.7 mm for excitation and emission, respectively. The emission decay curves were carried out in a phosphorimeter equipped with Xe (5 J/pulse) lamp. By applying the Judd–Ofelt theory that describes the behavior of f-f electronic transitions in Ln3+ by a forced electric dipole mechanism, it is possible to infer on the radiative decay probabilities (Arad) of the 5D0 emitting state and the Ω2 and the Ω4 intensity parameters. Arad was calculated by using Eq. 3, where A01 = 14.65 n3 in s−1 [32], and n is the refractive index, equal to 1.657 for BaAl2O4 [33], I is the integrated area under the 5D0 → 7FJ transitions in the emission spectra, and hυ0-J is the energy barycenter of the electronic transition. Thus, Arad is the sum of radiative rates of all electronic transitions coming from the 5D0 state (Arad = \(\sum A_{0 - J}\)).
The intensity parameters were calculated by using Eq. 4, where \(\left| {{}_{{}}^{7} F_{J} U^{\left( \lambda \right)} {}_{{}}^{5} D_{0} } \right|^{2}\) represents the square reduced matrix elements whose values are equal to 0.0032 to Ω2 and 0.0023 to Ω4; h is the Planck's constant, e is the electron charge, c is the speed of light in vacuum, ω is the angular frequency of the incident radiation field, ε0 is vacuum permittivity constants, and X is the Lorentz local field correction equal to n(n2 + 2)2/9 [34].
Results and discussion
Structural characterization
Precursors of both methods were characterized by thermogravimetry aiming the determination of the BaAl2O4 phase formation temperature, Fig. 1. For the sol–gel-derived precursor, the thermal event starting at 200 °C and peaking at 280 °C is correlated with the thermal decomposition of the organic matter coming from the precursors. On the other hand, for the Pechini precursor, the first thermal event with maximum at 100 °C is due to water evaporation, while the second one at 200 °C is related to the thermal degradation of nitrates and organic matter. Due to its high basicity, Ba2+ tends to form BaCO3 in such environmental conditions, whose formation ranges within 400–500 °C [35]. For both precursors, the thermal event at 900 °C is associated with the BaAl2O4 phase formation, which was confirmed by XRD data.
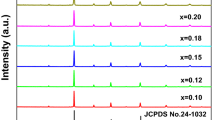
The X-ray diffraction patterns of samples synthesized at 1,000 °C, Fig. 2, are indexed as the hexagonal BaAl2O4 structure (JCPDS-17–0306), space group P6322. All diffraction peaks are sharp and well defined, confirming that the phase is highly crystalline. However, for the sol–gel-derived samples with low Eu3+ doping percentage, a low-intensity peak at 24° is observed, characteristic of the diffraction plan of the BaCO3 (JCPDS-45–1471) spurious phase (see the magnification of this region in supplementary material, Figure S2). The BaCO3 phase formation is common for compounds with high basicity cations such as Ba2+, being intermediated by CO2 coming from organic matter decomposition and/or air atmosphere, as reported for the synthesis of BaTiO3 [36, 37] or Ba2SiO4 [25]. The sol–gel route itself normally leads to particles displaying porous surface that can easily react with CO2 from atmosphere, favoring the formation of carbonate groups. Moreover, the sol–gel-derived xerogel precursor based on Si–O bonds is often reported [38,39,40] as a porous structure that can confine organic groups, making it difficult for CO2 to be released due to thermolysis, which favors the BaCO3 formation. Interestingly, it is observed a smooth peak at 24° in the XRD data reported by Chatterjee and co-workers for sol–gel-derived BaAl2O4 [15], which may be assigned to the BaCO3 phase.
In BaAl2O4, Ba2+ occupies two different sites with the same coordination number (CN = 9), Fig. 2c, but in the Ba1 site (C3 point group), the Ba–O bond distance lies within the 2.86 – 2.87 Å, while for the Ba2 site (C1 point group), the Ba–O bond distance is 2.69 Å. Moreover, the Ba1 site occurs at about three times more than the Ba2 one. On the other hand, Al3+ is inserted into four different tetrahedral sites featuring Al-O bond distances close to 1.77, 1.74, 1.72, and 1.83 Å. [9] Since Eu3+ (ionic radius of 126 pm, CN = 9) and Ba2+ (ionic radius of 161 pm) have a better ionic radii match, Eu3+ replaces Ba2+ sites in the BaAl2O4 structure according to the Hume-Rothery rules, which states that the ionic radius difference between solvent and solute ions should be close to 15% [9]. It is worth pointing out that although the ionic radii difference between Eu3+ and Ba2+ is larger than 15%, doping takes place since no Eu2O3 diffraction peaks are observed in the XRD diffractogram (considering the detection limit of 1wt.%), Fig. 2, confirming the formation of a solid solution. Interestingly, Ba2+ replacement by lanthanide(III) in inorganic matrices has already been reported, for instance, for the Ba2SiO4:Eu3+ [25] and Ba2SiO4:Tb3+ [4] systems.
