Abstract
Ion co-doped photocatalysts with In2O3/TiO2 heterostructure (In2O3/TiO2(m)) were prepared through a new polymeric precursor method. The In2O3/TiO2(m) precursor solution is of salient advantage in coating photocatalysts on various substrates and multiple modifications. The photocatalysts were facilely obtained after the calcination of precursors. Mass ratio of In/Ti was optimized according to their photocatalytic performances, and IT(m)-3 (wIn/Ti = 3%, modified by N, B co-doping) showed the best photocatalytic activity. The band gap was successfully narrowed to 2.37 eV, and In2O3/TiO2 heterostructure was successfully constructed in the photocatalysts. Visible light absorption and separation of photoexcited carriers were significantly enhanced. Under visible light, NOX (20 mg/L in the water) was 100% photodegraded in 10 min, with 100% TOC removed in 25 min. The IT(m)-3-loaded quartz fabrics (ITQ) also showed excellent photocatalytic performance. The photocatalytic activity of ITQ was repeatable and stable in 45 rounds of recycle without centrifugation. Synergetic effect of heterostructure and element doping was considered contributing to the outstanding photocatalysis performance. The polymeric precursor method proposed in this work showed great potential in large-scale preparation and application of immobilized photocatalysts. The synergetic improvement through co-modifications showed its proper compatibility with modifications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Antibiotics have been utilized as an elixir to protect human beings and animals from various pathogens for more than a half of century, and the production and consumption are both escalating in recent years [1]. The emission of antibiotics from hospitals and farms is increasingly detrimental to water environments [2,3,4]. Norfloxacin (NOX), belonging to the third generation of fluoroquinolone antibiotics (FQAs), has been repeatedly found in various aqueous environments [5,6,7,8]. The omnipresent and increasing concentration of NOX in aquatic environments may induce occurrence of microbial pathogens with antibiotic resistance, rendering the extant antibiosis treatment futile [9,10,11,12]. In order to remove the antibiotics from wastewater, numerous techniques have been exerted, such as microbial degradation process [13], sorption [5, 14, 15] or Fenton process [16]. However, due to the stability and poor biodegradability of the quinolone ring of NOX [17], traditional treatment cannot achieve efficient degradation of NOX or demands tremendous time and money [18].
Recently, photocatalysis has been receiving considerable concern as an effectual method in the field of water treatment [19,20,21,22,23], which is able to overcome the disadvantages of low efficient degradation and secondary pollution for its complete degradation of organic contaminates into H2O and CO2 [24,25,26]. TiO2 is generally recognized as the promising photocatalyst for its nontoxicity, stability, low-cost and high photocatalytic activity [27]. However, pristine TiO2 shows hardly any photocatalysis under visible light due to its intrinsic wide band gap (3.2 eV) and frequent recombination of excited carriers during the photodegradation [28]. Therefore, numerous researches have been conducted to improve the visible-light photocatalytic activity and restrain the recombination of photogenerated carriers, such as self-doping [29], ion doping [30,31,32] and heterojunction constructing [28, 33,34,35,36]. The strategy of ion doping is a convenient and fruitful way to narrow the band gap by the substitution of Ti 3d or O 2p states [37]. Moreover, multiple ions co-doping, such as N-B co-doping [38], N–Cl co-doping [39], N–Ir co-doping [40] and N-F co-doping [41], has been studied and proved to be more effective than single-element doping. Besides, heterostructure constructing with semiconductors with narrow band gap is also an ideal candidate because their typical structure is conducive to charge separation and transfer [42]. Indium oxide, with a band gap of 2.8 eV, is an indirect band semiconductor and known as a transparent conducting material which has been diffusely applied in solar cells and optical or antistatic coatings [43]. Moreover, In2O3 has also been proved to be competent in improving the photocatalytic efficiency of other semiconductors by improving absorption of visible light region [44, 45] and In2O3/TiO2 heterostructrue is reported to be in favor of increasing the photocatalytic activity due to the lower bandgap of In2O3 and band alignments of both semiconductor materials [46]. Mohammad et al. [47] prepared mesoporous In2O3-TiO2 nanocomposites by a sol–gel method, and the sample 0.1% In2O3-TiO2 achieved the best photocatalytic performance under UV light. However, the product in this work showed no improved visible light absorption in terms of its ultraviolet–visible diffuse reflectance spectra (UVDRS), and no photodegradation efficiencies under visible light were provided. Du et al. [48] prepared core–shell-structured TiO2@In2O3, and it showed enhanced photocatalytic activity under visible light compared with the samples for reference. In2O3 significantly enhanced visible-light absorption of TiO2 and photocatalytic activity of decomposing methyl orange in this work. The final product of the above two studies was in the form of powders or particles, limiting their practical application because the posttreatment recovery of TiO2 in powder form is both time and money consuming [49]. An et al. [50] prepared immobilized TiO2@In2O3 by a two-step hydrothermal method and adopted photoelectrochemical (PEC) measurements to investigate the capacity of water splitting and degradation of BPA. Though compared with TiO2 nanorods (NR), the TiO2@In2O3 showed improved photocatalytic performance according to the H2 evolution reaction (HER) rates; no improvement in photodegradation efficiency can be discerned through PEC decomposition of bisphenol A and methyl orange. Moreover, the UVDRS also showed no redshift to longer wavelength.
