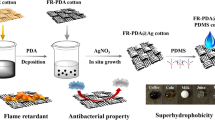
Abstract
Flame-retardant and superhydrophobic coatings over cotton fabric are fabricated by solution immersion method using green-based flame retardants, i.e. DNA, silver nitrate and octadecyltriethoxysilane are used to get flame-retardant cotton with superhydrophobic nature. The results of this work showed that the as-prepared cotton fabric with a maximal contact angle of 157° was obtained and surfaces before and after were characterised by scanning electron microscopy, X-ray photoelectron spectroscopy and FT-IR studies. Some typical flame tests and TGA/DTG studies were done to evaluate the flame retardant property and thermal stability of the coated cotton samples. These results demonstrated that solution immersion strategy is a relatively simple and convenient method to fabricate superhydrophobic and flame-retardant cotton fabric.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Among the various types of textiles, such as cotton, silk, wool and other synthetic fabrics, cotton is widely used fabric for apparel, domestic (upholsteries, mattresses, etc.), medical and industrial applications [1, 2] due to its extreme outstanding properties such as high breathability, wearable comfortability in all climatic conditions and softness. It is already known that cotton fabrics are made up of cellulose fibres, most abundant biodegradable polymer in world [3]. However, it is one of the highly flammable textile fabrics, and it causes more commercial fire accidents, serious civilian deaths and many property losses. Therefore, many researchers and scientists are trying to find the ways to render the cotton textiles with less flammable/inflammable in order to safeguard people from fire accidents in an eco-friendly and cost-effective manner [4, 5].
Recently, a number of methods are reported for production of flame-retardant textiles such as surface chemical modification, surface photo-induced chemical grafting method and addition of flame-retardant additive into fibre blending and spinning processes. Due to the availability of instrumentation facilities and production of textile fabrics with higher efficiency, surface modification is one of the most widely accepted fabrication methods for the production of flame-retardant fabrics [6]. Organo-phosphorous, halogenated organic compounds, nitrogen-based compounds and inorganic compounds are commonly used flame retardants in textile industries. The major identified drawbacks of halogenated organic compounds and organo-phosphorous compounds are the production of toxic gases during thermal degradation process, which causes many respiratory problems to human, and also they require some more additives for the strong adhesion over cotton surface [7].
As an alternative to traditional approaches, the biomacromolecules like proteins and nucleic acids are considered as better environmentally friendly, renewable green flame retardants. In past few years, many green alternatives such as proteins, caseins [8,9,10], hydrophobins [11], deoxyribonucleic acid (DNA) [12, 13] as well as some of the bio-based flame retardants such as phytic acid [14] and cyclodextrin [15] were used as green alternatives for the fabrication of flame-retardant textiles. The major advantages of these types of biomacromolecules in flame retardancy are: (1) significant enhancement, (2) low environmental impact, (3) less toxicity, (4) less or no volatile organic carbon production and (5) ease of availability [16]. Thus, many researchers utilised these types of bio-based flame retardants as effective approaches in textile applications.
For example, Alongi and his co-workers used caseins and hydrophobins as novel green flame retardants for the fabrication of flame-retardant cotton fabrics [11]. In 2014, Zang and his co-workers used chitosan/phytic acid polyelectrolyte complex as flame retardant for ethylene–vinyl acetate co-polymer [17], and Carosio et al. employed DNA and chitosan as flame retardants over cotton fabric [18]. In addition, Quartinello and his co-workers increase the flame retardancy of enzymatic functionalised PET and nylon fabrics via DNA immobilisation [19]. When DNA is subjected to heat flow, it forms a carbonaceous material, i.e. char. This acts as a physical barrier which is able to limit the heat, fuel and oxygen transfer between the flame and polymer [20].
Usually, intumescent material has three components such as (1) acid source (which releases phosphoric acid), (2) a carbon source (pentaerythritol, arabitol, sorbitol, inositol, cyclodextrins, saccharides, polysaccharides, etc.) and (3) blowing agent (guanidine, melamine, etc.) which upon heating produces greater amounts of non-combustible gases like water vapour, ammonia, nitrogen and carbon dioxide [13].
