Abstract
Proficient visible light-driven photocatalyst for waste water remediation has gained immense attention in decades. Herein, novel foamed zinc oxide–silica spheres and carbon nitride nanocomposite (FZSS@CN) were fabricated by hydrothermal and calcination treatments. The resulting nanocomposite was passed through complete characterization profiling for structural, optical and kinetics properties. The photocatalytic performance was investigated by the degradation of textile dyes such as methylene blue (MB) and Rhodamine B (RhB) under visible light. The optimized nanocomposite (FZSS@CN-10) exhibited the superior photocatalytic degradation performance up to 95% for MB and 90% for RhB with rate constant (k) = 0.0516 min−1 and 0.0464 min−1, respectively. This excellent performance was attributed to the hierarchical morphology, high surface area and mesoporous channels with open cavities. This uniqueness is responsible to enhance the optical and kinetic capabilities of the material for effective photocatalysis. More interestingly, the crystalline structure of the recycled sample has more sharp peaks than fresh sample. This phenomenal behavior leads to a new method for the fabrication of effective materials in this field.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Environmental issues are enormously increasing with time; generally, the release of industrial effluents into the aqueous atmosphere has great influence on living environment and irritates society progression [1, 2]. The waste materials released from the industries include inert dyes that disturb the water quality. Hence, there is a need to detoxify these wastes before releasing into water [3,4,5]. Nowadays, photocatalysis based on semiconductors is conveniently applied for the waste water treatment and environmental protection [6,7,8]. Among the semiconductors, zinc oxide (ZnO) was considered as an ideal photocatalytic system because of its high optical capabilities and photo-stability [9, 10].
Nevertheless, there are still certain shortcomings that confine to reduce the photocatalytic performance of ZnO; these are wide band gap energy and high recombination rate of photo-generated charges [11]. To address these issues, great efforts have been devoted, consisting of structural design, chemical as well as surface modification and developing heterojunctions. Recently, graphitic carbon nitride (g-C3N4) seemed to be a potential photocatalyst due to its suitable band gap energy (2.7 eV), facile synthesis and chemical stability [12,13,14,15]. Therefore, copious interest has been focused to enhance the photocatalytic performance of ZnO by modifying with g-C3N4. Wang et al. [16] structured g-C3N4 nanosheets coupled with oxygen-defective ZnO nanorods, which led to improve photocatalytic performance in the degradation of 4-chlorophenol. Guo et al. [17] prepared carbon dots (CDs)/g-C3N4/ZnO nanocomposite by facile impregnation–thermal process and exhibited superior photocatalytic performance. Rong et al. [18] built a ZnO-Ag2O/pg-C3N4 porous compound and concluded that it has comparatively narrow band gap energy, which is very helpful for the rapid separation of photo-induced charge carriers. Regardless of tremendous development, it is still a challenge for the researchers to explore a photocatalytic system which has excellent visible light photocatalytic efficiency. To date, various morphological-based ZnOs have been fabricated, including nanowires [19], nanowhiskers [20], nanorods [21], nanotube [22], nanospheres [23], star-like [24], flower-like [25], ring-like [26] and foams [27]. Among these, the hierarchical porous structures get more significance to enhance the corresponding optical and kinetic properties. Similarly, foamed and hollow materials also received widespread attention because of good surface permeability, high surface area-to-volume ratio and low mass density [28,29,30,31,32]. All these properties are very helpful to absorb a large number of visible light photons to boost the photocatalytic reaction [33,34,35]. Recently, our group has successfully synthesized the foamed materials by one-step micro-emulsification followed by calcination techniques [28, 36, 37].
Herein, we successfully prepared the foamed zinc oxide–silica spheres (FZSS) coupled with g-C3N4 (CN) nanosheets by hydrothermal followed by calcination treatment. The n-octane was used as the co-solvent and CTAB as the template to get foamed and porous structure. This structure displayed a large number of open cavities responsible to absorb more visible light and enhance the charge transportation efficiency during photocatalytic reaction. The resulting photocatalyst is used for the visible light-induced photocatalytic degradation of two organic dyes such as methylene blue (MB) and Rhodamine B (RhB). The crystalline structure of the recycled sample was investigated, and its behavior leads to a new fabrication method. Hence, this investigation is based on the fabrication and photocatalytic degradation performance and opens a gate for a new fabrication method.
Experimental section
Reagents
Aqueous NH3 (25 wt%) and C2H5OH (A.R 99.7%) were purchased from Beijing Chemical Reagent Co., Ltd. Tetraethyl orthosilicate, TEOS (A.R 99%), was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Melamine (A.R 99%), zinc nitrate hexahydrate Zn(NO3)2.6H2O (A.R 99%) and cetyltrimethylammonium bromide CTAB (A.R 99%) were purchased from Aladdin Industrial Corporation. n-octane (A.R 99%) was purchased from Tianjin Fuchen Chemical Reagents Industry, China.
Fabrication of bulk CN
In a typical synthesis, the melamine was dropped into Al2O3 crucible and calcined at 550 °C for 4 h@3 °Cmin−1. When it cooled down, the sample was grinded to very tiny light yellow powder of bulk CN [38].
Fabrication of FZSS
Generally, 0.7 g CTAB was dropped in 30 mL D.I water for 30 min at 30 °C. Then, 7 mL aqueous ammonia (3 molL−1) and 10 mL n-octane were added to the solution under vigorous stirring to form the white micro-emulsion [39]. Subsequently, the 10 mL D.I water containing 0.97 g Zn (NO3)2·6H2O was added into the micro-emulsion and stirred for 3 h. Finally, the TEOS as the SiO2 source was added to the micro-emulsion with stirring. The blend was transferred into the Teflon-lined autoclave and heated at 100 °C for 24 h. Then, it was filtered by centrifugation, washed with water and ethanol several times and air-dried. After drying, the samples were burned in a muffle furnace at 500 °C for 3 h @3 °Cmin−1 to obtain the final crystallized product.