From the analysis of Fig. 2, there is no shift of the diffraction peaks related to the BaAl2O4 phase in the XRD data for the Pechini-derived samples (Figure S2), suggesting that neither the doping nor the synthetic route led to significant changes in the BaAl2O4 crystalline structure. Yet, for the sol–gel-prepared ones, slight shifts of the diffraction peaks (Figure S2) may be correlated with the spurious phase that influences the BaAl2O4 lattice organization.
The crystallite size values determined by the Scherrer equation shown in Table 2 are comparable with those ones of other oxide compounds prepared by humid routes [35, 41, 42] and they also reveal a non-isotropic crystallite grown tendency. There is a tendency of increasing the average crystallite size due to the doping for the SG samples, while the values for the Pechini-derived ones have no statistical change. In this case, changes in the crystallite size may be correlated with two factors, the BaCO3 spurious phase (present only in the SG1 and SG3 samples) and the doping amount. The amount of BaCO3 phase may control the boundaries during the synthesis, determining the mass transfer through the grain boundaries [43]. On the other hand, since Eu3+ and Ba2+ have quite different ionic radii, the increase in the doping content may lead to smooth local changes around Eu3+ local sites, which influence the structure formation and as a consequence the crystallite size.
Representative SEM images of PC1 and PC5 samples, Fig. 3a and b, exhibit particle agglomerates, which are common to quite high-temperature annealing processes, resulting in sintered surfaces [44]. On the other hand, the sol–gel-derived particles, Fig. 3c and d, feature different shapes and sizes, with spheroidal-like particles on the surface. This behavior is associated with the acid-catalyzed sol–gel route, which typically leads to particle agglomerates from the coalescence of spheres [45]. Thus, the sol–gel synthesis enables better shape control, although both methods form a nanostructured bulk.
Optical band gap evaluation
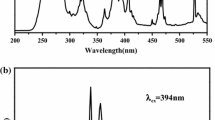
The optical absorption of all samples was evaluated through diffuse reflectance spectroscopy, Fig. 4. For all doped samples, three bands are noticed in the diffuse reflectance spectra; the first one at 220 nm (also noticed in the excitation spectra, Fig. 5) is assigned to the Eu3+ → O2− charge transfer band (CTB), and its relative intensity enhances as the Eu3+ content increases; the second at 262 nm is the electronic transition from the valence band (VB) to the conduction band (CB) of the matrix (the optical bandgap), and the third at 361 nm, also observed in the non-doped samples, may be associated with absorptions to electronic states arising from structural defects.
Indeed, it is usual to notice absorption in inorganic matrices ranging in the near-UV spectral region due to the presence of traps or intrinsic defects that may arise from the thermal decomposition of organic solvents or organic products of the sol–gel or Pechini syntheses [25, 35]. Moreover, due to the isoelectronic doping, three Ba2+ sites are replaced by only two Eu3+ ions, leading to one Ba2+ vacancy according to Eq. 5. Those vacancies increase the concentration of structural defects, leading to the appearance of defect-related electronic states within the forbidden band, as represented in Fig. 4c. For sol–gel-derived samples, the higher is the doping percentage, the stronger is the defect-related absorption band at 350 nm, ensuring that the structural defect concentration increases. On the other hand, for Pechini-based samples, this behavior is not observed due to the larger amount of organic matter within the precursor coming from sorbitol and citric acid, which also induces structural defects, decreasing the importance of defects arising from the doping process.
The optical bandgap of samples was calculated accordingly to the Kubelka–Munk approximation, Figure S1, and the values are statistically the same (4.3 ± 0.1 eV) independent on the doping concentration or the synthesis, in accordance with other values reported so far for the BaAl2O4 host [46].