On direct comparison with the above system, DNA has all three components such as phosphate groups which are able to produce phosphoric acid, the deoxyribose units acting as a carbon source and the nitrogen base such as adenine (A), guanine (G), cytosine (C) and thymine (T) which may act as blowing agent and produce ammonia. Thus, DNA serves as a better option for the application of intumescent flame retardants over cotton fabric [20]. Other than flammability, high water absorbance is also a major drawback for cotton fabric. Thus, fabrication of materials with both superhydrophobicity and flame retardancy is still a challenging task. The fabrication of dual functional textile like flame retardancy and superhydrophobicity increases the usage of cotton fabrics in all applications and also extends the lifespan of cotton fabric.
Superhydrophobic surfaces have water contact angle of 150° and sliding angle of 10°. The superhydrophobic textiles possess water/liquid repellence, self-cleaning, anti-sticking and anti-contaminant properties [21]. The major requirement for the fabrication of superhydrophobic surfaces is micro-/nano-surface roughness with low surface energy [22]. Recently, many synthetic and bio-approaches were used for surface functionalisation of cellulosic materials as well as other different materials such as PLA fabrics [23]. In this work, DNA is used as flame-retardant and silver nitrate and octadecyltriethoxysilane (ODTS) are used in order to render superhydrophobic property to cotton fabric.
Experimental section
Materials
Degreased cotton fabric (180 GSM) was obtained from local market. DNA sodium salt from calf thymus (molecular biology grade, containing 41.9 mol % G-C; 58.1 mol % A-T and highly polymerised double-stranded DNA) was purchased from Sigma-Aldrich. Silver nitrate (AgNO3) (ACS reagent grade) and ethanol were obtained from CDH. Hydrazine hydrate (99 + % purity) and octadecyltriethoxysilane (ODTS) (> 85% GC) were purchased from Alfa Aesar.
Deposition of DNA–Ag–ODTS over cotton fabric
For impregnation of cotton fabric with DNA, cotton fabric was immersed in DNA suspension for about 12 h. The DNA suspension was prepared by dissolving DNA powder (3 wt.%) in acidified distilled water. After 12 h, it was taken out from acidified water. Then, the DNA-coated fabric was immersed in 1 mM AgNO3 solution for about an hour and it was further reduced by 300 μL of hydrazine hydrate to deposit Ag over DNA-coated cotton. Finally, DNA–Ag-coated cotton fabric was immersed in ethanolic solution of ODTS for 12 h, washed with ethanol and dried in air.
Characterisation techniques
The scanning electron microscopic images of uncoated and coated cotton were obtained using a VEGA3-TESCAN Scanning Electron Microscopy (SEM) with working distance in the range of 9.24–1.09 mm and beam voltage in the range of 4.0–8.0 kV. The chemical elements deposited on cotton surface were identified by energy-dispersive X-ray spectrometer (EDAX) (Bruker, Nano GMBH X’ Flash Detector, 5010 model, Germany). Further, the as-coated cotton surface was characterised using ATR Fourier transform infrared spectroscopy (FT-IR) in the region of 4000–400 cm−1 (Jasco FT-IR 4700 and resolution is 0.4 cm−1, 64 scans per sec was performed in this work).
The surface wettabilities of normal and coated cotton surface were assessed by goniometer (ramé-hart instrument co., USA). The water contact angle (WCA) of cotton surface was evaluated by placing exactly 15 μL of distilled water on different areas of cotton surface for about 60–80 s. The average value was considered for this study.
The thermal stability of normal and modified cotton textiles was evaluated by thermogravimetric analysis (TGA) using thermogravimetric analyser (SDT Q600 V20 9 Build 20) and alumina sample pans (40 µL, 90 µL and has horizontal purge gas flow) under nitrogen atmosphere from the temperature range of 0–600 °C at the heating rate of 10 °C/s, and TGA experiment for each sample is repeated continuously for three times in order to calculate decomposition of residual mass of each sample.