Fabrication of FZSS@CN
For the synthesis of FZSS@CN, first, 0.7 g CTAB was dispersed in 30 mL D.I water for 30 min at room temperature. Then, 7 mL of ammonia (3 molL−1) and 10 mL n-octane were added to the resulting solution. After that, homogenized CN was added to the above microemulation with constant stirring of 2 h. Then, 0.97 g Zn (NO3)2·6H2O was dissolved in 10 mL water as ZnO precursor and added dropwise to the above micro-emulsion and stirred for 3 h followed by adding TEOS with constant stirring. Subsequently, the mixture was transferred to the reactor and hydrothermally treated at 100 °C for 24 h. Then, the resulting product was purified by centrifugation, washed with water and ethanol and air-dried. After drying, the sample was calcined in a muffle furnace at 500 °C for 3 h @3 °Cmin−1 and the as-prepared sample was denoted as FZSS@CN nanohybrid. Through this method, samples with different CN to ZnO weight loadings (5%, 10%, 15%) were also synthesized and labeled as FZSS@CN-5, FZSS@CN-10 and FZSS@CN-15, respectively.
State-of-the-art characterization techniques
The XRD analysis was performed on the Rigaku D/MAX-2500 VBZ +/PC diffractometer with Cu Kα radiations; the voltage and the current were 40 kV and 40 mA. The XPS was carried out by the Thermo VG ESCALAB-250 with monochromatic Al-Kα X-ray radiations. The UV–Vis/DRS was taken by the UV-3600 spectrophotometer (Shimadzu), and the BaSO4 was used as the reflectance background. The SEM images, surface elemental composition and mapping were taken by the SEM, Hitachi S-4700, equipped with EDS spectroscopy. The microstructure was taken by the transmission electron microscopy (HRTEM, JEM-3010) with an accelerating voltage of 300 kV measurements. The N2 sorption isotherms were determined by the Micromeritics ASAP2020 M instrument for specific surface area and pore size distribution. ESR spectra were taken by the Bruker EPR A300 spectrometer under visible light irradiations (λ > 420 nm) at ambient temperature. DMPO was used as the spin-trapping reagent in the methanolic and aqueous media.
Photocatalytic performance measurements
The photocatalytic activity was investigated by the degradation of MB and RhB as the toxic organic pollutants under the visible light irradiations. The 300-W halogen lamp with cutoff filter AM 1.5G filter (Newport 81094) was used as visible light source with light intensity of 100 mW cm−2. In a typical reaction, 0.02 g of the photocatalyst was suspended in 100 mL mixed aqueous solution containing 20 mg L−1 MB and RhB of each dye in a Pyrex reactor. Prior to the irradiations, the suspension was steadily stirred in dark for 30 min to ensure the adsorption desorption equilibrium at ambient temperature. Then, the visible light was focused onto the mixture with continuous agitation. At certain time intervals, the quantitative aliquots were extracted and then centrifuged at 4000 rpm to separate the photocatalyst. The concentration of supernatant was examined by the Shimadzu UV-3600 spectrophotometer at 664 nm for MB and 552 nm for RhB. The change in the concentration of residual dyes was calculated from their respective absorption peaks compared with the standard calibration curve. Ultimately degraded quantity of each pollutant at particular irradiation time intervals was calculated by plotting C/Co versus time. The Co is the initial concentration, and C is the equilibrium concentration at reaction time t.
Results and discussion
Characterization of the material
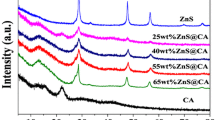
The XRD patterns were used to describe the crystallographic structure, crystallize size and the phase identification of the synthesized CN, FZSS, FZSS@CN-5, FZSS@CN-10 and FZSS@CN-15 nanocomposite as displayed in Fig. 1. The XRD of pure CN exhibited two pronounced diffraction peaks at 13.1° and 27.8° indexed to the (100) and (002) planes, indicating the tri-s-thiazine units and the characteristic interlayer stacking reflection of aromatic rings [40]. The XRD of pure FZSS exhibited the nine sharp peaks located at 31.6°, 34.2°, 36.2°, 47.4°, 56.4°, 62.8°, 67.9°, 68.9° and 77.3°, respectively, indexed as (100), (002), (101), (102), (110), (103), (112), (201) and (202) (JCPDS No. 36-1451) of wurtzite hexagonal structure of ZnO [35]. The XRD of FZSS@CN nanocomposite with the different amount of CN gives rise to the characteristic XRD peaks of both ZnO and CN. The peaks of CN become stronger by successive mass loading without obstructing the crystalline structure of ZnO. Notably, there was no variation in the position and widths of the characteristic diffraction peaks of FZSS@CN nanocomposite by doping with CN. The crystallite size of ZnO was calculated by the Debye–Scherrer formula (D = Kλ/βcosθ), and average size was noted as 2.11 nm [41]. There is gradual reduction in the relative peak intensity of ZnO noted by successive loading of the mass of CN nanosheets. This phenomenon was explained by overlapping and hybridization between CN nanosheets and ZnO nanospheres which leads to decreased peak intensity [39, 42]. Moreover, it has been interestingly seen the XRD of recycled sample (after four consecutive photocatalytic degradation cycles) has more pronounced peaks of ZnO and CN, indicating its best crystalline structure as compared to fresh sample. This is attributed to ultrasonication and irradiations for longtime during the photocatalytic reaction that affects the van der Waal forces between the CN layers. This phenomenon was depicted to the surface termination defects that seem to make a strong bond with OH group of ZnO and triazine unit of CN leading to crystalline perfection [42].