Luminescence
The excitation spectra of samples monitored at emission of Eu3+, Fig. 5, are dominated by a broad and intense excitation band within the short-wavelength region assigned to the Laporte’s allowed Eu3+ → O2− CTB, in accordance with reflectance diffuse spectra, confirming that the BaAl2O4 host sensitizes the Eu3+ luminescence. Moreover, the lower-intensity excitation lines ranging in the long-wavelength region are attributed to the Laporte’s forbidden intraconfigurational Eu3+ f-f transitions [47]. In this sense, the phosphors may be excited from the deep UV to the blue spectral window, crossing the near UV spectral region.
The emission spectra of all phosphors, Fig. 6, are characterized by a set of sharp emission bands within the orange-red spectral region assigned to electronic transitions coming from the 5D0 state of Eu3+ to 7F0-4 ones, where the band peaking at 617 nm assigned to the 5D0 → 7F2 transition is the most intense one [47]. Interestingly, the Pechini-derived phosphors also feature a broad and weak emission band at 420 nm, which is assigned to the matrix emission accordingly to the emission spectrum of the undoped BaAl2O4 (see Figure S3); this band arises from the aforementioned defect-related electronic states according to the diffuse reflectance data and its stronger intensity in the Pechini-derived phosphors is related to the higher concentration of organic matter in the Pechini synthesis, which may induce a higher concentration of structural defects in the matrix.
From the colorimetric point of view, the emission color of phosphors was characterized by the 1,931 Commission Internationale de l'éclairage (CIE) color coordinates, Fig. 6 and Table S1. The emission color of all sol–gel-derived phosphors lies within the red spectral region with color purity near to 100% although, for the Pechini-derived samples, there is a shift toward the pink due to the combination of the weak matrix blue luminescence and the Eu3+ characteristic red emission.
Besides the red luminescence, Eu3+ may act as a spectroscopic probe since some of its f-f electronic transitions are influenced by the ligand field, enabling the evaluation of the local Eu3+ microssimetry by the relative intensity and the number of components of emission bands [48]. In the emission spectra shown in Fig. 6, the most intense band arises from the 5D0 → 7F2 transition, which occurs by a forced electric dipole mechanism, suggesting that Eu3+ is inserted in a low-symmetric site, in accordance with Eu3+ replacing Ba2+ local sites (C1 or C3 point group) within the BaAl2O4 lattice.
Eu3+ also enables a deep evaluation of the number of non-equivalent Eu3+ local sites with Cnv, Cn, and Cs point group since the 5D0 state is non-degenerated, leading to only one component for the 5D0 → 7F0 band for each non-equivalent Eu3+ local site according to the J rule (2 J + 1) [49]. To get further insight on the Eu3+ local microssimmetry, low-temperature (77 K) selective excitation spectra were carried out in the range of the 5D0 → 7F0 band for representative SG1, SG5, PC1, and PC5 phosphors, Fig. 7. PC1 and PC5 feature 1 and 2 5D0 → 7F0 transition components, respectively, suggesting that Eu3+ is inserted in at least two non-equivalent Eu3+ local sites, in accordance with Eu3+ replacing both Ba2+ sites in the BaAl2O4 lattice. On the other hand, SG1 and SG5 display 2 and 3 5D0 → 7F0 transition components, respectively, confirming that Eu3+ is inserted into 2 and 3 sites lacking inversion center. In this case, it must be highlighted that Eu3+ may replace Ba2+ into the BaCO3 spurious phase found in low amounts in the sol–gel-derived samples.
Based on the Judd–Ofelt theory that describes the f-f emission of Ln3+ by the forced electric dipole mechanism, it is possible to determine Ω2 and Ω4 intensity parameters, Table 3. A study carried out by Malta and coworkers [50] has shown that the Ω2 parameter is more influenced by angular distortions of the Eu3+-ligand bond, in other words, its local microssymmetry, while Ω4 suffers stronger influence of the Eu3+-ligand bond polarizability.
The quite low Ω4 values for samples reveal a predominant ionic nature of the Eu3+–O2− bonds, as expected since the Ba2+-O2− bonds are mainly ionic. For the Pechini-derived samples, the Ω4 parameter increases from PC1 to PC3 and then, it decreases, suggesting an increase in the bond covalent degree up to the doping concentration of 3%. On the other hand, for the samples prepared by the sol–gel route, except for the SG3, the values are practically invariant; in these samples, it must be highlighted that the BaCO3 spurious phase plays an important role in the crystal growth as discussed by XRD, influencing the organization of the BaAl2O4 lattice.
