Abstract
Unique structure and performance in prominent high-entropy ceramics (HECs) have attracted enormous attentions recently. In this work, we have successfully synthesized pure-phase (Bi1/6Na1/6Sr1/6Ba1/6Pb1/6Ca1/6)TiO3 (BNSBPC) and (Bi1/6La1/6Na1/6K1/6Sr1/6Ba1/6)TiO3 (BLNKSB) HECs by conventional solid-state method. Rietveld refinement results show that BNSBPC composition possesses tetragonal phase (P4mm) while the BLNKSB one exhibits cubic phase (Pm \(\overline{3}\) m). Dielectric characterization reveals distinct relaxation behaviors between BNSBPC and BLNKSB compositions. Relaxor-like nature in BNSBPC and Debye medium in BLNKSB one are verified by dielectric, ferroelectric and Raman spectra characterizations. Maximal electrocaloric effect (ECE, ΔTmax) reaches 0.63 K with relatively narrow temperature span (ΔTspan) of ~ 20 K at 60 kV cm−1 for BNSBPC ceramic, while the BLNKSB one possesses superior temperature stability (ΔTspan = 100 K) with ΔTmax = 0.14 K. Room-temperature ECE is also obtained by home-made adiabatic calorimeter, which indicates a positive ECE for both compositions. Finally, phase structure, dielectric properties and ECE in two high-entropy compositions are comparatively discussed to explore structure–ECE relationships in HECs. The superior thermal stability of ECE in BLNKSB samples provides a broad prospect for designing solid-state refrigeration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Conventional compression refrigeration devices have disadvantages of complex structure, large volume and low efficiency with production of greenhouse gas, which retards a progress of integrations, miniaturizations and creation of environment-friendly society [1]. To overcome these shortcomings, solid-state refrigeration technology has stepped onto the stage of history. This technology is based on the electrocaloric effect (ECE), which is defined as temperature variation in polar materials with application and removal of electric field [2]. Since it has merits of easy miniaturization, low cost/noise and high efficiency, it gradually becomes a promising candidate in application of refrigeration devices. After years of silence, Mischenko et al. reported a large adiabatic temperature change (ΔT) of 12 K in PbZr0.95Ti0.05O3 thin films, thus igniting passions in exploring novel material with a large ECE [3]. Taking heat-transfer efficiency into account, high-ECE researches in bulk materials are mainstream, both lead-containing such as Pb(Mg1/3Nb2/3)O3-PbTiO3 (PMN-PT) [4, 5] and lead-free ceramics such as BaTiO3– (BT) [6,7,8,9,10,11,12], K0.5Na0.5NbO3– (KNN) [13,14,15], NaNbO3– (NN) [16, 17] and Bi0.5Na0.5TiO3-based (BNT) [18,19,20]. However, there are some weakness that hiding behind excellent performances. For instance, lead-containing ceramics have a high ECE but are not friendly to the environment. BT bulk ceramics produce large ΔT under electric field, the maximal ECE deviates from room temperature with narrow working temperature range yet. Ferroelectric–relaxor phase transition boosts ECE in BNT-based ceramics. However, the contribution of polar nanoregions (PNRs) leads to temperature instability. When modulating the transition temperature near room temperature, temperature stability has been improved. However, ECE peak value is much reduced due to missing of latent heat [20]. Therefore, it is necessary to explore a novel material with a large ΔT accompanied by wide ΔTspan.
Enlightened by high-entropy alloys (HEA) in metallurgy, high-entropy ceramics (HECs) have drawn increasing attractions due to their unique structure and performances [21,22,23]. Introducing high configurational entropy opens up an exciting and numerous unexpected performances. High-entropy oxide (HEO) (Mg0.2Zn0.2Cu0.2Co0.2Ni0.2)O ceramic was reported by Rost et al. [24], which provided a guideline for property engineering via deliberately designing configurational disorder. Afterward, various HEO ceramics are synthesized, such as spinel–structure (Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4 [25], fluorite–oxide (Ce0.2Zr0.2Hf0.2Sn0.2Ti0.2)O2 with low thermal conductivity [26] and perovskite–structure Sr(Zr0.2Sn0.2Ti0.2Hf0.2Nb0.2)O3 [27]. The HEO ceramics have also shown peculiar performances in colossal dielectric relaxation [28], superconductivity [29] and catalytic [30, 31]. However, research on ECE in HEO is scarce thus far. Pu et al. reported a kind of (Na0.2Bi0.2Ba0.2Sr0.2Ca0.2)TiO3 ceramic, which demonstrated possible applications in solid-state refrigeration and energy-storage devices [32]. Du et al. also synthesized Ba(Ti1/6Sn1/6Zr1/6Hf1/6Nb1/6Ga1/6)O3 ceramic with substitution of six different cations at B–site and phase formation and dielectric properties in this ceramic were analyzed [33].