Flame retardant properties of uncoated and coated cotton samples were measured by simple vertical flame testing method [24]. In this method, the samples of (2.0 cm × 2.3 cm) were exposed to burning flame for 30 s in open atmospheric condition. The flame retardant ability of coated and uncoated cotton samples was observed after 30-s burning process.
Durability studies were done to evaluate the industrial and practical application of as-fabricated cotton textiles. The chemical durability of flame-retardant hydrophobic cotton was studied by immersion in various pH solutions for about 32 h. Then, the samples were dried in hot air oven at 100 °C for about 2 h. The WCAs of each sample were measured to assess the chemical stability of cotton samples.
The washing durability of coated samples was evaluated by modified washing method [25]. To assess the washing durability, the modified samples were washed with 3% detergent solution and stirred for about 1 h at 900 rpm and then the samples were dried in hot air oven for about 1 h. After each cycle, the WCA was measured.
Mechanical stability was evaluated by scratch test [7]. The as-modified samples were placed under 100 g of weight and scratched with sandpaper in an abrasive length of 15 cm/s. The mechanical stability of as-coated samples was evaluated by measuring the WCA after each scratch cycle.
Results and discussion
The procedure used for the fabrication of environmentally friendly superhydrophobic and flame-retardant fabric by using biomacromolecule, viz. DNA, is shown in Fig. 1. As mentioned earlier, DNA contains all three major components of an intumescent material in a single compound. Thus, the use of DNA as a flame retardant for cotton fabric was preferred [26].
But considering the hydrophilic nature of DNA, there was a likelihood of the waterproofing ability of cotton fabric getting affected. Thus, further treatment with silver and ODTS became necessary for this work. According to the literature survey, Ag metal particles have strong interaction with nucleotide bases of DNA, and also deposition of Ag along with DNA is a desirable approach for the immobilisation of DNA–Ag over fabric surface through strong electrostatic interactions [27,28,29]. The deposition of Ag over DNA-coated fabric showed hydrophobic characteristic by the creation of dual (micro/nano) surface roughness. Then, further deposition of ODTS over DNA–Ag-coated cotton surface made the surface as superhydrophobic one by lowering the surface energy.
SEM analysis
In order to observe the changes obtained after deposition of DNA, Ag and ODTS over cotton fabric, the as-coated cotton surfaces were compared with normal cotton using SEM analysis. The SEM images of normal cotton, DNA-coated, DNA–Ag-coated and DNA–Ag–ODTS-coated flame-retardant superhydrophobic cotton fabric are shown in Fig. 2.
The SEM images of normal cellulose fabric revealed that the surface has criss-crossing and porous structure with the high-magnification image showing the smooth surface. The low- and high-magnification images of normal cotton are shown in Fig. 2a, b. The surface morphology is changed after deposition of DNA over cotton surface which is depicted in Fig. 2c.
The deposition of Ag over DNA-coated cotton surface turns surfaces as rougher one which is shown in Fig. 2d. According to SEM image of superhydrophobic and flame-retardant fabric using DNA–Ag–ODTS deposition (Fig. 2e), the cotton surface became rougher after deposition of ODTS, and the image showed that each fibre surface is completely wrapped up with ODTS.
EDAX analysis
Figure 3a shows the EDAX analysis of DNA-coated cotton surface. The successful deposition of chemical elements over cotton fabric was confirmed using EDAX analysis. The peaks corresponding to phosphorous, sulphur and nitrogen confirmed the deposition of DNA over cotton surface. The deposition of Ag over DNA-coated cotton surface was confirmed by finding the additional peak corresponding to silver in Fig. 3b. Finally, in superhydrophobic cotton the peak corresponding to phosphorous, sulphur, silver, nitrogen and silicon confirmed the successful deposition of DNA, Ag and ODTS over cotton surface (Fig. 3c). Further, these results confirmed that all chemical elements were distributed very well all over the cotton surface.