The Ω2 values are close for all Pechini-derived samples, suggesting that the Eu3+ concentration does not lead to significant changes on the Eu3+ local microssimetry, in accordance with the XRD data. However, samples synthesized by sol–gel display significant changes in the Ω2 parameter as the Eu3+ amount increases, especially the SG3 sample. Again, in this case, the spurious phase may influence the network organization during the synthesis. As observed by the XRD data, Figure S2(a), the relative intensity of the peak at 24° assigned to BaCO3 is not the same for all samples, suggesting that the weight fraction in the mixture is different. In this context, for the SG3 sample, the BaCO3 weight fraction should be quite distinct from the others, causing deviations of the typical crystalline structure, especially modifications of Eu – O bond angles whose behavior influences the Ω2 value.
The 5D0 state lifetime values, Table 3, were obtained through a biexponential adjustment of the emission-decay curves shown in Figure S4. In this case, two 5D0 state lifetime values were found and they were used to calculate an average lifetime by applying Eq. 6, where τ is the 5D0 state lifetimes and A is a pre-exponential value obtained from the biexponential adjustment. The main importance of the lifetime value is the balance between the non-radiative (Anrad) and radiative (Arad) decay probabilities from the 5D0 emitting state (1/τ = Arad + Anrad). Thus, apart from the 5D0 state lifetime value and the Arad determined from Eq. 3, the intrinsic emission quantum yield of Eu3+ is defined as \(\phi_{Ln}^{Ln} = A_{rad} /\left( {A_{rad} + A_{nrad} } \right)\).
For all doping concentrations, the Pechini-derived phosphors feature the highest \(\phi_{Ln}^{Ln}\), confirming that the Pechini synthesis is the best route to get phosphors with better luminescent features. In the Pechini-derived series, \(\phi_{Ln}^{Ln}\) increases from 1 to 3% of doping and then, it decreases, while for the phosphors obtained by the sol–gel route, \(\phi_{Ln}^{Ln}\) enhances from 1 to 5%, and then it decreases, suggesting that the optimal doping concentration is around 3–5%, which is in accordance with a previous study reported for BaAl2O3:Eu3+ by R. Chatterjee and coworkers [15], implying that a quenching mechanism by concentration takes place from that on. This mechanism occurs because as the Eu3+ doping concentration increases, there occurs a shortening of the Eu–Eu distance within the BaAl2O4 lattice, increasing the energy transfer between them. However, this energy transfer may find network defects into the lattice, favoring non-radiative processes, enhancing the non-radiative decay probabilities, and as a consequence, decreasing \(\phi_{Ln}^{Ln}\).
Usually, this energy transfer among Ln3+ ions occurs non-radiatively by exchange or multipolar mechanisms [4]. The exchange mechanism contribution gains importance as the Ln3+-Ln3+ distance decreases below 5 Å, since this mechanism occurs by electron transfer and requires a wide orbital overlap. Thus, the critical Ln3+-Ln3+ distance within the matrix is estimated by means of Eq. 7, where nA is equal to (x.n)/V, and V is the unit cell volume (V = 830 Å3, from the JCPD-17–0306 card) [15], x is the dopant concentration and n is the Ba2+ mol number per unit cell (n = 6 from the JCPD-17–0306 card) [15]. As the concentration increases, the Eu3+-Eu3+ distance becomes shorter, suggesting that the exchange mechanism becomes relevant at higher doping concentrations.
To evaluate if the multipolar mechanism contributes to the energy transfer, the theoretical model developed by Van Uitert [51] was applied which is showed in Eq. 8, where x is the Eu3+ concentration, I is the emission intensity (Arad was considered in this case), and β is a constant. In Eq. 8, θ is equal to 6 for dipole–dipole (D–D) interactions, 8 for electric dipole–quadrupole interactions, and 10 for electric quadrupole–quadrupole (Q–Q) interactions. In this sense, in a graphic of log(I/x) versus log(x), the slope of the curve is equal to –θ/3. For both Pechini and sol–gel-derived samples, θ is 3 (Figure S5, supplementary material), suggesting that the multipolar mechanism is not relevant for the Eu3+-Eu3+ energy transfer. Thus, the exchange mechanism is dominant on the Eu3+-Eu3+ energy transfer in the samples featuring high Eu3+ concentration.