The morphological features of the as-prepared samples were investigated by using scanning electron microscopy (SEM). Figure 2a reveals that the foamed zinc oxide–silica spheres (FZSS) possess the distinctive foamy porous surface with cavities [43]. The hollow interiors and the mesoporous channels are perpendicularly oriented to the surface as shown in Fig. 2b. The FZSS@CN nanocomposite exhibited the clearer foamy structure by coupling with the CN. These unique foamy characteristics and cavity-based porous corrugation surface were due to the disintegration of CN into NH3 and simultaneously flew away from n-octane and CTAB during calcination.
The opening of the cavities of FZSS@CN becomes smaller as compared to the pure FZSS as shown in Fig. 2c, d. The magnified image displayed that the average diameter of FZSS@CN is about 300–400 nm. These morphological changes demonstrated the successfully incorporation of CN nanosheets to the hollow and foamy nanospheres. Such type of cavities-based morphology acts as reflection centers for the absorption of visible light.
The HRTEM images were used to illustrate the microstructure of the FZSS and FZSS@CN. Figure 3a indicates that FZSS is composed of hollow structure surrounded by fibrous shells. The cavity diameter and the outer dimensions of porous shell are about 100 nm. There are also small internal hollow spherical structures due to the assembling of characteristic microspheres on the inner surface of the mesoporous shells. The HRTEM image of the FZSS@CN provided in Fig. 3b reveals the intrinsic structure of the FZSS that does not encounter damage by adding CN. Obviously, the open cavities of FZSS@CN become narrower than FZSS because the CN nanosheets were evenly overlapped and embedded on the surface of the FZSS. The SAED pattern in Fig. 3c with concentric rings further confirms the bonding between two basic precursors. The d spacing of 0.325 nm (002) and 0.26 nm (002) corresponds to the crystal planes of CN and tetragonal ZnO, respectively, as shown in Fig. 3d. Consequently, the HRTEM measurements indicate the profound interfacial connection between the components in the FZSS@CN nanocomposite rather than a simple physical mixture. Upon calcination, the bulk CN is converted into the ultrathin nanosheets and established the intimate interfacial connection with FZSS. Consequently, the FZSS@CN nanocomposite is perfectly constructed by the chemical linkage between Zn and N atoms which facilitate the transportation of the photo-generated electron–hole pairs in the photocatalytic degradation reaction (consistent with XPS).
The EDS spectroscopy was carried out to elucidate the surface elements’ composition of the as-prepared samples. Figure 4 displays the distinctive and strong peaks of C, N, Si, Zn, O atoms indicating the coexistence of these elements on the surface of the FZSS@CN nanocomposite. In addition, the corresponding mapping images of the FZSS@CN exhibited that all the elements are distributed uniformly to confirm the firmly insertion of the CN nanosheets into FZSS. The mass composition of each element calculated by EDS is depicted in Table S2 (supporting information).
To investigate the thermostability and the amount of CN contents in obtained FZSS@CN nanocomposites, the thermogravimetric analysis (TGA) was performed in the range from room temperature to 900 °C at 10 °C min−1 under nitrogen atmosphere. The TGA curves of FZSS, FZSS@CN-10 (representative photocatalyst) and CN are shown in Fig. 5. A negligible weight loss in the case of FZSS was detected in TGA curve over the whole temperature range indicating its high thermal stability, while for pure CN, the rapid weight loss appeared in the range of 500 °C to 730 °C that is ascribed to the typical combustion and decomposition of CN phase showing the instability. The CN nanosheets almost entirely decomposed and loss its 100% weight at 730 °C. The weight loss in FZSS@CN-10 nanocomposite is 10%, affirming the real CN contents in FZSS@CN-10 [44]. Correspondingly, the relative CN contents for FZSS@CN-5 and FZSS@CN-15 composites were calculated to be 5% and 15%, respectively.
The UV–Vis DRS was used to measure the optical absorption capability for CN, FZSS, FZSS@CN-5, FZSS@CN-10 and FZSS@CN-15 photocatalysts as displayed in Fig. 6a. It reveals that the pure CN exhibited optical absorption band edge located at 460 nm conforming to the expected benchmark absorption position of the CN [45]. The FZSS absorption peak was located at 400 nm. The significant redshift has been noted for FZSS@CN nanocomposite with sufficient visible light absorption as compared to FZSS and CN. This phenomenon was due to the favorable combination of the CN nanosheets with the foamed zinc oxide–silica spheres [46, 47]. Due to this chemically bonded interaction of the individual components in the FZSS@CN nanocomposite, the electron transportation takes place between zinc oxide and CN [48, 49]. In addition, the successive loading of CN puts tremendous effect on the visible light absorption. The absorption trends have some dramatic variations by successive loading of CN. It was noted that the value of the absorption band edge among the FZSS@CN nanocomposites first increased and then decreased due to the strong interactions between CN and FZSS. The maximum absorption was found in FZSS@CN-10 while the reduction was observed in FZSS@CN-15. This is caused by the surface saturation with CN nanosheets which disturb the active sites on the surface of FZSS; hence, electronic excitations were inhibited under the visible light [50]. In particular, the FZSS@CN-10 nanocomposite has the smallest band gap energy and the strongest optical absorption capability. Figure 6b presents the plots of (αhυ)2 vs hυ; the intercept of the tangent of corresponding plots represents the band gap energy (Eg) of the as-prepared samples. The corresponding band gap energy (Eg) was estimated about 2.69 eV, 3.1 eV, 2.30 eV, 1.98 eV and 2.19 eV for the CN, FZSS, FZSS@CN-5, FZSS@CN-10 and FZSS@CN-15, respectively. Based on the above analysis, the FZSS@CN-10 nanocomposite has the lowest Eg value related to the excellent visible light absorption and hence boosts its photocatalytic activity than other counterparts.