Inspired by these researches, high-entropy perovskite oxides with six components at A–site is fabricated for exploring possible peculiar performances. In this work, two kinds of high-entropy perovskite oxides (Bi1/6Na1/6Sr1/6Ba1/6Pb1/6Ca1/6)TiO3 (BNSBPC) and (Bi1/6La1/6Na1/6K1/6Sr1/6Ba1/6)TiO3 (BLNKSB) with equimolar proportions of six elements at A–site are firstly reported. These materials have a configurational entropy (ΔSconfig) of 1.79R (\(\Delta S_{{{\text{config}}}} = - R\mathop \sum \limits_{i = 1}^{N} x_{i} {\text{ln}}x_{i}\), where R is the gas constant of 8.314 J mol−1 K−1, xi is the molar concentration of each element), which is higher than 1.5R (minimal value of high entropy in definition) [34]. The influence of A–site multi-element incorporations on phase/local structure, dielectric/ferroelectric properties and ECE are discussed in detail.
Experimental section
Synthesis
(Bi1/6Na1/6Sr1/6Ba1/6Pb1/6Ca1/6)TiO3 and (Bi1/6La1/6Na1/6K1/6Sr1/6Ba1/6)TiO3 ceramics were prepared through a conventional solid-state method by using high purity raw chemicals Bi2O3(≥ 99%), Na2CO3(≥ 99.8%), SrCO3(≥ 99%), BaCO3(≥ 99%), PbO(≥ 99%), CaCO3(≥ 99%), K2CO3(≥ 99%), La2O3(≥ 99.99%) and TiO2(≥ 98%). Na2CO3 and K2CO3 raw materials were dried for 12 h at 150 °C in advance for removing water absorption. Firstly, raw chemicals were mixed in stoichiometric ratios and ball milled for 18 h. The resultant powders were dried at 100 °C for 12 h. Then, BNSBPC and BLNKSB powders were calcined at various temperatures for four hours to obtain a single-phase compound. After calcination, powders were ball-milled again for 15 h, and then dried powders were ground with 8 wt.% PVA as a binder. The weight ratio of PVA to powder is about 1:4. The granulated powders were pressed into pellets with 10 mm in diameter and 1 mm in thickness with cold-isostatic pressure of 6 MPa. After removing the PVA binder, compacted pellets were sintered at 1260 °C with soaking time of three hours. The heating rate is 3 °C min−1 and they were cooled with furnace when sintering was finished. The polished pellets for both compositions are about 0.4 mm in thickness with an area of ~ 59.4 mm2.
Measurement
The lattice structure of HECs was measured by X-ray diffractometer (XRD, Rigaku Smart–lab Beijing Co, Beijing, China). The sample morphology was observed by a scanning electron microscope (SEM, Regulus 8230; Hitachi Co, Tokyo, Japan). Sample surface was polished and thermally etched at 1150 °C for 30 min. Archimedes method was used to measure samples’ density. The density of BNSBPC and BLNKSB ceramic was measured to be 5.73 g cm−3 and 5.48 g cm−3, respectively. Temperature-dependent dielectric properties were measured by Wayne Kerr 6500B impedance analyzer (Wayne Kerr Electronic Instrument Co., Shenzhen, China) with a Partulab DMS–2000 apparatus (Partulab Technology Co, Wuhan, China) in the frequency range of 0.1 kHz ~ 1 MHz. Temperature-dependent polarization–electric field hysteresis loops (P–E) were measured with frequency of 1 Hz by a ferroelectric test system (Precision LC, Radiant Technologies, Inc. Albuquerque, NM) with Delta–9023 device. Specific heat capacity Cp was measured by a differential scanning calorimeter (DSC, TA Instruments Q2000, USA). Raman spectroscopy was measured by Renishaw Raman microscope with a 532 nm laser and a temperature controller (Linkam THMSE 600). Direct ECE was measured by using a home-made adiabatic calorimeter, which can be referred to [35, 36].
