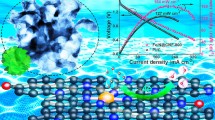
Abstract
Oxygen evolution reaction (OER) for water splitting has a sluggish kinetics, thus significantly hindering the reaction efficiency. So far, it is still challenging to develop a cost-efficient and highly active catalyst for OER processes. To address such issues, we design and synthesize NiP2/FeP heterostructural nanoflowers interwoven by carbon nanotubes (NiP2/FeP@CNT) by a hydrothermal reaction followed by phosphating. The NiP2/FeP@CNT catalyst delivers excellent OER performance: it displays an ultralow Tafel slope of 44.0 mV dec−1 and a relatively low overpotential of 261 mV at 10 mA cm−2, better than RuO2 commercial catalyst; it also shows excellent stability without observable decay after 20-h cycling. The outstanding OER property is mainly attributed to its special 3D stereochemical structure of CNT-interwoven NiP2/FeP heterostructural nanoflowers, which is highly conductive and guarantees considerable active sites. Such nanostructure greatly facilitates the charge transfer, which significantly improves its electrocatalytic activity. This work offers a simple method to synthesize non-precious transition metal-based phosphide electrocatalysts with a unique hierarchical nanostructure for water splitting.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The increasing energy consumption and the serious environmental pollution forces us to develop sustainable and environmentally friendly hydrogen energy [1,2,3,4]. Water splitting has attracted much attention as a green strategy for hydrogen energy production. However, the water-splitting efficiency is reduced by the slow dynamics of the multiple electron transfer in the oxygen evolution reaction (OER) process [5,6,7,8,9]. At present, RuO2 or IrO2 is still considered the mature catalyst in all respects, although the high price and scarcity hinder its large-scale applications [10,11,12,13,14]. Hence, it is urgent and essential to develop earth-abundant non-precious electrocatalysts for OER [15,16,17].
At present, great efforts have been made to explore non-noble metal catalyst materials, such as transition metal phosphates, sulfides, oxides, hydroxides and non-metallic catalysts [18,19,20]. Among various catalysts, transition metal phosphides have aroused the interest of many researchers because of their durable stability and remarkable OER performances [21]. In particular, NiFe-based phosphate is the most outstanding as a highly efficient catalyst for OER. Because the electronic interaction between Ni and Fe can regulate the affinity of the catalyst material surface to oxygen, so as to achieve the optimal conditions to promote the generation of oxygen molecules. At the same time, the morphology and structure of nanomaterials are also important factors to determine whether the performance is excellent. The catalyst materials with a three-diensonal structure increase the electrochemical surface area during the reaction. Therefore, it is very important to develop a three-dimensional transition metal phosphide catalyst [22].
Wang et al. [23] synthesized heterostructural nanoparticles growing on nanosheets which provide rich active surfaces, the overpotential (at 10 mA cm−2) and the Tafel slope are 295 mV, and 59.1 mV dec−1, respectively. Luo et al. [24] by hydrothermal method and phosphating prepared a novel nanostructure based on graphene oxide as a carrier, showing a small overpotential of 221 mV. However, the Tafel slope reached 105.7 mV dec−1. Xuan et al. first synthesized Prussian blue analogues of NiFe, then phosphated its precursors, and finally produced the morphology of nanocube, which exposed more active sites. The overpotential was 271 mV (10 mA cm−2), and the corresponding Tafel slope was 53 mV dec−1 [25]. The comparison of these reported studies shows that the transition metal phosphates have outstanding electrocatalytic activities for OER. However, the long-term stability of metal phosphides is still not so satisfactory compared to commercial RuO2 or IrO2 catalyst. Therefore, it is urgently desired to rationally design and facilely synthesize transition metal phosphide-based electrocatalysts with unique nanostructure and high efficiency for OER.
To address such issues, herein, NiP2/FeP heterostructural nanoflowers interwoven by carbon nanotubes (NiP2/FeP@CNT), are synthesized via a facile hydrothermal reaction followed with phosphating. The nanoflowers appear to unique 3D nanoporous architecture constructed by NiP2/FeP heterostructural nanoflowers interwoven by highly conductive carbon nanotubes, this porous structure has good gas permeability and is conducive to the generation of oxygen. The NiP2/FeP@CNT catalyst delivers outstanding OER performance with a very low Tafel slope of 44.0 mV dec−1 and overpotential of 261 mV @ 10 mA cm−2 and excellent conductivity. This work provides a strategy for facile synthesis of catalysts with low cost, high conductivity and outstanding performance, which is promising for large-scale industrial application.