The XPS spectra were determined to evaluate the surface chemical composition and valence states of FZSS and FZSS@CN nanocomposite. The survey spectra in Fig. 7a reveal FZSS and FZSS@CN are mainly comprised of Zn, O and Si, while FZSS@CN possesses the more obvious N and C peaks, verifying the effective combination of CN nanosheets with the foamed zinc oxide–silica spheres. Figure 7b, c depicts the high-resolution spectrum of C1s and N1s of FZSS@CN. The C1s high-resolution spectrum was distinguished into three individual peaks sited at 284.84 eV, 285.85 eV and 288.50 eV as illustrated in Fig. 7b. The peak at 284.84 eV originated from the sp2 (C=C) bonds, whereas the other two peaks at 285.85 eV and 288.50 eV are identified as the sp3 tertiary carbon atom (C–(N)3) and sp2 hybridized carbon (N–C=N) bonds in triazine rings of the CN [51]. The N1 s high-resolution spectra given in Fig. 7c are fitted into two major peaks at 398.67 eV and 399.56 eV. The peak at 398.67 eV is associated with the sp2 nitrogen (C–N=C) groups, and the peak at 399.56 eV is arising from the bridging nitrogen (N–(C)3) or (H–N–(C)2) bonds, respectively [52]. Figure 7d shows the comparative spectrum of Zn 2p of the FZSS and FZSS@CN. The two characteristic peaks for FZSS appeared at 1021.74 eV and 1044.78 eV are assigned to the Zn2p3/2 and Zn2p1/2, while for the FZSS@CN the peaks are centered at the 1022.25 eV and 1045.30 eV, respectively. The binding energy values are accorded with the zinc ions existed in the heterojunction as the positive bivalent states. The shifting of the FZSS@CN toward the high binding energy than FZSS validates the successful formation of the covalent bonds between zinc and nitrogen atoms. It provides the authentic conformation of the chemical connection of the foamed zinc oxide–silica spheres with the CN nanosheets. This intimate interfacial connection of two phases is favorable for the transportation of the photo-generated charge carriers [53].
The BET isotherms and pore size distribution curves of FZSS and FZSS@CN are presented in Fig. 8a, c, respectively. It is evident that the N2 sorption isotherms of FZSS and FZSS@CN are belonging to type-IV isotherms and categorized as type H3 hysteresis loops that indicated the typical mesoporous features and slit-like pores of the photocatalysts. At the relative pressure P/P0 > 0.9, adsorption amount rapidly raises owing to the nitrogen adsorbed on the irregular mesopores and intergranular porosity between the spheres. In addition, the pore size distribution curves in Fig. 8b, d displayed that FZSS has a narrow peak with the average pore size of 14 nm. However, the average pore size of the FZSS@CN is mainly concentrated at 19 nm with wide peak indicating the homogeneous mesoporous size. Furthermore, the specific surface areas of FZSS and FZSS@CN were calculated by the BET equation which are 115 m2 g−1 and 74 m2 g−1, respectively, as listed in Table S1 (supporting information). There is a decrease in specific surface areas of FZSS/CN than FZSS due to the coupling of CN and FZSS. This phenomenon confirmed the incorporation of CN nanosheets into the FZSS that extends its cavities and widening of the pore diameters resulting in the reduction in the surface area. This was due to the high weight of the CN than ZnO; hence, its integration into the internal mesoporous surface decreased the surface area [54]. The overall N2 sorption isotherms of FZSS@CN shifted to downward under the entire pressure range that also demonstrated the reduction in specific surface area [55]. Therefore, it might be probable to deduce that the relatively high specific surface area, unique mesoporous channels and controllable pore size are valuable to the adsorption of dye molecules and facilitate the charge transportation during photocatalytic reaction.
Mechanism of the fabrication of FZSS@CN
Herein, the fabrication mechanism of the FZSS@CN is depicted in scheme 1. First, the CTAB, aqueous ammonium, the n-octane and the water formed the milky white micro-emulsion under vigorously stirring. The hydrophobic n-octane is easily combined with the hydrophobic carbon chain of the CTAB. The n-octane as swelling agent easily enters into the interior of the CTAB micelles to swell them leading to minimize the surface energy of the micelles, and the aqueous ammonia hydrolyzed the TEOS to produce silicate anions. The silicate anions and the quaternary ammonium cations on the surface of the spherical micro-micelles are combined and converted to small silica hollow microspheres by electrostatic interactions. Upon the excessive addition of n-octane and water, more micro-emulsion forms under vigorous stirring, so these small silica hollow microspheres will be transformed into spherical shells and start to make aggregates at the surface of micro-emulsion droplets. By adding zinc nitrate and bulk CN, the mesoporous shells of silicate anions coated with CTAB symmetrically attract toward them. After calcination, the templating agent CTAB and n-octane decomposed off, creating the open cavities and distinctive porous corrugation on the surface. Meanwhile, the bulk CN converts into ultrathin nanosheets and made a chemically bond with the OH group of ZnO during calcination.
Photocatalytic degradation performance
The photocatalytic degradation performance of FZSS@CN-5, FZSS@CN-10, FZSS@CN-15, FZSS and CN has been investigated by selecting two hazardous colored dyes MB and RhB under the visible light irradiations. Before irradiating the reaction system, an adsorption desorption equilibrium was established by stirring for 30 min and the concentration of each dye decreased depending upon their surface area and porous nature of the corresponding samples. After that, light was switched on and the photocatalytic activity of the FZSS@CN nanocomposite and other reference samples has been investigated to ensure the influence of light and catalyst on degradation performance. Corresponding results have been taken as triplicate, and the average results are depicted in Fig. 9a, c. The FZSS has relatively weak decolorization ability under visible light than other counterparts because of its absorption edge in blue region. After connecting with CN, it absorbs more visible light and hence enhances the photocatalytic degradation efficiency of the FZSS@CN nanocomposite than individual components. This was attributed to the distinctive coupling of foamed zinc oxide–silica spheres with CN that has great potential to enhance the optical capabilities as well as charge transportations during the photocatalytic degradation reaction. Moreover, the hierarchical surface morphology, mesoporous channels and the open cavities have a great deal to improve the light trapping capacity due to multiple reflection sites and hence favor the photocatalytic performance [56]. The photocatalytic performance of FZSS@CN first increased and then decreased from FZSS@CN-5 to FZSS@CN-15 by successive loading of CN. The FZSS@CN-10 with the optimal CN contents (10%) has great potential to degrade the MB and RhB dyes up to 95% and 90% under visible light, respectively. Noteworthy, upon the excessive CN loading on the foamed zinc oxide–silica spheres (FZSS@CN-15), the photocatalytic degradation efficiency decreases because the accumulations of CN on the FZSS surface shielded active sites leading to slow down the transportation rate of charge carriers [57]. Moreover, we have also compared the photocatalytic activity of our nanocomposite with reported photocatalysts under visible light as enlisted in Table 1. It is observed that our as-made FZSS@CN nanocomposite has outperformed than the corresponding reported samples.