By using \(\phi_{Ln}^{Ln}\) as a figure of merit, Table 4, the phosphor efficiency is comparable with other typical red-emitting phosphors featuring high intrinsic emission quantum yield. Moreover, the value reported by us is also larger than the only value reported for BaAl2O4:Eu3+ by R. J. Wiglusz and coworkers, [11] which confirm that the Pechini route and the charge compensation mechanism are desirable strategies toward enhancing the luminescent features.
Finally, from the structural point of view, both sol–gel and Pechini methods enable the formation of highly crystalline and nanostructured BaAl2O4:Eu3+ red-emitting phosphor, although the sol–gel process also leads to the formation of traces of the BaCO3 spurious phase. Moreover, the Pechini route is better to optimize the phosphor synthesis, leading to the highest intrinsic emission quantum yield (86% for PC3) and high emission color purity, which qualifies it for application as a UV-to-red downshifting converter phosphor.
Conclusions
Herein, UV-to-red downshifting converter phosphors based on BaAl2O4:Eu3+ were synthesized by modified sol–gel or Pechini methods, performing a detailed investigation of luminescence, particle shape and structure. Both methods lead to highly crystalline samples and Eu3+ replaces Ba2+ within the BaAl2O4 lattice; yet, the sol–gel-derived samples display traces of BaCO3 spurious phase. Moreover, under UV excitation, all phosphors feature emission with high color purity within the red spectral region as a result of electronic transitions from the Eu3+ 5D0 state to the 7F0-4 ones. Finally, the Pechini-derived sample doped with 3% of Eu3+ displays the largest intrinsic emission quantum yield (86%) reported so far for BaAl2O4:Eu3+, qualifying it for application as a red-emitting phosphor.
References
Moscardini SB, Sverzut L, Massarotto WL, Nassar EJ, Rocha LA (2020) Multi-color emission from lanthanide ions doped into niobium oxide. J Mater Sci Mater Electron 31:5241–5252. https://doi.org/10.1007/s10854-020-03084-5
Kim CH, Kwon IE, Park CH, Hwang YJ, Bae HS, Yu BY, Pyun CH, Hong GY (2000) Phosphors for plasma display panels. J Alloys Compd 311:33–39. https://doi.org/10.1016/S0925-8388(00)00856-2
Edgar A (2004–2021) Luminescent Materials. In: Kasap S, Capper P (eds) Springer Handbook of Electronic and Photonic Materials. Springer, Cham., pp. 1–1.
Bispo-Jr AG, Lima SAM, Pires AM (2018) Energy transfer between terbium and europium ions in barium orthosilicate phosphors obtained from sol-gel route. J Lumin 199:372–378. https://doi.org/10.1016/j.jlumin.2018.03.057
Wang L, Xie RJ, Suehiro T, Takeda T, Hirosaki N (2018) Down-Conversion nitride materials for solid state lighting: recent advances and perspectives. Chem Rev 118:1951–2009. https://doi.org/10.1021/acs.chemrev.7b00284
Bispo-Jr AG, Lima SAM, Carlos LD, Ferreira RAS, Pires AM (2020) Red-emitting coatings for multifunctional UV/Red emitting LEDs applied in plant circadian rhythm control. ECS J Solid State Sci Technol 9:016008. https://doi.org/10.1149/2.0122001JSS
Bispo-Jr AG, Saraiva LF, Lima SAM, Pires AM, Davolos MR (2021) Recent prospects on phosphor-converted LEDs for lighting, displays, phototherapy, and indoor farming. J Lumin 237:118167. https://doi.org/10.1016/j.jlumin.2021.118167
Carlos LD, Ferreira RAS, Bermudez VZ, Julian-Lopez B, Escribano P (2011) Progress on lanthanide-based organic–inorganic hybrid phosphors. Chem Soc Rev 40:536–549. https://doi.org/10.1039/c0cs00069h
Kaur J, Jaykumar B, Dubey V, Shrivastava R, Suryanarayana NS (2015) Optical properties of rare earth-doped barium aluminate synthesized by different methods-A Review. Res Chem Intermed 41:2317–2343. https://doi.org/10.1007/s11164-013-1349-z