Experimental section
Synthesis of NiFe-LDH@CNT
NiFe-LDH@CNT was prepared via a one-pot solvothermal method. First, 1.2 g of CNT slurry was placed in a beaker containing 60 ml of deionized water and ultrasonized for 30 min. Precisely, 1 mmol of Ni(NO3)2·6H2O, 1 mmol of Fe(NO3)3·9H2O, 8 mmol NH4F and 20 mmol of CO(NH2)2 were weighed and dissolved in the solution described above. The mixture was magnetically stirred for another 30 min. Then, after the mixture was evenly mixed, the mixture was transferred into 100 mL of the stainless steel Teflon-lined autoclave, and then heated to 120 °C for 12 h. After cooling down naturally, the obtained black product was washed several times with ethanol and DI water, then placed overnight in a vacuum freeze-dryer. At the end of the freeze-drying process, the sample is taken out and carefully ground into a powder for further use. For comparison, the synthesis of NiFe-LDH was identical to NiFe-LDH@CNT without the addition of CNT slurry.
Synthesis of NiP2/FeP@CNT nanoflowers
The as-prepared NiFe-LDH@CNT and NaH2PO2·H2O were placed in a tube furnace with two separate porcelain boats for annealing treatment under Ar atmosphere at 400 °C with temperature rising rate of 3 °C min−1 for 2 h. When the temperature of the tubular furnace naturally cools to the surrounding environment, the resulting product is named NiP2/FeP@CNT. NiFeP is obtained in the same way, but the precursor is NiFe-LDH instead of NiFe-LDH@CNT.
Electrocatalytic measurements
The OER performances were performed on an electrochemical workstation (CHI660D) equipped with three electrodes in 1 M KOH solution at ambient temperature (298 K). Generally, a graphite rod was used as the counter electrode; and the Hg/HgO electrode was used as the reference electrode in OER; the glassy carbon electrode with the sample was used as a working electrode. The preparation method of the electrode is shown as follows. About 750 μL water and 250 μL ethanol were used to disperse 4 mg of the catalyst solid. Ultrasonication was performed for 10 min to evenly disperse the sample. Next, add 50 μL of 5 wt% Nafion solution to the above solution and continue ultrasonicated for a period of time. Finally, 5 μL of liquid was applied to the glass carbon electrode with a pipette gun and then dried in an oven at 60 °C for several minutes. In all measurements, potential values were changed to a reversible hydrogen electrode (RHE): ERHE = EHg/HgO + 0.923 V. Before all electrochemical tests, an activation scan of minimum of 20 rounds of cyclic voltammetry (CV) cycles was performed at 100 mV s−1 to obtained stable curves. The linear sweep voltammetry of NiP2/FeP@CNT was tested at 1 mV s−1 to eliminate the redox peak, and the remaining samples were measured at 5 mV s−1. And, the double-layer capacitance (Cdl) was also calculated from cyclic voltammogram (CV) data in 1 M KOH solution, which require sweeping rates from 20 to 200 mV s−1 over a range of potential tests, with a step of 20 mV s−1 for each curve. The electrochemical impedance spectroscopy (EIS) tests were performed from 100 kHz to 0.1 Hz. The long-time stability of NiP2/FeP@CNT was evaluated by continuous chronoamperometry using nickel foam (1 × 1 cm).
Results and discussion
The preparation of the NiP2/FeP@CNT electrocatalyst is shown in the schematic diagram in Fig. 1, including a hydrothermal step followed by a phosphating reaction. Initially, the Teflon-lined autoclave with reactants was put in a hydrothermal oven for 12 h (120 °C) to get NiFe-LDH@CNT. Then, the appropriate amount of the above sample was placed together with sodium hypophosphite into a high-temperature tubular furnace at 400 °C for 2 h under continuous argon flow. After natural cooling to room temperature, the sample NiP2/FeP@CNT was obtained.
The sample structure was performed by X-ray diffractions (XRD). As displayed in Fig. 2a, XRD patterns of NiFeP and NiP2/FeP@CNT shows characteristic peaks at 31.6°, 35.9°, 40.7°, 47.9° and 55.4°, corresponding to the (200), (210), (211), (220) and (311) planes of NiP2, respectively.