The kinetic study for the degradation of MB and RhB was carried out by fitting the experimental data in rate equation (ln(Co/C) = kt) as shown in Fig. 9b, d, where the Co and C represent the initial and final concentrations for MB and RhB, respectively, t represents the irradiation time and k represents the reaction rate constant (min−1). The photocatalytic degradation kinetic data followed the pseudo-first-order reaction. Obviously, the reaction rate constant for the FZSS@CN-10 was higher than other counterparts; thus, it has the most prominent photocatalytic degradation performance (Tables S3–4). The improvement in the photocatalytic efficiency is primarily ascribed to the following factors: (I) Favorable synergistic coupling and well-defined interfacial connection of the FZSS and CN. (II) Improvement in the optical responses in the visible light region. (III) Unique foamed zinc oxide–silica spheres with substantial cavities-based morphology have enough space for the adsorption of dyes molecules and ideal transportation of the photo-generated charges.
Photocatalytic degradation mechanism
On account of the above-mentioned outcomes, a conceivable photocatalytic degradation mechanism of FZSS@CN under visible light irradiations is shown in scheme 2. Advanced oxidation process (AOPs) involves the generation of very reactive oxygen species (ROSs) to degrade the organic pollutants. During photocatalysis, the photo-excitation of FZSS and CN surfaces generated photo-excited electron–hole pairs due to suitable edge potentials. The band edge level of the FZSS and CN was calculated by cyclic voltammetry under scan rate of (+ 3 to − 3 V) to calculate the CB or VB potential levels as shown in Fig. S2 (supporting information). The CB of CN (electron donor) was calculated by equation ECB (eV) = − e (4.8 − EFC + Eoxd) + Eg, where Eg is the CB − VB band and Eoxd is the oxidation potential. EFC = (Eoxd + Ered)/2 = 0.5 is the energy level of standard ferrocene. Correspondingly, the CB of FZSS (electron acceptor) was calculated by ECB (eV) = − e (4.8 − EFC + Ered) + Eg, where Ered is the reduction potential of FZSS. The VB potentials were calculated by Eg = ECB − EVB. The corresponding CB and VB potential levels of CN were located at − 1.04 eV and + 1.65 eV and FZSS at − 0.45 eV and + 2.67 eV. The transportation mechanism of the photo-generated charge carriers is as follows: The holes are generated when electrons are excited from VB to CB of FZSS and CN. As the CB edge level of FZSS is lower than CN, the excited electrons travelled from the CN to the FZSS and the holes in the VB of FZSS travelled to CN due to potential difference, henceforth taking a part in photocatalytic degradation reactions. The accumulated electrons on the surface of ZnO could react with adsorbed O2 and produce the superoxide radical species O·−2. The ·OH radicals are further generated from the O·−2 by chain reactions [65]. Therefore, much of these reactive radicals are available to interact with the organic dye molecules which ultimately degraded it to harmless components. Furthermore, the holes present in the valance band of CN are capable of reacting directly with the dyes to degrade it.
Measurements of reactive oxygen species
The trapping experiments were carried out to detect the chief reactive radical species generated during photo-degradation reaction. Here, the benzoquinone (BQ), isopropanol (IPA) and ammonium oxalate (AO) were used for the O·−2, ·OH and h+ radical scavengers, respectively. The degradation activities were hindered by these three quenchers relatively compared to the same composite with no scavenger as shown in Fig. 10a. The addition of BQ and IPA scavengers apparently suppresses the photo-degradation performance. It could be inferred that the O·−2 and ·OH played as the most predominant reactive oxygen radicals that have prominent influence on the photocatalytic process. Moreover, the h+ has relatively less contribution to the photo-degradation.
To further investigate the photo-degradation pathway, the electron spin resonance (ESR) was carried out for identifying the nature of reactive oxygen species. Here, ESR measurements were conducted in dark and visible light by utilizing the DMPO as the spin-trapping reagent presented in Fig. 10b, c. No apparent signals for DMPO-O·−2 and DMPO-·OH adducts were seen under the dark environment, but the prominent signals of O·−2and ·OH radicals were found under visible light irradiations [66]. It is concluded the visible light is very necessary for the production of the active radicals. Consequently, the superoxide and hydroxyl radicals are paramount reactive oxygen species used in the visible light-induced photocatalytic degradation reaction [16].
Stability of the material
Besides the catalytic behavior, the long-term stability of the photocatalyst is a crucial matter to evaluate its degradation performance under the visible light illuminations. We have conducted the recycling experiments of FZSS@CN-10 nanocomposite. The amount of the photocatalyst and the concentration of the dye remain unchanged for each run. Obviously, photocatalytic degradation performance has little variations after four successive stability cycles as presented in Fig. 10d. Therefore, the as-prepared FZSS@CN was not affected upon illuminations and regarded to the venerable photo-stability and repeatability for practical applications. Besides this, the crystalline structure and surface morphology of recycled sample have been investigated after completing four photocatalytic reactions. Figure S1 displays the SEM images of recycled sample seem to be little corrosive and aggregated after longtime exposure to visible light, but its crystalline structure has some dramatic behavior and looks like perfect with more prominent peaks as compared to fresh sample. This has been discussed in detail in XRD section (Fig. 1). It could be inferred that our as-made FZSS@CN photocatalyst displayed the outstanding potential in the further waste water remediation.