ATR FT-IR analysis
The successful deposition of DNA, Ag and ODTS over cotton fabric was further confirmed by the use of ATR FT-IR analysis. Figure 4 shows the ATR spectra of the DNA-coated, DNA–Ag-coated and DNA–Ag–ODTS-coated cotton fabrics. In ATR spectrum of DNA-coated cotton fabric (Fig. 4a), the three bands located at 1682 cm−1, 1217 cm−1 and 1069 cm−1 could be attributed to P=O, asymmetric and symmetric vibration modes of PO2−, whereas less intense bands at 1107 cm−1 and 969 cm−1 correspond to P-O-C groups and ribose-phosphate skeletal motions of DNA molecule. Furthermore, the presence of purine and pyrimidine bases was confirmed by the bands corresponding to C=C and C=N stretching modes (1712, 1540, 1456, 1372, 1003, 810, 662 and 622 cm−1), which confirmed the deposition of DNA over cotton surface [26]. After deposition of silver over DNA, the spectral changes observed at bands for nitrogenous bases (1712 cm−1 and 1372 cm−1) showed that silver was intercalated with nitrogenous bases of DNA (Fig. 4b) [18]. Moreover, the new bands observed at 2927 cm−1, 2850 cm−1 and 786 cm−1 were assigned to -CH2, -CH3 and Si–O bonds of ODTS [7], respectively, which strongly supported the successful deposition of ODTS over DNA–Ag-coated cotton surface.
XPS analysis
The XPS analysis was performed to further confirm the successful deposition of chemical elements over cotton surface. The typical XPS spectrum of DNA–Ag–ODTS-coated cotton fabric is shown in Fig. 5. The peaks corresponding to C, N, O, P, Ag and Si were identified in the DNA–Ag–ODTS-coated cotton fabric. In Fig. 5, the presence of C 1s, N 1s, O 1s, P (2s and 2p), Ag (3d5/2, 3d3/2) and Si 2s confirmed the successful deposition of DNA, Ag and ODTS over cotton surface. Further, the elemental ratio on DNA–Ag–ODTS-coated cotton was found to be 87.5, 0.2, 0.08, 2.8 and 5.2 for C, N, P, AgO and Si, respectively.
Superhydrophobicity property of coated samples
The WCA measurements are used to assess the wettability nature of as-fabricated cotton surfaces. The WCA images of normal and as-coated cotton surfaces are shown in Fig. 6. As already known, normal cotton has high water wettability due to the presence of number of hydroxyl groups on its surface. Thus, it showed the WCA of 0°. After deposition of DNA over cotton surface, the WCA was found to be 78.1°. This result showed that DNA-coated cotton surface also exhibited the hydrophilic character. In the case of DNA–Ag-coated cotton surface, the WCA was found to be 138°. This increase in the WCA showed that the deposition of Ag over DNA-coated cotton turned the hydrophilic cotton surface into hydrophobic surface. Finally, the surface was turned into superhydrophobic with a WCA of 157° after ODTS deposition over DNA–Ag-coated cotton surface. The results of WCA measurements clearly showed that the surface roughness and lower surface energy were obtained by deposition of Ag and ODTS, respectively.
Thermal stability and decomposition studies
The thermal stability and thermo-oxidative stability of solid samples are generally investigated by using thermogravimetric method [30]. It is an effective and direct method to study the degradation process of pure and as-coated cotton surfaces by measuring the weight loss as a function of temperature. Figure 7 represents the TGA and DTG of all coated samples. From TGA and DTG curves, parameters such as the initial degradation temperature, the maximum weight loss temperature of treated and untreated cotton samples were calculated. TGA for each sample was recorded three times in order to find the decomposition temperature and char residue.
The TGA and DTG analysis showed the effect of DNA coating over cotton surface. The pyrolysis of DNA-coated cotton sample started at 220 °C ± 0.5 °C, whereas other coated samples started at 280 °C ± 0.4 °C. The char residue left by DNA-coated cotton sample was significantly higher than that of pure cotton. (Char residue measured for DNA-coated and pure cotton showed 65.48 ± 0.41% and 47.49 ± 0.64%, respectively.) These findings agreed well with previous results of Alongi et al. [26]. According to early reports, phosphate groups of DNA decompose at low temperature (220 °C) and release phosphoric acid which induces the dehydration of cellulose towards the formation of aromatic carbon soot. As a consequence of this process, the production of volatile products, viz. levoglucosan and furan derivatives, is limited. Because of this levoglucosan limitation, the activation energy of the burning process was reduced, through which the thermally stable char formation was enhanced. The presence of nitrogen bases in DNA releases ammonia, which dilutes the flammable gases and inhibits combustion reactions. This also helps to turn carbon-rich deposits into a slow-burning protective layer.