The other signals at 32.7°, 37.2° and 48.3°can be attributed to the (011), (111), (211) planes of FeP, respectively [23, 24]. Then, the Raman spectrum of the NiP2/FeP@CNT is provided, considering the signal of carbon can not be detected by XRD characterization. As shown in Fig. 2b, characteristic Raman peaks can be clearly seen at 1350 cm−1 and ~1600 cm−1, which can be assigned to the D, and G bands of carbon nanotubes, respectively [25]. In order to get the information of the surface properties of NiP2/FeP@CNT, the BET test was acquired through N2 adsorption–desorption-isotherm as displayed in Fig. 2c, d. The NiP2/FeP@CNT shows a BET surface area of 68.36 m2 g−1, considerably larger than that of NiFeP (23.07 m2 g−1) [26, 27]. Additionally, The distribution of pore size obtained from the Barrett–Joyner–Halenda (BJH) method is presented in Fig. 2d. The NiP2/FeP@CNT displays great probabilities at the range of 20–60 nm, confirming the overwhelming existence of mesopores, which is rarely observed in the sample NiFeP. The larger surface area and higher mesoporous percentage are the result of the specific microtopography of nanoflower as well the carbon nanotube networks. More importantly, from a microstructure point of view, the larger surface area and higher mesoporous percentage are generally favored in catalysis due to the greatly increase in active sites and facilitate mass transfer [20].
X-ray photoelectron spectroscopy (XPS) analysis was performed for NiP2/FeP@CNT. As shown in Fig. S2 and Fig. 3, the XPS survey spectrum of NiP2/FeP@CNT shows the coexistence of Fe, Ni C, P and O. The XPS spectrum of C 1 s in Fig. 3a exhibits three peaks at 289.7, 285.0 and 283.8 eV, which corresponds to O–C = O, C–O and C–C bonds, respectively [23, 26]. The XPS spectrum of Fe 2p is shown in Fig. 3b. It can be observed that there are two peaks at 706.5 and 724.1 eV, matching with the Fe 2p3/2 and Fe 2p1/2 of FeP, respectively. The wider peak at 710.0 eV and 713.3 eV are ascribed to the oxidation peak of Fe and shakeup satellite. Figure 3c shows the XPS spectrum of Ni 2p. There are three peaks at 853.6, 856.1 and 860.4 eV, corresponding to Ni2+, Ni3+ and the satellite peaks of Ni 2p3/2, respectively. Ni2+, Ni3+ and the satellite peaks in Ni 2p1/2 are located at 870.4, 874.3 and 879.1, respectively [28]. In P 2p spectrum, as shown in Fig. 3d, the characteristic signal of P 2p3/2 and P 2p1/2 with peaks at 128.8 and 129.6 eV reflect the formation of phosphide, and the other peak at 133.8 eV belong to the P–O band [26, 31].
The morphologies of the samples can be clearly revealed by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). As shown in Fig. 4a, NiP2/FeP@CNT shows the morphology of nanoflowers supported by a network of carbon nanotubes, which is similar to the structure of neural networks in the human body [25]. In Fig. S1a, b, the obtained NiFe-LDH@CNT presents as homogeneous nanoflowers dispersed in the CNT network. After the phosphating treatment, these nanoflowers were tightly wrapped with carbon nanotubes, with the size and shape well maintained (Fig. 4a). The SEM images of the other reference samples are displayed in Fig. S1. In Fig S1. a and b, we can clearly see the interweaving of nanoflowers and carbon nanotubes, which proves that the morphology of the sample remains the same after phosphating. Meanwhile, this is also confirmed by the TEM image (Fig. 4b). High-resolution TEM (HRTEM) image of NiP2/FeP@CNT (Fig. 4c, d) shows lattice spacing of 0.188 and 0.219 nm, which are consistent with the plane (211) of FeP and (211) of NiP2. The EDX spectrum and the content of the element can prove the existence of C, P, Ni and Fe elements (Fig. S4, Table S1). From the element mapping images (Fig. 4e–i), it can be further concluded that these elements are evenly distributed in the sample.