Conclusions
In conclusion, a novel FZSS@CN nanocomposite was prepared by one-step micro-emulsification followed by hydrothermal and calcination treatments. The FZSS@CN entirely degrades MB and RhB under the visible light irradiations compared to the individual FZSS and CN components. Moreover, the CN has tremendous effect on photocatalytic performance and the FZSS@CN with 10% CN exhibited the outstanding photocatalytic activity for MB (95%) and RhB (90%). The exceptional photocatalytic degradation performance was due to the improvement in the visible light absorption and efficient transportation of the photo-generated electron–hole pairs. These distinctive features are due to well-built interfacial connections between individual components and the porous morphological features. Besides this, the FZSS@CN possessed the venerable photo-stability and repeatability. One interesting aspect is that XRD structure of recycled sample has more crystallinity as compared to fresh samples. This behavior opens a gate to develop a new methodology and simultaneously delivers an innovative way in designing the active photocatalyst for pollution remediation.
References
Ashar A, Iqbal M, Bhatti IA, Ahmad MZ, Qureshi K, Nisar J, Bukhari IH (2016) Synthesis, characterization and photocatalytic activity of ZnO flower and pseudo-sphere: nonylphenol ethoxylate degradation under UV and solar irradiation. J Alloys Compd 678:126–136
Iqbal M (2019) Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: a review. Chem Int 5:1–80
Iqbal M (2016) Vicia faba bioassay for environmental toxicity monitoring: a review. Chemosphere 144:785–802
Abbas M, Adil M, Ehtisham-ul-Haque S, Munir B, Yameen M, Ghaffar A, Shar GA, Tahir MA, Iqbal M (2018) Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: a review. Sci Total Environ 626:1295–1309
Ata S, Shaheen I, Qurat ul A, Ghafoor S, Sultan M, Majid F, Bibi I, Iqbal M (2018) Graphene and silver decorated ZnO composite synthesis, characterization and photocatalytic activity evaluation. Diam Relat Mater 90:26–31
Chen X, Wu Z, Liu D, Gao Z (2017) Preparation of ZnO photocatalyst for the efficient and rapid photocatalytic degradation of azo dyes. Nanoscale Res Lett 12:143
Wang Y, Zhu S, Chen X, Tang Y, Jiang Y, Peng Z, Wang H (2014) One-step template-free fabrication of mesoporous ZnO/TiO2 hollow microspheres with enhanced photocatalytic activity. Appl Surf Sci 307:263–271
Yu L, Chen W, Li D, Wang J, Shao Y, He M, Wang P, Zheng X (2015) Inhibition of photocorrosion and photoactivity enhancement for ZnO via specific hollow ZnO core/ZnS shell structure. Appl Catal B 164:453–461
Sun H, Wang L, Chu D, Ma Z, Wang A, Zheng Y, Wang L (2014) Facile template-free hydrothermal fabrication of ZnO hollow microspheres for gas sensing applications. Ceram Int 40:16465–16473
Qureshi K, Ahmad MZ, Bhatti IA, Zahid M, Nisar J, Iqbal M (2019) Graphene oxide decorated ZnWO4 architectures synthesis, characterization and photocatalytic activity evaluation. J Mol Liq 285:778–789
Alam U, Khan A, Raza W, Khan A, Bahnemann D, Muneer M (2017) Highly efficient Y and V co-doped ZnO photocatalyst with enhanced dye sensitized visible light photocatalytic activity. Catal Today 284:169–178
Shen R, Xie J, Zhang H, Zhang A, Chen X, Li X (2017) Enhanced solar fuel H2 generation over g-C3N4 nanosheet photocatalysts by the synergetic effect of noble metal-free Co2P cocatalyst and the environmental phosphorylation strategy. ACS Sustai Chem Eng 6:816–826
Lu D, Wang H, Zhao X, Kondamareddy KK, Ding J, Li C, Fang P (2017) Highly efficient visible-light-induced photoactivity of Z-scheme g-C3N4/Ag/MoS2 ternary photocatalysts for organic pollutant degradation and production of hydrogen. ACS Sustain Chem Eng 5:1436–1445
Miao X, Ji Z, Wu J, Shen X, Wang J, Kong L, Liu M, Song C (2017) g-C3N4/AgBr nanocomposite decorated with carbon dots as a highly efficient visible-light-driven photocatalyst. J Colloid Interface Sci 502:24–32
He Y, Wang Y, Zhang L, Teng B, Fan M (2015) High-efficiency conversion of CO2 to fuel over ZnO/g-C3N4 photocatalyst. Appl Catal B 168:1–8
Wang J, Xia Y, Zhao H, Wang G, Xiang L, Xu J, Komarneni S (2017) Oxygen defects-mediated Z-scheme charge separation in g-C3N4/ZnO photocatalysts for enhanced visible-light degradation of 4-chlorophenol and hydrogen evolution. Appl Catal B 206:406–416
Guo F, Shi W, Guan W, Huang H, Liu Y (2017) Carbon dots/g-C3N4/ZnO nanocomposite as efficient visible-light driven photocatalyst for tetracycline total degradation. Sep Purif Technol 173:295–303
Rong X, Qiu F, Jiang Z, Rong J, Pan J, Zhang T, Yang D (2016) Preparation of ternary combined ZnO-Ag2O/porous g-C3N4 composite photocatalyst and enhanced visible-light photocatalytic activity for degradation of ciprofloxacin. Chem Eng Res Des 111:253–261
Rackauskas S, Barbero N, Barolo C, Viscardi G (2017) ZnO nanowire application in chemoresistive sensing: a review. Nanomaterials 7:381