Results and discussion
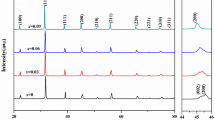
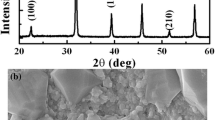
To investigate the phase formation and lattice structure, XRD patterns of BNSBPC and BLNKSB powders calcined at various temperatures are displayed in Fig. 1a and c. XRD patterns show that secondary phase appears at a lower calcined temperature and intensity of miscellaneous peaks decrease gradually with increasing temperature. Decrease in second-phase peak intensity with rising temperature is due to the decreased concentration of nonperovskite–structure composition. When temperature reaches 1193 K, BNSBPC powders form a single-phase solid solution, while the BLNKSB calcined powders show absence of secondary phase until 1273 K. This phenomenon is explained as both entropy and enthalpy drive the material to form a stable single-phase structure [32]. Decrease in entropy and increase in enthalpy can reduce Gibbs free energy and thereby enhancing thermal stability for HEC system. Considering the formation of second phase and easy volatilization of Bi/Pb/Na/K element at low/high temperature respectively, the calcined temperature is determined to be 1193 K and 1273 K for BNSBPC and BLNKSB systems. The experimental XRD patterns for crushed pellets are shown in Fig. 1b and d, which demonstrates the formation of pure perovskite structure. To get in-depth understanding of phase structure, Rietveld refinement of XRD patterns are performed. The experimental, calculated and different profiles of BNSBPC and BLNKSB composition are shown by selecting models of tetragonal (T) phase with space groups P4mm and cubic (C) phase with space groups Pm \(\overline{3}\) m, respectively. The goodness of fit Rwp and Rp is lower than 15%, which indicates that fitting results are credible. The insets in Fig. 1b and d show the lattice structure of T and C phase for BNSBPC and BLNKSB, respectively. Furthermore, Jiang et al. reported that tolerance factor (~ 1) was perhaps a necessary condition to form a single phase in high-entropy perovskite materials [37]. Tolerance factor is calculated by using the following formula [38]:
where RA, RB, RO are the effective ionic radii of A–site cation, B–site cation and oxygen anion, respectively. Tolerance factor in both HECs are calculated to be 1.006, which further confirms that the easier formation of a single-phase high-entropy ceramics.
XRD patterns of calcined powders at various temperatures ranging from 1073 to 1273 K for (a) BNSBPC and (c) BLNKSB. Rietveld refinement patterns of (b) BNSBPC and (d) BLNKSB samples with lattice-structure illustrations in their insets, respectively (The black crosses represent the observed data for corresponding crushed pellets, red lines are the calculated profiles, and blue lines are the difference between the observed and calculated diffraction patterns; the vertical ticks indicate the peak position of each phase)
Figure 2 shows the surface morphology of thermally-etched BNSBPC and BLNKSB ceramics with corresponding elemental mapping. Obviously, both samples exhibit dense microstructures with absence of pores and voids, as shown in Fig. 2a and h. Besides, grain size in BNSBPC ceramic (~ 1.29 μm) is to some extent larger than that of BLNKSB one (~ 0.82 μm). The elemental mapping images of BNSBPC and BLNKSB samples demonstrate that all A–site elements are homogeneously distributed (Fig. 2b–g and Fig. 2i–n).
Temperature dependence of dielectric constant (ɛr) and loss tangent (tanδ) for BNSBPC and BLNKSB ceramics are shown in Fig. 3a and c. As shown, maximal dielectric constant (ɛm) of BNSBPC ceramic slightly decreases with increasing frequency and the temperature corresponding to ɛm (Tm) has weak relationship with frequency. It is usually considered to be an intermediate state between normal and relaxor ferroelectrics [39]. Interestingly, this composition also possesses a spontaneous ferroelectric–relaxor phase transition at ~ 403 K. On the contrary, Tm and ɛm value in BLNKSB ceramic distinctly depends on frequency, in which Tm increases and ɛm decreases with increasing frequency, as shown in Fig. 3c. The BLNKSB system is denoted as typical relaxor ferroelectric [40]. To further describe the degree of relaxation, a power law is adopted [41, 42]:
where γ and δc are degree of relaxation and broadening parameter, respectively. The parameter γ reveals phase transition character: The system is an ideal normal ferroelectric as γ = 1 and an ideal relaxor as γ = 2. ɛm is dielectric constant at Tm. The dielectric curves for two samples above Tm are fitted, as shown in the inset of Fig. 3a–c. The parameter γ are fitted to be 1.59 and 1.68 for BNSBPC and BLNKSB ceramics, respectively. This confirms that both ceramics have a diffuse phase transition (DPT) behavior. Interestingly, the relaxor degree of BNSBPC is less than that of BLNKSB. Though incorporation of other cations induces local random field, BNSBPC behaves like a normal ferroelectric material, which is probably due to a strong hybridization between Pb 6 s and O 2p orbitals. Therefore, the BLNKSB ceramic is a relaxor ferroelectric material while the BNSBPC one is classified as a relaxor-like ferroelectric material [43].