Considering the nanoflower morphology of NiP2/FeP@CNT, good catalytic performance is expected. The OER electrochemical activity of NiP2/FeP@CNT was evaluated in a conventional three-electrode setup in alkaline electrolyte. Figure 5a shows the linear sweep voltammetry (LSV) plots of NiP2/FeP@CNT, NiFeP, NiFe-LDH@CNT, NiFe-LDH and RuO2. NiP2/FeP@CNT needs 261 mV to deliver 10 mA cm−2, while NiFeP, NiFe-LDH@CNT, NiFe-LDH and RuO2 require 319, 319, 414 and 295 mV, respectively. Compared NiFe-LDH@CNT, the performance of NiP2/FeP@CNT is greatly improved, which proves that phosphating plays a very significant role. Figure 5b shows the Tafel slopes of NiP2/FeP@CNT, NiFeP, NiFe-LDH@CNT, NiFe-LDH and RuO2. Besides, NiP2/FeP@CNT delivers the lowest Tafel slope of is 44 mV dec−1. The order of Tafel slope is NiP2/FeP@CNT (44 mV dec−1) < NiFe-LDH@CNT (62 mV dec−1) < NiFeP(68 mV dec−1) < RuO2 (73 mV dec−1) < NiFe-LDH (76 mV dec−1). Obviously, NiP2/FeP@CNT shows the best OER activity, making this unique 3D nanoflower interwoven by carbon network as an excellent non-noble metal-based OER catalyst material [23, 29, 30].
a LSV curves in 1 M KOH solution and b corresponding Tafel plots of NiP2/FeP@CNT, NiFeP, NiFe-LDH@CNT, NiFe-LDH and RuO2. c Estimated Cdl values and relative electrochemically active surface areas, and d Nyquist plots of NiP2/FeP@CNT, NiFeP, NiFe-LDH@CNT and NiFe-LDH. The inset of (d) is the magnification of the EIS spectrum of NiP2/FeP@CNT
Electrochemical active surface area (ECSA) is a key factor of catalyst property, which is usually characterized by the double-layer capacitance (Cdl). The Cdl was obtained by performing CV scans with different rates over a certain voltage range (Fig. S3). Notably, the Cdl values of NiP2/FeP@CNT and NiFe-LDH@CNT are very similar, which is consistent with the similar morphology between the two materials. This result indicates that the optimal performance of NiP2/FeP@CNT is attributed to an enhanced catalytic activity instead of the surface area change. Electrochemical impedance spectroscopy (EIS) was usually used to characterize the charge transfer status during the OER process. The obtained Nyquist plots are shown in Fig. 5d. NiP2/FeP@CNT has the smallest charge transfer resistance (Rct), indicating a smooth charge transfer and thus a rapid reaction.
The mechanism for the high performance of NiP2/FeP@CNT can be summarized as below. First of all, the addition of carbon nanotube networks improved the electrical conductivity of the material. Secondly, the three-dimensional structure of the nanoflower structure exposed more active areas and active sites. Finally, the presence of heterojunctions offers a strong synergistic effect, which further enhances the catalytic activity.
In addition, enduring stability is another important factor in evaluating the performance of catalyst materials. As shown in Fig. 6b, the current density maintained almost unchanged during the 20 h test. The disadvantage is that a higher voltage is displayed after 3000 cycles. Despite this, NiP2/FeP@CNT still has great prospects for OER in alkaline media [32].
Conclusion
In summary, NiP2/FeP@CNT heterostructural nanoflowers interwoven by carbon nanotubes are synthesized by the strategy of hydrothermal and subsequent phosphating. The NiP2/FeP@CNT hybrid shows an ultralow overpotential (261 mV) to deliver 10 mA cm−2 and a small Tafel slope (44 mV dec−1). The outstanding properties are assigned to the 3D structure of CNT-interwoven NiP2/FeP heterostructural nanoflowers, which provides rich active sites and enhanced conductivity. Significantly, the synergistic effect between the ultrathin NiP2/FeP heterostructural flowers and the skeleton carbon nanotubes favors charge transfer and ion diffusion. This work offers a simple method to synthesize non-precious transition metal-based phosphide electrocatalysts with a unique hierarchical nanostructure for water splitting.