Ma X-Y, Zhang W-D (2009) Effects of flower-like ZnO nanowhiskers on the mechanical, thermal and antibacterial properties of waterborne polyurethane. Polym Degrad Stabil 94:1103–1109
Chen HM, Chen CK, Lin CC, Liu R-S, Yang H, Chang W-S, Chen K-H, Chan T-S, Lee J-F, Tsai DP (2011) Multi-bandgap-sensitized ZnO nanorod photoelectrode arrays for water splitting: an X-ray absorption spectroscopy approach for the electronic evolution under solar illumination. J Phys Chem C 115:21971–21980
Samadipakchin P, Mortaheb HR, Zolfaghari A (2017) ZnO nanotubes: preparation and photocatalytic performance evaluation. J Photochem Photobiol A Chem 337:91–99
Yang C, Yu J, Li Q, Yu Y (2017) Facile synthesis of monodisperse porous ZnO nanospheres for organic pollutant degradation under simulated sunlight irradiation: the effect of operational parameters. Mater Res Bull 87:72–83
Peng Z, Dai G, Chen P, Zhang Q, Wan Q, Zou B (2010) Synthesis, characterization and optical properties of star-like ZnO nanostructures. Mater Lett 64:898–900
Sun Y, Guo H, Zhang W, Zhou T, Qiu Y, Xu K, Zhang B, Yang H (2016) Synthesis and characterization of twinned flower–like ZnO structures grown by hydrothermal methods. Ceram Int 42:9648–9652
Li F, Ding Y, Gao P, Xin X, Wang ZL (2004) Single-crystal hexagonal disks and rings of ZnO: low-temperature, large-scale synthesis and growth mechanism. Angew Chem Int Ed 43:5238–5242
Kovačič S, Anžlovar A, Erjavec BT, Kapun G, Matsko NB, Žigon M, Žagar E, Pintar A, Slugovc C (2014) Macroporous ZnO foams by high internal phase emulsion technique: synthesis and catalytic activity. ACS Appl Mater Interfaces 6:19075–19081
Zhang J, Li B, Han C, Liu J (2014) One-pot synthesis of foamed titania–silica composite and its photocatalytic performance. Mater Lett 129:50–53
Wang J, Pei C, Cheng L, Wan W, Zhao Q, Yang H, Liu SF (2016) Responses of three-dimensional porous ZnO foam structures to the trace level of triethylamine and ethanol. Sens Actuators B Chem 223:650–657
Ding D, Lan W, Yang Z, Zhao X, Chen Y, Wang J, Zhang X, Zhang Y, Su Q, Xie E (2016) A simple method for preparing ZnO foam/carbon quantum dots nanocomposite and their photocatalytic applications. Mater Sci Semicond Process 47:25–31
Jhao J-J, Yang M-H, Tseng WJ (2014) Effect of solids concentration on pore structure of ZnO-foams prepared by particle-stabilized foaming route. Ceram Int 40:4649–4654
Lu P, Xue D (2011) Bubble-assisted nanofabrication of macroporous ZnO foams. Nanosci Nanotechnol Lett 3:394–399
Yin M, Liu S (2014) Preparation of ZnO hollow spheres with different surface roughness and their enhanced gas sensing property. Sens Actuators B Chem 197:58–65
Tian Y, Lu H-B, Liao L, Li J-C, Liu C (2009) Synthesis and evolution of hollow ZnO microspheres assisted by Zn powder precursor. Solid State Commun 149:456–460
Bao Y, Wang C, Ma J (2016) Trisodium citrate as bridging and suppressing agent to control synthesis of ZnO hollow hierarchical microspheres and their photocatalytic properties. Ceram Int 42:1746–1755
Shakeel M, Arif M, Yasin G, Li B, Khan AU, Khan FU, Baloch MK (2018) Hollow mesoporous architecture: a high performance Bi-functional photoelectrocatalyst for overall water splitting. Electrochim Acta 268:163–172
Shakeel M, Li B, Arif M, Yasin G, Rehman W, Khan AU, Khan S, Khan A, Ali J (2018) Controlled synthesis of highly proficient and durable hollow hierarchical heterostructured (Ag-AgBr/HHST): a UV and visible light active photocatalyst in degradation of organic pollutants. Appl Catal B 227:433–445
Arif M, Yasin G, Shakeel M, Fang X, Gao R, Ji S, Yan D (2018) Coupling of Bifunctional CoMn-Layered Double Hydroxide@ Graphitic C3N4 Nanohybrids towards Efficient Photoelectrochemical Overall Water Splitting. Chem Asian J 13:1045–1052
Shakeel M, Li B, Yasin G, Arif M, Rehman W, Khan HD (2018) In situ fabrication of foamed titania carbon nitride nanocomposite and its synergetic visible-light photocatalytic performance. Ind Eng Chem Res 57:8152–8159
Liu Q, Chen T, Guo Y, Zhang Z, Fang X (2017) Grafting Fe(III) species on carbon nanodots/Fe-doped g-C3N4 via interfacial charge transfer effect for highly improved photocatalytic performance. Appl Catal B 205:173–181
Mahfooz-ur-Rehman Rehman W, Waseem M, Shah BA, Shakeel M, Haq S, Zaman U, Bibi I, Khan HD (2019) Fabrication of titanium-tin oxide nanocomposite with enhanced adsorption and antimicrobial applications. J Chem Eng Data 64:2436–2444
Niu P, Zhang L, Liu G, Cheng H-M (2012) Graphene-like carbon nitride nanosheets for improved photocatalytic activities. Adv Funct Mater 22:4763–4770
Yoo WC, Stein A (2011) Solvent effects on morphologies of mesoporous silica spheres prepared by pseudomorphic transformations. Chem Mater 23:1761–1767
Sun J-X, Yuan Y-P, Qiu L-G, Jiang X, Xie A-J, Shen Y-H, Zhu J-F (2012) Fabrication of composite photocatalyst g-C3N4-ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans 41:6756–6763