The relaxation behavior is usually related with polar nanoregions (PNRs) [36]. At high temperature, relaxors possess non-polar paraelectric (PE) phase and then transform into an ergodic relaxor (ER) state during cooling, in which PNRs appear in this process. The dynamics of PNRs slow down and their size grows and finally freeze into a nonergodic state (NER) at freezing temperature (Tf). The Tf is obtained by using Vogel–Fulcher (V–F) law [44]:
where f0 is attempt frequency, Ea is activation energy, Tf is static freezing temperature and k is Boltzmann constant. Figure 3b shows the V–F fitting for BLNKSB ceramic and the Tf is fitted to be 111 K. The f0 value is about 5.83 × 1014 Hz, which is an order higher than that of its upper physical limit (~ 1012–1013) [45]. This indicates V–F law is unsuitable for interpreting relaxation behaviors for BLNKSB system. Interestingly, a parameter p is adopted to characterize the dielectric relaxation strength (DRS) in relaxor ferroelectric, which has been introduced by Cheng et al. [45]:
where ω0 is attempt frequency and T0 is the temperature of activation energy. Figure 3d shows the fitting result and the p−1 value is calculated to be 0.34, which indicates the BLNKSB system is probably a Debye medium state. In Debye medium, there is no interaction between dipoles and they can be frozen only at absolute zero temperature theoretically [45].
In-situ temperature-dependent Raman spectra are performed to clarify the local structure evolution, as shown in Fig. 4(a, c). All Raman spectra are corrected by using Bose–Einstein temperature factor [20]. The corrected Raman intensity is calculated by the formula:
where Ic(w) is calculated Raman intensity and \(n\left( {w,T} \right) = \left[ {1/{\text{exp}}\left( {\frac{\hbar \omega }{{kT}} - 1} \right)} \right]\) is temperature factor. Obviously, all Raman spectra are broad and diffuse, which is due to random occupations of various cations at A–site. In general, Raman spectra can be divided into four regions within 100–1000 cm−1: (a) mixed A–site vibrations within 100–200 cm−1; (b) Ti–O bond vibrations in the range of 200–400 cm−1; (c) TiO6 octahedral vibrations within 400–700 cm−1 (d) the region above 700 cm−1 is correlated with the A1 and E overlapping modes [20, 46,47,48,49]. To analyze temperature-dependent phase transition, Raman spectra are deconvoluted into Lorentzian peaks from 100 cm−1 to 700 cm−1 to obtain the band position and full width at half maximum (FWHM). As well known, analysis of band position and FWHM evolution in Raman spectra can deeply understand the local structure changing as temperature increases. The deconvolution of peaks is shown in the bottom of Fig. 4b and d for BNSBPC and BLNKSB system, respectively.
Since 200–400 cm−1 wavenumber region is associated with Ti–O bands vibrations, it is usually selected to probe phase transitions in perovskite-type ceramics [49]. The evolution of band position and FWHM within 200–400 cm−1 is plotted in Fig. 5. Obviously, the wavenumber and FWHM exhibit a discontinuity at 403 K for BNSBPC composition (as highlighted by the shadow area), which signifies the appearance of phase transition. This is also in accordance with the spontaneous ferroelectric–relaxor phase transition temperature (see dielectric curves). The missing of anomaly in wavenumbers and FWHM evolution within 93 ~ 313 K demonstrates an absence of ferroelectric–relaxor phase transition. Therefore, phase transition character in BLNKSB composition is strikingly different to that of BNSBPC.
Figure 6(a-b, d-e) shows the temperature dependence of polarization–electric field (P–E) hysteresis loops and current density–electric field (J–E) loops for BNSBPC and BLNKSB ceramics (E = 60 kV cm−1). As shown in Fig. 6a, BNSBPC sample has a relatively slim P–E loop at high temperature and gradually transforms to a slanted shape and finally to a square shape with decreasing temperature. As well known, thermal agitation tends to destroy long-range polar correlations into fragmented nanodomains (ND) and finally PNRs [20]. The ferroelectric state revives with decreasing temperature due to re-connections of ND and PNRs. Combined with J–E loops (Fig. 6b) and contour map of absolute J–E (Fig. 6c), the single current peak is observed when the temperature decreases to 403 K, which demonstrates a relaxor-ferroelectric phase transition. However, ferroelectric property in BLNKSB ceramic is quite different, as shown in Fig. 6e, f. P–E loops are always slim within the temperature range of 213 ~ 423 K accompanied by rectangle J–E loops, which means an absence of domain switching under electric field. Notably, maximal polarization (Pmax) increases gradually with decreasing temperature for both samples since the reduction of thermal disturbance [14].