References
Huo D, Song F, Hu J, Yuan J, Niu L, Wang A-j (2021) One-step synthesis of carbon-encapsulated nickel phosphide nanoparticles with efficient bifunctional catalysis on oxygen evolution and reduction. Int J Hydrog Energy 46(12):8519–8530
Li D, Liu C, Ma W et al (2021) Fe-doped NiCoP/prussian blue analog hollow nanocubes as an efficient electrocatalyst for oxygen evolution reaction. Electrochim Acta 367:137492
Li Y, Ge L, Zhou Y et al (2020) KB-templated in situ synthesis of highly dispersed bimetallic NiFe phosphides as efficient oxygen evolution catalysts. Inorg Chem Front 7(24):4930–4938
Lim D, Lim C, Hwang M, Kim M, Shim SE, Baeck S-H (2021) Facile synthesis of flower-like P-doped nickel-iron disulfide microspheres as advanced electrocatalysts for the oxygen evolution reaction. J Pow Sour 490:229552
Xu H, Ye K, Zhu K et al (2020) Transforming carnation-shaped MOF-Ni to Ni–Fe prussian blue analogue derived efficient bifunctional electrocatalyst for urea electrolysis. ACS Sustain Chem Eng 8(42):16037–16045
Cui Z, Lin J, Wu J, Yu J, Si J, Wang Q (2021) N- doped CoP nanoparticles embedded in electrospun N-doped porous carbon nanofiber as high-efficiency oxygen evolution electrocatalysts. J Alloys Compd 854:156830
Fei L, Sun H, Ran R, Zhou W, Shao Z (2021) Self-supported nickel phosphide electrode for efficient alkaline water-to-hydrogen conversion via urea electrolysis. Ind Eng Chem Res 60(3):1185–1193
Wang C, Shang H, Xu H, Du Y (2021) Nanoboxes endow non-noble-metal-based electrocatalysts with high efficiency for overall water splitting. J Mater Chem A 9(2):857–874
Chen D, Chen Y, Zhang W, Cao R (2021) Nickel selenide from single-molecule electrodeposition for efficient electrocatalytic overall water splitting. New J Chem 45(1):351–357
Yu J, Cheng G, Luo W (2017) Hierarchical NiFeP microflowers directly grown on Ni foam for efficient electrocatalytic oxygen evolution. J Mater Chem A 5(22):11229–11235
Wang J, Chen C, Cai N, Wang M, Li H, Yu F (2021) High topological tri-metal phosphide of CoP@FeNiP toward enhanced activities in oxygen evolution reaction. Nanoscale 13(2):1354–1363
Wei B, Xu G, Hei J, Zhang L, Huang T (2021) PBA derived FeCoP nanoparticles decorated on NCNFs as efficient electrocatalyst for water splitting. Int J Hydrog Energy 46(2):2225–2235
Anantharaj S, Sugime H, Chen B, Akagi N, Noda S (2020) Boosting the oxygen evolution activity of copper foam containing trace Ni by intentionally supplementing Fe and forming nanowires in anodization. Electrochim Acta 364:137170
Wu X, Li J, Li Y, Wen Z (2021) NiFeP-MoO2 hybrid nanorods on nickel foam as high-activity and high-stability electrode for overall water splitting. Chem Eng J 409:128161
Tong J, Li C, Bo L et al (2021) Bimetallic Fe–Co chalcogenophosphates as highly efficient bifunctional electrocatalysts for overall water splitting. Int J Hydrog Energy 46(5):3354–3364
Kang Y, Wang S, Zhu S et al (2021) Iron-modulated nickel cobalt phosphide embedded in carbon to boost power density of hybrid sodium–air battery. Appl Catal B Environ 285:119786
Liu X, Yao Y, Zhang H et al (2020) In situ-grown cobalt-iron phosphide-based integrated electrode for long-term water splitting under a large current density at the industrial electrolysis temperature. ACS Sustain Chem Eng 8(48):17828–17838
Suryawanshi UP, Ghorpade UV, Lee DM et al (2020) Colloidal Ni2P nanocrystals encapsulated in heteroatom-doped graphene nanosheets: a synergy of 0D@2D heterostructure toward overall water splitting. Chem Mater 33(1):234–245
Shuai C, Mo Z, Niu X et al (2020) Nickel/cobalt bimetallic phosphides derived metal-organic frameworks as bifunctional electrocatalyst for oxygen and hydrogen evolution reaction. J Alloys Compd 847:156514