Flammability test
The flammable properties of as-coated cotton samples have been evaluated by vertical flame test [24], by applying a flame to the coated as well as uncoated specimens for about 30 s. When uncoated sample was exposed to direct flame, the fabric immediately caught fire and burnt completely within 5 s without any ash. In contrast, the flame-retardant superhydrophobic cotton fabric was directly exposed to flame, it started to burn very slowly and self-extinguished after removal of fire source and the black charred layer (char length of 0.7 cm) formed over the superhydrophobic cotton showed the flame retardant property of the as-coated cotton sample.
Furthermore, it is also possible to observe the change happened after ignition process of flame-retardant cotton by SEM analysis. The SEM observation showed that fabric structure was still maintained, and the fibre surface was fully covered with coating. The digital images of flammable property of coated, uncoated as well as SEM images of burnt surface of DNA–Ag–ODTS-coated cotton fabric are shown in Fig. 8. The SEM image of burnt surface showed that the surface morphology of DNA–Ag–ODTS-coated cotton surface was completely disturbed after burning, and the surface was covered with dense charred layer and became uneven.
Oil absorption studies
Majority of superhydrophobic surface has superoleophilic character. Thus, the superoleophilic character of DNA–Ag–ODTS-coated cotton surface was tested with few oil drops. The oil absorption ability of as-coated cotton sample is shown in Fig. 9. The quick absorption ability of oil showed the surface to be having a good superoleophilic character and could be employed for oil absorption studies. For this test, the as-fabricated superhydrophobic and flame-retardant cotton fabric was placed in oil-contaminated water surface. Due to quick absorption behaviour of oil, the DNA–Ag–ODTS-coated cotton surface got completely wetted by oil and left the water surface as clean one.
Durability studies
The laundering durability of as-coated cotton samples against water and detergent is shown in Fig. 10. The washing and chemical durability studies showed that the as-fabricated surface has limited washing durability in both water and detergent wash. It loses their superhydrophobic nature after 4 washing cycles in the case of detergent wash and 6 cycles in the case of water wash. After 10 washing cycles, the superhydrophobic surface was turned into hydrophobic surface in both water and detergent washes.
The changes in WCA with respect to various pH solutions are shown in Fig. 11. The chemical durability studies showed that the WCA angle of as-coated samples was maintained above 150° in all pH solutions. This result showed that the as-fabricated cotton fabric has good chemical durability in all pH ranges.
Similarly, the mechanical durability was examined by strong abrasions with sand paper. The mechanical durability of as-fabricated sample is shown in Fig. 12. After the severe abrasion process, the cotton surface was completely distorted, but still the water droplets attained spherical shape over the damaged cotton surface.
The durability studies thus clearly showed the flame retardant and superhydrophobic nature of DNA–Ag–ODTS-coated cotton which also has excellent chemical and mechanical stability, but limited washing durability.
Conclusions
To summarise, DNA, Ag and ODTS were successfully deposited onto cotton fabrics by simple immersion, imparting superhydrophobic and flame retardant properties. After deposition of ODTS, the superhydrophilic cotton fabric turned into a superhydrophobic one, with a WCA of 157°. The TGA results and flame test studies suggested that the deposition of DNA over cotton increases the thermal stability of cotton fabric and decreases the spread speed of fire. Moreover, durability studies demonstrated the excellent chemical as well as mechanical durability of as-coated cotton samples. Results of the present study showed that the scope of this work can be enlarged for future development of commercial grade textiles.