The indirect method is used to calculate temperature change according to Maxwell relation, which is expressed by the following equation:
where ρ is ceramic density, Cp is specific heat capacity and its evolution as temperature increases is shown in Fig. 7a, b. In contrast to BLNKSB ceramic, the gentle heat capacity peak near the Curie temperature illustrates relaxor-like behaviors for BNSBPC ceramics [50]. This may signal that BNSBPC composition is in an intermediate state of first-order and second-order phase transition. E1 and E2 is initial and final electric–field strength, respectively. The value of \(\left( {\partial P/\partial T} \right)_{E}\) is gained from a seven-order polynomial fitting of raw Pmax–T (extracted from Fig. 6(a, d)) at various external electric fields, as shown in Fig. 7c, d. Figure 7e, f shows profiles of adiabatic temperature change versus temperature (ΔT–T) under different electric fields. Obviously, ΔT of both samples increases with increasing electric field strength from E = 1 ~ 6 kV mm−1. Interestingly, ΔT is found to increase first and then decrease as temperature surpasses to 413 K for BNSBPC ceramic. As shown in Fig. 7e, the ΔTmax is 0.63 K at 413 K under E = 6 kV mm−1, which is comparable to other systems [7, 14, 17, 27, 32, 51]. Since distinct entropy change takes place across the phase transition, high ECE is hardly maintained over a wide temperature window [14]. The ECE peak occurs in the vicinity of ferroelectric–relaxor phase transition and the ΔTspan (defined as ± 10% variation of ΔTmax) is only 20 K, which limits its application in solid-state cooling devices. In contrast, BLNKSB ceramic possesses ΔTmax ~ 0.14 K and an ultrawide temperature span (~ 100 K) ranging from 223 to 323 K, as shown in Fig. 7f, which is superior to other lead-free ceramics [14, 17, 52, 53]. The large ΔTspan is related to the Debye medium character of BLNKSB since an absence of phase transition. The directly measured room-temperature ECE in both ceramics are shown in insets of Fig. 7(e, f) (E = 60 kV cm−1). As electric field is applied, there appears an upward exothermic peak, and when electric field is removed, there is a downward endothermic peak. This presents an obvious positive ECE characteristic. ΔT is about 0.08 K for BNSBPC and 0.03 K for BLNKSB ceramics at room temperature, which is slightly different to that of indirect method and likely because of the hysteretic response for calorimeter [53]. As a matter of fact, both indirect and direct method are adopted to calculate the ECE in ferroelectric materials. Notably, the variation trend matches well for both methods though ECE value may exist some differences [13, 14, 53].
Specific heat capacity Cp as a function of temperature: (a) BNSBPC ceramic; (b) BLNKSB ceramic. Maximal polarization versus temperature (Pmax–T) for (c) BNSBPC and (d) BLNKSB at various electric field. ECE versus temperature (ΔT–T) at different electric fields by indirect method for (e) BNSBPC and (f) BLNKSB ceramic. The insets of (e–f) show the ΔT measured by direct method under 60 kV cm−1 at room temperature, respectively
The electrocaloric strength (dT/dE) is an important parameter to estimate ECE properties under electric field. Based on the thermodynamic theory, Lu et al. obtained the electrocaloric strength (dT/dE) equation [54, 55]:
where β is the phenomenological coefficient (~ 4.71 × 105 K−1 and 3.83 × 105 K−1 for BNSBPC and BLNKSB, respectively), ɛ0 is vacuum dielectric constant (~ 8.85 × 10–12 F m−1), ɛr is relative dielectric constant and Cv is the specific heat per unit volume. Figure 8 shows the dT/dE versus temperature at 3 MV m−1 and 6 MV m−1 for BNSBPC and BLNKSB ceramics, respectively. With increasing electric field, the dT/dE also show an increase. Obviously, dT/dE first increases and then decreases for BNSBPC ceramic, and maximal dT/dE is 0.379 K m MV−1 near 420 K. In terms of BLNKSB ceramics, dT/dE gradually decreases with increasing temperature and maximal dT/dE is 0.064 K m MV−1 near 215 K. The calculated electrocaloric strength at room temperature (~ 0.048 K m MV−1 and 0.049 K m MV−1) for BNSBPC and BLNKSB ceramics are larger than that of practical measured values (~ 0.013 K m MV−1 and 0.005 K m MV−1), which maybe due to the heat dissipation in the ECE measurement.
Conclusion
In summary, we have successfully synthesized two kinds of pure-phase high-entropy ceramics with perovskite structure. The BNSBPC ceramic possesses a tetragonal structure with relaxor-like dielectric behaviors and the BLNKSB one has a cubic phase with Debye medium relaxation. The dielectric and Raman spectra analysis also verify the phase transition for both ceramics. The BNSBPC ceramic shows ΔTmax = 0.63 K with a relatively narrow ΔTspan ~ 20 K under E = 60 kV cm−1, while the BLNKSB exhibits excellent temperature stability with ΔTspan = 100 K and ΔTmax = 0.14 K. The high temperature stability for the BLNKSB ceramic is conducive to cooling device applications, and its ECE value deserves to be further improved yet. This work guides us to further explore high-performance ECE in HEC materials and also opens a way to developing novel materials in solid-state refrigeration applications.