Shimokawa Y, Sakaida S, Iwata S, Inoue K, Honda S, Iwamoto Y (2015) Synthesis and characterization of Eu3+ doped CaZrO3-based perovskite type phosphors part II: PL properties related to the two different dominant Eu3+ substitution sites. J Lumin 157:113–118. https://doi.org/10.1016/j.jlumin.2014.08.042
Wiglusz RJ, Grzyb T (2013) Sol–gel synthesis of micro and nanocrystalline BaAl2O4:Eu3+ powders and their luminescence properties. Opt Mater 36:539–545. https://doi.org/10.1016/j.optmat.2013.10.029
Binnemans K (2015) Interpretation of europium(III) spectra. Coord Chem Rev 295:1–45. https://doi.org/10.1016/j.ccr.2015.02.015
Nakauchi D, Okada G, Kato T, Kawaguchi N, Yanagida T (2020) Crystal growth and scintillation properties of Eu:BaAl2O4 crystals. Radiat Meas 135:106365. https://doi.org/10.1016/j.radmeas.2020.106365
Raia RK, Upadhyay AK, Kher RS, Dhoble SJ, Mehta M (2011) BaAl2O4: Eu - Phosphor for mechanoluminescence dosimetry. Radiat Meas 46:1393–1396. https://doi.org/10.1016/j.radmeas.2011.08.016
Chatterjee R, Saha S, Sen D, Panigrahi K, Ghorai UK, Das GC, Chattopadhyay KK (2018) Neutralizing the Charge Imbalance Problem in Eu3+-Activated BaAl2O4 Nanophosphors: Theoretical Insights and Experimental Validation Considering K+ Codoping. ACS Omega 3:788–800. https://doi.org/10.1021/acsomega.7b01525
Marí B, Singh KC, Verma N, Mollar M, Jindal J (2015) Luminescence Properties of the Eu2+/Eu3+ activated barium aluminate phosphors with Gd3+ concentration variation. Trans Ind Ceram Soc 74:1–5. https://doi.org/10.1080/0371750X.2015.1082932
Grzeta B, Lutzenkirchen-Hecht D, Vrankic M, Bosnar S, Saric A, Takahashi M, Petrov D, Biscan M (2018) Environment of the Eu3+ ion within nanocrystalline Eu-Doped BaAl2O4: correlation of X-ray diffraction, mossbauer spectroscopy, X-ray absorption spectroscopy, and photoluminescence investigations. Inorg Chem 57:1744–1756. https://doi.org/10.1021/acs.inorgchem.7b02322
Rezende MVS, Montes PJR, Andrade AB, Macedo ZS, Valerio MEG (2016) Mechanism of X-ray excited optical luminescence (XEOL) in europium doped BaAl2O4 phosphor. Phys Chem Chem Phys 18:17646. https://doi.org/10.1039/C6CP01183G
Rezende MVS, Montes PJ, Valerio MEG, Jackson RA (2012) The optical properties of Eu3+ doped BaAl2O4: a computational and spectroscopic study. Opt Mater 34:1434–1439. https://doi.org/10.1016/j.optmat.2012.02.050
Rezende MVS, Andrade AB, William C, Paschoal A (2018) Co-doping effect of Ca2+ on luminescent properties of BaAl2O4:Eu3+ phosphors. J Electron Spectros Relat Phenomena 225:62–65. https://doi.org/10.1016/j.elspec.2018.04.002
Araujo RM, Mattos EFS, Júnior BFS, Rezende MVS, Valerio MEG, Jackson RA (2021) Optical spectroscopy study of Eu-doped ions in BaAl2O4 phosphors. J Lumin 236:118011. https://doi.org/10.1016/j.jlumin.2021.118011
Gomes MA, Andrade AB, Rezende MVS, Valerio MEG (2017) Production of Eu-doped BaAl2O4 at low temperature via an alternative solgel method using PVA as complexing agent. J Phys Chem Solids 102:74–78. https://doi.org/10.1016/j.jpcs.2016.11.010
Verma N, Marí B, Singh KC, Jindal J, Yadav S, Mittal A (2019) Enhanced luminescence by tunable coupling of Eu3+ and Tb3+ in ZnAl2O4:Eu3+:Tb3+ phosphor synthesized by solution combustion method. J Aust Ceram Soc 55:179–185. https://doi.org/10.1007/s41779-018-0223-2
Kumar N, Marí B, Jindal J, Mittal KK, Maken S (2019) Near ultraviolet excited down conversion Eu and Er co-doped CaAl2O4 color tunable nano-phosphors: Structural, morphological and photolumniscent characteristics. Mater Today Proc 19:646–649. https://doi.org/10.1016/j.matpr.2019.07.747