References
Laufer G, Kirkland C, Morgan AB, Grunlan JC (2012) Intumescent multilayer nanocoating, made with renewable polyelectrolytes, for flame-retardant cotton. Biomacromol 13:2843–2848. https://doi.org/10.1021/bm300873b
Shen Q, Song L, Pan H, Pan Y, Hu Y, Lu Y et al (2016) Fabrication of flame retardant coating on cotton fabric by alternate assembly of exfoliated layered double hydroxides and alginate. RSC Adv 6:111950–111958. https://doi.org/10.1039/c6ra21804k
Zhang M, Zang D, Shi J, Gao Z, Wang C, Li J (2015) Superhydrophobic cotton textile with robust composite film and flame retardancy. RSC Adv 5:67780–67786. https://doi.org/10.1039/c5ra09963c
Chen S, Li X, Li Y, Sun J (2015) Al CET. Intumescent Flame-Retardant and coatings on cotton fabric. ACS Nano. https://doi.org/10.1021/acsnano.5b00121
Carosio F, Laufer G, Alongi J, Camino G, Grunlan JC (2011) Layer-by-layer assembly of silica-based flame retardant thin film on PET fabric. Polym Degrad Stab 96:745–750. https://doi.org/10.1016/j.polymdegradstab.2011.02.019
Becher EM, Patel H, Zhang D, Nasir Z, Shrestha SB, Peng X et al (2017) Flame retardant and hydrophobic coatings on cotton fabrics via sol-gel and self-assembly techniques. J Colloid Interface Sci 505:892–899. https://doi.org/10.1016/j.jcis.2017.06.087
Suryaprabha T, Sethuraman MG (2018) Fabrication of superhydrophobic and enhanced flame-retardant coatings over cotton fabric. Cellulose 25:3151–3161. https://doi.org/10.1007/s10570-018-1757-8
Zhang S, Jin X, Gu X, Chen C, Li H, Zhang Z et al (2018) The preparation of fully bio-based flame retardant poly(lactic acid) composites containing casein. J Appl Polym Sci 135:46599. https://doi.org/10.1002/app.46599
Faheem S, Baheti V, Tunak M, Wiener J, Militky J (2019) Comparative performance of flame retardancy, physiological comfort, and durability of cotton textiles treated with alkaline and acidic casein suspension. J Ind Text 48:969–991. https://doi.org/10.1177/1528083717750885
Faheem S, Baheti V, Tunak M, Wiener J, Militky J (2019) Flame resistance behavior of cotton fabrics coated with bilayer assemblies of ammonium polyphosphate and casein. Cellulose 26:3557–3574. https://doi.org/10.1007/s10570-019-02296-1
Alongi J, Carletto RA, Bosco F, Carosio F, Di Blasio A, Cuttica F et al (2014) Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym Degrad Stab 99:111–117. https://doi.org/10.1016/j.polymdegradstab.2013.11.016
Alongi J, Carletto RA, Di Blasio A, Cuttica F, Carosio F, Bosco F et al (2013) Intrinsic intumescent-like flame retardant properties of DNA-treated cotton fabrics. Carbohydr Polym 96:296–304. https://doi.org/10.1016/j.carbpol.2013.03.066
Alongi J, Di Blasio A, Milnes J, Malucelli G, Bourbigot S, Kandola B et al (2015) Thermal degradation of DNA, an all-in-one natural intumescent flame retardant. Polym Degrad Stab 113:110–118. https://doi.org/10.1016/j.polymdegradstab.2014.11.001
Cheng X-W, Guan J-P, Tang R-C, Liu K-Q (2016) Phytic acid as a bio-based phosphorus flame retardant for poly(lactic acid) nonwoven fabric. J Clean Prod 124:114–119. https://doi.org/10.1016/j.jclepro.2016.02.113
Feng JX, Su SP, Zhu J (2011) An intumescent flame retardant system using β-cyclodextrin as a carbon source in polylactic acid (PLA). Polym Adv Technol 22:1115–1122. https://doi.org/10.1002/pat.1954
Malucelli G, Bosco F, Alongi J, Carosio F, Di Blasio A, Mollea C et al (2014) Biomacromolecules as novel green flame retardant systems for textiles: an overview. RSC Adv 4:46024–46039. https://doi.org/10.1039/C4RA06771A