References
Ma RJ, Zhang ZY, Tong KW, Huber D, Kornbluh R, Ju YS, Pei QB (2017) Highly efficient electrocaloric cooling with electrostatic actuation. Science 357(6356):1130–1134
Valant M (2012) Electrocaloric materials for future solid-state refrigeration technologies. Prog Mater Sci 57(6):980–1009
Mischenko AS, Zhang Q, Scott JF, Whatmore RW, Mathur ND (2006) Giant electrocaloric effect in thin-film PbZr0.95Ti0.05O3. Science 311(5765):1270–1271
Hagberg J, Uusimaki A, Jantunen H (2008) Electrocaloric characteristics in reactive sintered 0.87Pb(Mg1/3Nb2/3)O3-0.13PbTiO3. Appl Phys Lett 92(13):132909
Lu B, Jian XD, Lin XW, Yao YB, Tao T, Liang B, Luo HS, Lu SG (2020) Enhanced electrocaloric effect in single crystals via direct measurement 0.73Pb(Mg1/3Nb2/3)O3-0.27PbTiO3. Curr Comput-Aided Drug Des 10(6):451
Jian XD, Lu B, Li DD, Yao Y-B, Tao T, Liang B, Guo JH, Zeng Y-J, Chen JL, Lu SG (2018) Direct measurement of large electrocaloric effect in Ba(ZrxTi1–x)O3 Ceramics. ACS Appl Mater Interfaces 10(5):4801–4807
Li JN, Zhang DW, Qin SQ, Li TY, Wu M, Wang D, Bai Y, Lou XJ (2016) Large room-temperature electrocaloric effect in lead-free BaHfxTi1-xO3 ceramics under low electric field. Acta Mater 115:58–67
Luo ZD, Zhang DW, Liu Y, Zhou D, Yao YG, Liu CQ, Dkhil B, Ren XB, Lou XJ (2014) Enhanced electrocaloric effect in lead-free BaTi1-xSnxO3 ceramics near room temperature. Appl Phys Lett 105(10):102904
Bai Y, Han X, Ding K, Qiao LJ (2013) Combined effects of diffuse phase transition and microstructure on the electrocaloric effect Ba1-xSrxTiO3 in ceramics. Appl Phys Lett 103(16):162902
Han F, Bai Y, Qiao LJ, Guo D (2016) A systematic modification of the large electrocaloric effect within a broad temperature range in rare-earth doped BaTiO3 ceramics. J Mater Chem C 4(9):1842–1849
Weyland F, Eisele T, Steiner S, Fromling T, Rossetti GA, Rodel J, Novak N (2018) Long term stability of electrocaloric response in barium zirconate titanate. J Eur Ceram Soc 38(2):551–556
Hanani Z, Merselmiz S, Danine A, Stein N, Mezzane D, Mb A, Lahcini M, Gagou Y, Spreitzer M, Vengust D, Kutnjak Z, El Marssi M, Luk’yanchuk IA, Gouné M (2020) Enhanced dielectric and electrocaloric properties in lead-free rod-like BCZT ceramics. J Adv Ceram 9(2):210–219
Li JT, Bai Y, Qin SQ, Fu J, Zuo RZ, Qiao LJ (2016) Direct and indirect characterization of electrocaloric effect in (Na, K)NbO3 based lead-free ceramics. Appl Phys Lett 109(16):162902
Yang JL, Zhao Y, Lou XJ, Wu JG, Hao XH (2020) Synergistically optimizing electrocaloric effects and temperature span in KNN-based ceramics utilizing a relaxor multiphase boundary. J Mater Chem C 8(12):4030–4039
Wang XJ, Wu JG, Dkhil B, Xu BX, Wang XP, Dong GH, Yang G, Lou XJ (2017) Enhanced electrocaloric effect near polymorphic phase boundary in lead-free potassium sodium niobate ceramics. Appl Phys Lett 110(6):063904
Tao H, Yang JL, Lv X, Hao XH, Wu JG (2019) Electrocaloric behavior and piezoelectric effect in relaxor NaNbO3-based ceramics. J Am Ceram Soc 102(5):2578–2586
Yu Y, Gao F, Weyland F, Du HL, Jin L, Hou L, Yang ZT, Novak N, Qu SB (2019) Significantly enhanced room temperature electrocaloric response with superior thermal stability in sodium niobate-based bulk ceramics. J Mater Chem A 7(19):11665–11672
Turki O, Slimani A, Seveyrat L, Sebald G, Perrin V, Sassi Z, Khemakhem H, Lebrun L (2016) Structural, dielectric, ferroelectric, and electrocaloric properties of 2% Gd2O3 doping (Na0.5Bi0.5)(0.94)Ba0.06TiO3 ceramics. J Appl Phys 120(5):054102
Weyland F, Acosta M, Koruza J, Breckner P, Rodel J, Novak N (2016) Criticality: concept to enhance the piezoelectric and electrocaloric properties of ferroelectrics. Adv Func Mater 26(40):7326–7333