Bispo-Jr AG, Ceccato DA, Lima SAM, Pires AM (2017) Red phosphor based on Eu3+-isoelectronically doped Ba2SiO4 obtained via sol–gel route for solid state lightning. RSC Adv 7:53752. https://doi.org/10.1039/C7RA10494D
Oliveira NA, Bispo-Jr AG, Shinohara GMM, Lima SAM, Pires AM (2021) The influence of the complexing agent on the luminescence of multicolor-emitting Y2O3:Eu3+, Er3+, Yb3+ phosphors obtained by the Pechini’s method. Mater Chem Phys 257:123840. https://doi.org/10.1016/j.matchemphys.2020.123840
Aflaki M, Davar F (2016) Synthesis, luminescence and photocatalyst properties of zirconita nanosheets by modified Pechini method. J Mol Liq 221:1071–1079. https://doi.org/10.1016/j.molliq.2016.06.067
Loghman-Estarki MR, Razavi RS, Edris H, Pourbafrany M, Jamali H, Ghasemi R, Lifetime of new SYSZ thermal barrier coatings produced by plasma spraying method under thermal shock test and high temperature treatment. Ceram Int 40:1405–1414. https://doi.org/10.1016/j.ceramint.2013.07.023
Patterson AL (1939) The Scherrer formula for X-ray particle size determination. Phys Rev 56:978. https://doi.org/10.1103/PhysRev.56.978
Murphy AB (2007) Band-gap determination from diffuse reflectance measurements of semiconductor films, and application to photoelectrochemicalwater-splitting. Sol Energy Mater Sol Cells 91:1326–1337. https://doi.org/10.1016/j.solmat.2007.05.005
Kubelka P, Munk F (1931) EinBeitragzurOptik der Farbanstriche. Z Tech Phys 15:593–601
Duarte AP, Gressier M, Menu M, Dexpert-Ghys J, Caiut JMA, Ribeiro SJL (2012) Structural and luminescence properties of silica-based hybrids containing new Silylated-Diketonato Europium(III) Complex. J Phys Chem C 116:505–515. https://doi.org/10.1021/jp210338t
Weber MJ, (2002) Handbook of Optical Materials, first ed., CRC Press.
Kodaira C, Brito HF, Malta OL, Serra OA (2003) Luminescence and energy transfer of the europium (III) tungstate obtained via the Pechini method. J Lumin 101:11–21. https://doi.org/10.1016/S0022-2313(02)00384-8
Bispo-Jr AG, Lima SAM, Lanfredi S, Praxedes FR, Pires AM (2019) Tunable blue-green emission and energy transfer properties in Ba2SiO4:Tb3+ obtained from sol-gel method. J Lumin 214:116604. https://doi.org/10.1016/j.jlumin.2019.116604
Phule PP, Risbud SH (1980) Low-temperature synthesis and processing of electronic materials in the BaO-TiO2 system. J Mater Sci 25:1169–2118. https://doi.org/10.1007/BF00585422
López MDCB, Fourlaris G, Rand B, Riley FL (1999) Characterization of barium titanate powders: Barium carbonate identification. J Am Ceram Soc 82:1777–1786. https://doi.org/10.1111/j.1151-2916.1999.tb01999.x
Andrade-Espinosa G, Escobar-Barrios V, Rangel-Mendez R (2010) Synthesis and characterization of silica xerogels obtained via fast sol–gel process. Colloid Polym Sci 288:1697–1704. https://doi.org/10.1007/s00396-010-2311-x
Brinker CJ, Scherrer GW (1990) Sol-gel science, the physics and chemistry of sol-gel processing. Academic, San Diego
Perullini M, Jobbagy M, Bilmes SA, Torriani IL, Candal R (2011) Effect of synthesis conditions on the microstructure of TEOS derived silica hydrogels synthesized by the alcohol-free sol–gel route. J Sol-Gel Sci Technol 59:174–180. https://doi.org/10.1007/s10971-011-2478-8
Amaral FA, Santana LK, Campos IO, Fagundes WS, Xavier FFS, Canobre SC, Pechini Synthesis of Nanostructured Li1.05M0.02Mn1.98O4 (M = Al3+ or Ga3+). Mater Res 18:250–259. doi: https://doi.org/10.1590/1516-1439.361514
Loghman-Estarki MR, Hajizadeh-Oghaz M, Edris H, RS, (2013) Comparative studies on synthesis of nanocrystalline Sc2O3–Y2O3 doped zirconia (SYDZ) and YSZ solid solution via modified and classic Pechini method. Cryst Eng Comm 15:5898–5909. https://doi.org/10.1039/C3CE40288F