Wang B, Chen Y, Wang X et al (2020) A microwave-assisted bubble bursting strategy to grow Co8FeS8/CoS heterostructure on rearranged carbon nanotubes as efficient electrocatalyst for oxygen evolution reaction. J Pow Sour 449:227561
Wang Y, Sun L, Lu L, Xu D, Hao Q, Liu B (2021) A sequential template strategy toward hierarchical hetero-metal phosphide hollow nanoboxes for electrocatalytic oxygen evolution. J Mater Chem A 9(6):3482–3491
Wang Z, Wei C, Zhu X, Wang X, He J, Zhao Y (2021) A hierarchical carbon nanotube forest supported metal phosphide electrode for efficient overall water splitting. J Mater Chem A 9(2):1150–1158
Wang H, Wang X, Zheng B, Yang D, Zhang W, Chen Y (2019) Self-assembled Ni2P/FeP heterostructural nanoparticles embedded in N-doped graphene nanosheets as highly efficient and stable multifunctional electrocatalyst for water splitting. Electrochim Acta 318:449–459
Luo S, Wang R, Hei P, Gao L, Yang J, Jiao T (2021) Self-assembled Ni2P nanosheet-implanted reduced graphene oxide composite as highly efficient electrocatalyst for oxygen evolution reaction. Coll Surf A Physicochem Eng Asp 612:125992
Wang B, Hu Y, Yu B, Zhang X, Yang D, Chen Y (2019) Heterogeneous CoFe–Co8FeS8 nanoparticles embedded in CNT networks as highly efficient and stable electrocatalysts for oxygen evolution reaction. J Pow Sour 433:126688
Li W, Chen Y, Yu B, Hu Y, Wang X, Yang D (2019) 3D hollow Co–Fe–P nanoframes immobilized on N, P-doped CNT as an efficient electrocatalyst for overall water splitting. Nanoscale 11(36):17031–17040
Wu Y, Xiao Z, Jin Z, Li X, Chen Y (2021) The cobalt carbide/bimetallic CoFe phosphide dispersed on carbon nanospheres as advanced bifunctional electrocatalysts for the ORR, OER, and rechargeable Zn-air batteries. J Coll Interface Sci 590:321–329
Song C, Liu Y, Wang Y, Tang S, Li W, Li Q, Zeng J, Chen L, Peng H, Lei Y (2021) Highly efficient oxygen evolution and stable water splitting by coupling NiFe LDH with metal phosphides. Sci China Mater 64(7):1662–1670
Wu Y, Li Y, Yuan M, Lü Z, Xu L, Wei B (2020) Direct growth of Ni–Fe phosphides nanohybrids on NiFe foam for highly efficient water oxidation. J Alloys Compd 847:156363
Liu H, Zhu S, Cui Z, Li Z, Wu S, Liang Y (2021) Ni2P nanoflakes for the high-performing urea oxidation reaction: linking active sites to a UOR mechanism. Nanoscale 13(3):1759–1769
Wang B, Chen Y, Wang X, Ramkumar J, Zhang X, Yu B, Yang D, Karpuraranjith M, Zhang W (2020) rGO wrapped trimetallic sulfide nanowires as an efficient bifunctional catalyst for electrocatalytic oxygen evolution and photocatalytic organic degradation. J Mater Chem A 8:13558–13571
Wang B, Chen Y, Wu Q, Lu Y, Zhang X, Wang X, Yu B, Yang D, Zhang W (2021) A co-coordination strategy to realize janus-type bimetallic phosphide as highly efficient and durable bifunctional catalyst for water splitting. J Mater Sci Technol 74:11–20. https://doi.org/10.1016/j.jmst.2020.10.009
Acknowledgements
This research was supported by the National Natural Science Foundation of China (Grant Nos. 52062045, 21773024), sichuan Science and Technology program (Grant Nos. 2020YJ0324, 2020YJ0262), Reformation and Development Funds for Local Region Universities from China Government in 2020 (Grant No. ZCKJ 2020-11) and China Postdoctoral Science Foundation (Grant No. 2019M653376).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts of interests.
Additional information
Handling Editor: Kyle Brinkman.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, Y., Wang, B., Lu, Y. et al. NiP2/FeP heterostructural nanoflowers interwoven by carbon nanotubes as highly efficient electrocatalyst for oxygen evolution reaction. J Mater Sci 56, 16000–16009 (2021). https://doi.org/10.1007/s10853-021-06282-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06282-6