Zhang W, Zhou L, Deng H (2016) Ag modified g-C3N4 composites with enhanced visible-light photocatalytic activity for diclofenac degradation. J Mol Catal A: Chem 423:270–276
Li X, Li M, Yang J, Li X, Hu T, Wang J, Sui Y, Wu X, Kong L (2014) Synergistic effect of efficient adsorption g-C3N4/ZnO composite for photocatalytic property. J Phys Chem Solids 75:441–446
Vignesh K, Kang S, Kwak BS, Kang M (2015) Meso-porous ZnO nano-triangles@ graphitic-C3N4 nano-foils: fabrication and Recyclable photocatalytic activity. Sep Purif Technol 147:257–265
Han X, Yu C, Yang J, Zhao C, Huang H, Liu Z, Ajayan PM, Qiu J (2016) Mass and charge transfer coenhanced oxygen evolution behaviors in CoFe‐layered double hydroxide assembled on graphene. Adv Mater Interfaces 3:1500782
Arif M, Yasin G, Shakeel M, Mushtaq MA, Ye W, Fang X, Ji S, Yan D (2019) Hierarchical CoFe-layered double hydroxide and gC3N4 heterostructures with enhanced bifunctional photo/electrocatalytic activity towards overall water splitting. Mater Chem Front 3:520–531
Nayak S, Mohapatra L, Parida K (2015) Visible light-driven novel gC3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction. J Mater Chem A 3:18622–18635
Wang J, Su F-Y, Zhang W-D (2014) Preparation and enhanced visible light photoelectrochemical activity of gC3N4/ZnO nanotube arrays. J Solid State Electrochem 18:2921–2929
Chang F, Xie Y, Li C, Chen J, Luo J, Hu X, Shen J (2013) A facile modification of g-C3N4 with enhanced photocatalytic activity for degradation of methylene blue. Appl Surf Sci 280:967–974
Zhu K, Neale NR, Miedaner A, Frank AJ (2007) Enhanced charge-collection efficiencies and light scattering in dye-sensitized solar cells using oriented TiO2 nanotubes arrays. Nano Lett 7:69–74
Wu N, Zhang W, Li B, Han C (2014) Nickel nanoparticles highly dispersed with an ordered distribution in MCM-41 matrix as an efficient catalyst for hydrodechlorination of chlorobenzene. Microporous Mesoporous Mater 185:130–136
Yu W, Xu D, Peng T (2015) Enhanced photocatalytic activity of gC3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: a direct Z-scheme mechanism. J Mater Chem A 3:19936–19947
Paramasivam I, Jha H, Liu N, Schmuki P (2012) A review of photocatalysis using self-organized TiO2 nanotubes and other ordered oxide nanostructures. Small 8:3073–3103
Nayak S, Mohapatra L, Parida K (2015) Visible light-driven novel g-C3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction. J Mater Chem A 3:18622–18635
Kumar S, Kumar A, Kumar A, Balaji R, Krishnan V (2018) Highly efficient visible light active 2D–2D nanocomposites of N-ZnO-g-C3N4 for photocatalytic degradation of diverse industrial pollutants. ChemistrySelect 3:1919–1932
Sun J-X, Yuan Y-P, Qiu L-G, Jiang X, Xie A-J, Shen Y-H, Zhu J-F (2012) Fabrication of composite photocatalyst gC3N4–ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans 41:6756–6763
Liu W, Wang M, Xu C, Chen S, Fu X (2013) Significantly enhanced visible-light photocatalytic activity of g-C3N4 via ZnO modification and the mechanism study. J Mol Catal A: Chem 368:9–15
Pawar RC, Son Y, Kim J, Ahn SH, Lee CS (2016) Integration of ZnO with g-C3N4 structures in core–shell approach via sintering process for rapid detoxification of water under visible irradiation. Curr Appl Phys 16:101–108
Zhu Y-P, Li M, Liu Y-L, Ren T-Z, Yuan Z-Y (2014) Carbon-doped ZnO hybridized homogeneously with graphitic carbon nitride nanocomposites for photocatalysis. J Phys Chem C 118:10963–10971
Zhang J, Ma Z (2018) Porous g-C3N4 with enhanced adsorption and visible-light photocatalytic performance for removing aqueous dyes and tetracycline hydrochloride. Chin J Chem Eng 26:753–760
Chen D, Wang K, Ren T, Ding H, Zhu Y (2014) Synthesis and characterization of the ZnO/mpg-C3N4 heterojunction photocatalyst with enhanced visible light photoactivity. Dalton Trans 43:13105–13114
Liu W, Wang M, Xu C, Chen S (2012) Facile synthesis of g-C3N4/ZnO composite with enhanced visible light photooxidation and photoreduction properties. Chem Eng J 209:386–393
Qin Q, Shi Q, Sun W, Wan J, Hu Z (2018) Fabrication and interfacial electron transfer of ultrathin gC3N4 nanosheet/TNT@ CNTs ternary nanostructure heterojunction for high-efficiency visible-light-driven photocatalysis. J Mater Sci: Mater Electron 29:8673–8687
Acknowledgements
This work was financially supported by National Natural Science Foundation of China (No. 21271017), National Science and Technology Supporting Plan of the Twelfth five-year (No. 2014BAE12B0101) and Fundamental Research Funds for the Central Universities (XK1802-6).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, X., Shakeel, M., Li, B. et al. Synthesis of foamed zinc oxide–silica spheres coupled with g-C3N4 nanosheets for visible light photocatalysis. J Mater Sci 54, 13118–13134 (2019). https://doi.org/10.1007/s10853-019-03848-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03848-3