Macedo WC, Bispo-Jr AG, Rocha KO, Albas AES, Pires AM, Teixeira SR, Longo E (2020) Photoluminescence of Eu3+-doped CaZrO3 red-emitting phosphors synthesized via microwave-assisted hydrothermal method. Mater Today Commun 24:100966. https://doi.org/10.1016/j.mtcomm.2020.100966
Hiratsuka RS, Santili CV, Pulcinelli SH, (1995) O processo sol-gel:uma visão físico-química. Quim. Nova 18:171–180. https://doi.org/10.21577/0100-4042.20170623
Oliveira HFN, Trinca RB, Gushikem Y (2009) Síntese e estudo de ortossilicatos de zinco luminescentes com aplicação da técnica sol-gel. Quim Nova 32:1346. https://doi.org/10.1590/S0100-40422009000500045
Zhang LW, Wang L, Zhu YF, Synthesis and performance of BaAl2O4 with a wide spectral range of optical absorption. Adv Funct Mater 17:3781–3790. doi: https://doi.org/10.1002/adfm.200700506
Carnall, WT, Crosswhite, H, Crosswhite, HM (1978) Energy level structure and transition probabilities in the spectra of the trivalent lanthanides in LaF2. USDOE Office of Science, United States.
Tanner PA (2013) Some misconceptions concerning the electronic spectra of tri-positive europium and cerium. Chem Soc Rev 42:5090–5101. https://doi.org/10.1039/C3CS60033E
Tanner PA, Yeung YY, Ning L (2013) What Factors Affect the 5D0 Energy of Eu3+? An investigation of nephelauxetic effects. J Phys Chem A 117:2771–2781. https://doi.org/10.1021/jp400247r
Moura RT, Neto ANC, Longo RL, Malta OL (2016) On the calculation and interpretation of covalency in the intensity parameters of 4f–4f transitions in Eu3+ complexes based on the chemical bond overlap polarizability. J Lumin 170:420. https://doi.org/10.1016/j.jlumin.2015.08.016
Uitert LG (1967) Characterization of energy transfer interactions between rare earth ions. J Electrochem Soc 14:1048–1053. https://doi.org/10.1149/1.2424184
Ting L, Qingyu M, Wenjun S (2015) Luminescent properties of Eu3+ doped NaY(WO4)2 nanophosphors prepared by molten salt method. J Rare Earths 33:915–921. https://doi.org/10.1016/S1002-0721(14)60505-6
Grzyb T, Wecławiak M, Rozowska J, Lis S (2013) Structural and spectroscopic properties of YOF:Eu3+ nanocrystals. J Alloy Compd 576:345–349. https://doi.org/10.1016/j.jallcom.2013.05.207
Som S, Das S, Dutta S, Visser HG, Pandey MK, Kumar P, Dubey RK, Sharma SK (2015) Synthesis of strong red emitting Y2O3:Eu3+ phosphor by potential chemical routes: comparative investigations on the structural evolutions, photometric properties and Judd-Ofelt analysis. RSC Adv 5:70887–70898. https://doi.org/10.1039/C5RA13247A
Bispo-Jr AG, Shinohara GMM, Pires AM, Cardoso CX (2018) Red phosphor based on Eu3+-doped Y2(MoO4)3 incorporated with Au NPs synthesized via Pechini’s method. Opt Mater 84:137–145. https://doi.org/10.1016/j.optmat.2018.06.023
Acknowledgements
Authors are thankful to the Brazilian agencies CNPq (Grant No. 304003/2018-2) and FAPESP for the financial research support. Laboratório de Microscopia Eletrônica de Varredura (FCT-UNESP, Prof. Dr. N. Alves), Laboratório de Materiais Cerâmicos (LaMaC, FCT-UNESP), and Laboratório Multiusuário de Análises Químicas (IQ/UNESP – Araraquara).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Andrea de Camargo.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Oliveira, N.A., Bispo-Jr, A.G., Lima, S.A.M. et al. Red-emitting BaAl2O4:Eu3+ synthesized via Pechini and sol–gel routes: a comparison of luminescence and structure. J Mater Sci 57, 170–184 (2022). https://doi.org/10.1007/s10853-021-06633-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06633-3