Li F, Li J, Zhai J, Shen B, Li S, Zhou M, Zhao K, Zeng H (2018) Influence of structural evolution on electrocaloric effect in Bi0.5Na0.5TiO3-SrTiO3 ferroelectric ceramics. J Appl Phys 124(16):164108
Miracle DB, Senkov ON (2017) A critical review of high entropy alloys and related concepts. Acta Mater 122:448–511
Oses C, Toher C, Curtarolo S (2020) High-entropy ceramics. Nat Rev Mater 5(4):295–309
Djenadic R, Sarkar A, Clemens O, Loho C, Botros M, Chakravadhanula VSK, Kubel C, Bhattacharya SS, Gandhif AS, Hahn H (2017) Multicomponent equiatomic rare earth oxides. Mater Res Lett 5(2):102–109
Rost CM, Sachet E, Borman T, Moballegh A, Dickey EC, Hou D, Jones JL, Curtarolo S, Maria JP (2015) Entropy-stabilized oxides. Nat Commun 6:8485
Dabrowa J, Stygar M, Mikula A, Knapik A, Mroczka K, Tejchman W, Danielewski M, Martin M (2018) Synthesis and microstructure of the (Co, Cr, Fe, Mn, Ni)3O4 high entropy oxide characterized by spinel structure. Mater Lett 216:32–36
Chen K, Pei X, Tang L, Cheng H, Li Z, Li C, Zhang X, An L (2018) A five-component entropy-stabilized fluorite oxide. J Eur Ceram Soc 38(11):4161–4164
Ge PZ, Tang XG, Liu QX, Jiang YP, Li WH, Luo J (2018) Energy storage properties and electrocaloric effect of Ba0.65Sr0.35TiO3 ceramics near room temperature. J Mater Sci-Mater Electron 29(2):1075–1081
Bérardan D, Franger S, Dragoe D, Meena AK, Dragoe N (2016) Colossal dielectric constant in high entropy oxides. Physica Status Solidi (RRL) Rapid Res Lett 10(4):328–333
Bérardan D, Franger S, Meena AK, Dragoe N (2016) Room temperature lithium superionic conductivity in high entropy oxides. J Mat Chem A 4(24):9536–9541
Chen H, Fu J, Zhang P, Peng H, Abney CW, Jie K, Liu X, Chi M, Dai S (2018) Entropy-stabilized metal oxide solid solutions as CO oxidation catalysts with high-temperature stability. J Mater Chem A 6(24):11129–11133
Zhai S, Rojas J, Ahlborg N, Lim K, Toney MF, Jin H, Chueh WC, Majumdar A (2018) The use of poly-cation oxides to lower the temperature of two-step thermochemical water splitting. Energy Environ Sci 11(8):2172–2178
Pu Y, Zhang Q, Li R, Chen M, Du X, Zhou S (2019) Dielectric properties and electrocaloric effect of high-entropy (Na0.2Bi0.2Ba0.2Sr0.2Ca0.2)TiO3 ceramic. Appl Phys Lett 115:223901
Du Q, Yan JH, Zhang XY, Li JS, Liu XY, Zhang JR, Qi XW (2020) Phase evolution and dielectric properties of Ba(Ti1/6Sn1/6Zr1/6Hf1/6Nb1/6Ga1/6)O3 high-entropy perovskite ceramics. J Mater Sci-Mater Electron 31(10):7760–7765
Xiang HM, Xing Y, Dai FZ, Wang HJ, Su L, Miao L, Zhang GJ, Wang YG, Qi XW, Yao L, Wang HL, Zhao B, Li JQ, Zhou YC (2021) High-entropy ceramics: Present status, challenges, and a look forward. J Adv Ceram 10(3):385–441
Li F, Chen GR, Liu X, Zhai JW, Shen B, Zeng HR, Li SD, Li P, Yang K, Yan HX (2017) Phase-composition and temperature dependence of electrocaloric effect in lead-free Bi0.5Na0.5TiO3-BaTiO3-(Sr0.7Bi0.2□0.1)TiO3 ceramics. J Eur Ceram Soc 37(15):4732–4740
Li F, Li J, Zhai J, Shen B, Li S, Zeng H (2019) Phase formation and electrocaloric effect in nonstoichiometric 0.94Bi0.5+xNa0.5TiO3-0.06BaTiO3 ceramics. J Mater Sci-Mater Electron 30(4):3465–3471
Jiang S, Hu T, Gild J, Zhou N, Nie J, Qin M, Harrington T, Vecchio K, Luo J (2018) A new class of high-entropy perovskite oxides. Scripta Mater 142:116–120
Goldschmidt VM (1926) Die Gesetze der Krystallochemie. Naturwissenschaften 14(21):477–485
Shvartsman VV, Kleemann W, Dec J, Xu ZK, Lu SG (2006) Diffuse phase transition in BaTi1-xSnxO3 ceramics: An intermediate state between ferroelectric and relaxor behavior. J Appl Phys 99(12):124111
Bokov AA, Ye ZG (2006) Recent progress in relaxor ferroelectrics with perovskite structure. J Mater Sci 41(1):31–52