Zhang T, Yan H, Shen L, Fang Z, Zhang X, Wang J et al (2014) Chitosan/phytic acid polyelectrolyte complex: a green and renewable intumescent flame retardant system for ethylene-vinyl acetate copolymer. Ind Eng Chem Res 53:19199–19207. https://doi.org/10.1021/ie503421f
Carosio F, Di Blasio A, Alongi J, Malucelli G (2013) Green DNA-based flame retardant coatings assembled through layer by layer. Polym Guildf 54:5148–5153. https://doi.org/10.1016/j.polymer.2013.07.029
Quartinello F, Kremser K, Vecchiato S, Schoen H, Vielnascher R, Ploszczanski L et al (2019) Increased flame retardancy of enzymatic functionalized PET and nylon fabrics via DNA immobilization. Front Chem 7:1–13. https://doi.org/10.3389/fchem.2019.00685
Alongi J, Milnes J, Malucelli G, Bourbigot S, Kandola B (2014) Thermal degradation of DNA-treated cotton fabrics under different heating conditions. J Anal Appl Pyrolysis 108:212–221. https://doi.org/10.1016/j.jaap.2014.04.014
Suryaprabha T, Sethuraman MG (2017) Fabrication of copper-based superhydrophobic self-cleaning antibacterial coating over cotton fabric. Cellulose 24:395–407. https://doi.org/10.1007/s10570-016-1110-z
Afzal S, Daoud WA, Langford SJ (2014) Superhydrophobic and photocatalytic self-cleaning cotton. J Mater Chem A 2:18005–18011. https://doi.org/10.1039/c4ta02764g
Wang X, Hu Y, Song L, Xuan S, Xing W, Bai Z et al (2011) Flame Retardancy and Thermal Degradation of Intumescent Flame Retardant Poly(lactic acid)/Starch Biocomposites. Ind Eng Chem Res 50:713–720. https://doi.org/10.1021/ie1017157
Xue C-H, Zhang L, Wei P, Jia S-T (2016) Fabrication of superhydrophobic cotton textiles with flame retardancy. Cellulose 23:1471–1480. https://doi.org/10.1007/s10570-016-0885-2
Suryaprabha T, Sethuraman MG (2017) Design of electrically conductive superhydrophobic antibacterial cotton fabric through hierarchical architecture using bimetallic deposition. J Alloys Compd 724:240–248. https://doi.org/10.1016/j.jallcom.2017.07.009
Alongi J, Carletto RA, Di Blasio A, Carosio F, Bosco F, Malucelli G (2013) DNA: A novel, green, natural flame retardant and suppressant for cotton. J Mater Chem A 1:4779–4785. https://doi.org/10.1039/c3ta00107e
Takeshima T, Tada Y, Sakaguchi N, Watari F, Fugetsu B (2015) DNA/ag nanoparticles as antibacterial agents against gram-negative bacteria. Nanomaterials 5:284–297. https://doi.org/10.3390/nano5010284
Pramanik S, Chatterjee S, Saha A, Devi PS, Suresh KG (2016) Unraveling the interaction of silver nanoparticles with mammalian and bacterial DNA. J Phys Chem B 120:5313–5324. https://doi.org/10.1021/acs.jpcb.6b01586
Basu S, Jana S, Pande S, Pal T (2008) Interaction of DNA bases with silver nanoparticles: assembly quantified through SPRS and SERS. J Colloid Interface Sci 321:288–293. https://doi.org/10.1016/j.jcis.2008.02.015
Zhang M, Wang C (2013) Fabrication of cotton fabric with superhydrophobicity and flame retardancy. Carbohydr Polym 96:396–402. https://doi.org/10.1016/j.carbpol.2013.04.025
Acknowledgements
The authors sincerely thank UGC-SAP for their financial support.
Funding
Funding was provided by UGC-RFSMS (Grand No. F.No.25-1/2014-15/(BSR)/7-225/2008/(BSR)).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Suryaprabha, T., Sethuraman, M.G. Fabrication of a superhydrophobic and flame-retardant cotton fabric using a DNA-based coating. J Mater Sci 55, 11959–11969 (2020). https://doi.org/10.1007/s10853-020-04911-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-020-04911-0