Sharma P, Kumar P, Kundu RS, Juneja JK, Ahlawat N, Punia R (2015) Structural and dielectric properties of substituted barium titanate ceramics for capacitor applications. Ceram Int 41(10):13425–13432
Sharma P, Berwal N, Ahlawat N, Maan AS, Punia R (2019) Study of structural, dielectric, ferroelectric and magnetic properties of vanadium doped BCT ceramics. Ceram Int 45(16):20368–20378
Xiong W, Zhang H, Cao S, Gao F, Svec P, Dusza J, Reece MJ, Yan H (2021) Low-loss high entropy relaxor-like ferroelectrics with A-site disorder. J Eur Ceram Soc 41(4):2979–2985
Viehland D, Jang SJ, Cross LE, Wuttig M (1990) Freezing of the polarization fluctuations in lead magnesium niobate relaxors. J Appl Phys 68(6):2916–2921
Cheng ZY, Zhang LY, Yao X (1996) Investigation of glassy behavior of lead magnesium niobate relaxors. J Appl Phys 79(11):8615–8619
Barick BK, Mishra KK, Arora AK, Choudhary RNP, Pradhan DK (2011) Impedance and Raman spectroscopic studies of (Na0.5Bi0.5)TiO3. J Phys D-Appl Phys 44(35):8615–8619
Jiang XJ, Wang BY, Luo LH, Li WP, Zhou J, Chen HB (2014) Electrical properties of (1–x)(Bi0.5Na0.5)TiO3-xKNbO3 lead-free ceramics. J Solid State Chem 213:72–78
Schutz D, Deluca M, Krauss W, Feteira A, Jackson T, Reichmann K (2012) Lone-pair-induced covalency as the cause of temperature- and field-induced instabilities in bismuth sodium titanate. Adv Func Mater 22(11):2285–2294
Haumont R, Gemeiner P, Dkhil B, Kiat JM, Bulou A (2006) Polar and chemical states at a nanometer scale in a PbSc1/2Nb1/2O3-PbTiO3 system investigated by Raman spectroscopy. Phys Rev B 73(10):104106
Li J, Li J, Qin S, Su X, Qiao L, Wang Y, Lookman T, Bai Y (2019) Effects of long- and short-range ferroelectric order on the electrocaloric effect in relaxor ferroelectric ceramics. Phys Rev Appl 11(4):044032
Wang JF, Yang TQ, Chen SC, Li G, Zhang QF, Yao X (2013) Nonadiabatic direct measurement electrocaloric effect in lead-free Ba, Ca(Zr, Ti)O3 ceramics. J Alloy Compd 550:561–563
Zhang L, Zhao C, Zheng T, Wu J (2021) Large electrocaloric response with superior temperature stability in NaNbO3-based relaxor ferroelectrics benefiting from the crossover region. J Mater Chem A 9(5):2806–2814
Li F, Li K, Long MS, Wang CC, Chen GH, Zhai JW (2021) Ferroelectric-relaxor crossover induce large electrocaloric effect with ultrawide temperature span in NaNbO3-based lead-free ceramics. Appl Phys Lett 118(4):043902
Lu SG, Li DD, Lin XW, Jian XD, Zhao XB, Yao YB, Tao T, Liang B (2020) Influence of electric field on the phenomenological coefficient and electrocaloric strength in ferroelectrics. Acta Physica Sinica 69(12):127701
Lu SG, Lin XW, Li J, Li DD, Yao YB, Tao T, Liang B (2021) Enhanced electrocaloric strengths at room temperature in (SrxBa1-x)(Sn0.05Ti0.95)O3 (lead-free ceramics. J Alloys Compd 871:159519
Acknowledgements
This work was supported by Anhui Provincial Natural Science Foundation (No. 2008085QE205), Anhui Provincial higher–education Natural Science research project (KJ2020A0019), the Open Project Program of Guangdong Provincial Key Laboratory of Electronic Functional Materials and Devices, Huizhou University (No. EFMD2020004Z), Guangxi Key Laboratory of Information Materials (Guilin University of Electronic Technology, No. 201006–K) and the National Natural Science Foundation of China (Nos. 51872001 and 51772211).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: David Cann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, W., Li, F., Chen, G. et al. Comparative study of phase structure, dielectric properties and electrocaloric effect in novel high-entropy ceramics. J Mater Sci 56, 18417–18429 (2021). https://doi.org/10.1007/s10853-021-06530-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06530-9