Hence, to further improve visible light response and photocatalytic activity of TiO2, incorporating ion doping and In2O3 heterostructures into the co-modification of TiO2 catalyst is promising, but the common viable approaches of ion doping or heterostructures in the previous studies demand long synthesis period and harsh condition which is detrimental to further massive production of catalysts, and the form of their products are mainly in powder which severely reduces the feasibility of practical application because of the expensive separation and recycle process for the powdered photocatalysts. Thus, it is imperative for academy and industry to find a convenient and effectual way of the ion doping or heterostructure construction. Feasible processing and stable recyclability will be a bonus for massive production and application in reality.
To resolve these scientific issues, polymeric precursor method [51] can be exerted whose product is homogeneous and long-term stable solution. This approach shows various advantages: (1) adjustable structure and composition; (2) mild synthesis conditions and short synthesis period; (3) facile to various modification and immobilization on substrates; (4) superior film forming ability and uniformity; (5) compatible with soft template to introduce pore structures. Based on the above superiorities, polymeric precursor method is suitable for the preparation of double elements co-doped photocatalyst with heterostructures and substrate-loaded photocatalyst or nanofiber membranes which facilitated the separation and reuse.
In this work, N–B co-doped TiO2 catalysts with In2O3/TiO2 heterostructure (In2O3/TiO2(m)) were prepared by a one-step polymeric precursor method. The physical property and chemical structure were characterized. The results of photodegradation tests showed that the photocatalytic capacity of the powered TiO2 catalyst and substrate-loaded catalysts were remarkable in decomposing NOX. The mechanism of how N, B co-doping and In2O3/TiO2 heterostructure ameliorated photocatalysis performance was also discussed.
Experiment
Preparation of In2O3/TiO2(m)
In2O3/TiO2(m) precursor solution was prepared by polymeric precursor method [51] (Fig. 1). Typically, titanium tetraisopropoxide (TTIP, Aldrich) was mixed with PEG-600 (Xilong Chemical Co. Ltd) and In(NO3)3 (Aladdin Industrial Co.). The mixture was heated at 120 °C for 2 h with the presence of drastic stir. After cooling to 90 °C, boric acid (BA, Sinopharm Chemical Reagent Co.) was added in and heated at 110 °C for 4 h. Then, acetamide (Acam, Sinopharm Chemical Reagent Co.) was added in the solution, which was heated at 120 °C for 4 h and then cooled to 85 °C. After that, acetylacetone (Acac, Sinopharm Chemical Reagent Co.) and a mixture of H2O and n-propanol (Sinopharm Chemical Reagent Co.) (mass ratio of H2O/n-propanol is 1/4) were sequentially added dropwise. Then, the mixture was refluxed for 2 h to accomplish the preparation of In2O3/TiO2(m) precursor. Afterward, the solvent of as-prepared precursor solution was evaporated and the obtained In2O3/TiO2(m) resins were calcined at 450 °C for 30 min with a heating rate of 3 °C/min. After calcination, the obtained In2O3/TiO2(m) chips were manually ground into powders. The molar ratio of the TTIP/BA/Acam/Acac/H2O was 1.0/0.24/2.0/0.3/0.5, and the mass ratio of In/Ti varied from 1%, 3% and 5%, denoting as IT(m)-1, IT(m)-3 and IT(m)-5, respectively. Besides, sample IT-10 (In2O3/TiO2 sample without the ion doping, mIn/mTi = 10%), sample TiO2(m) (TiO2, co-doped with N and B, without the addition of indium nitrate) and pristine TiO2 (without the ion doping and the addition of indium nitrate) were also prepared by similar method for comparison.
Preparation of coated quartz fabrics
Quartz fabric (QF) wool was preheated at 450 °C in muffle oven, removing the sizing agent on the surface. The heat-treated QF was tailored to a size of 50 mm × 30 mm as substrate with a weight of 0.15 g controlled by thickness. The IT(m)-3 precursor was diluted with n-propanol, content controlled by Ti mass ratio of 3%, Then, polyvinylpyrrolidone (PVP, 1% of mass of diluted precursor) was added and the system was heated at 100 °C for 1 h. The tailored QF was dipped into the 50 g diluted precursor and heated for 1 h at 100 °C. Then, it was extracted and the solvent was evaporated. The resulting precursor-coated QF was calcined at 450 °C for 30 min; the resulted IT(m)-3 coated-QF labeled as ITQ. The mass of loaded catalysts was 30 mg.
Characterization
Crystalline structures were probed by X-ray diffraction (XRD, Rigaku D/max 2500, Cu Ka source, 40 kV 200 mA). Chemical structure was studied by X-ray photoelectron spectroscopy (XPS, ESCALab220i-XL). The morphology was investigated by a field-emission scanning electron microscope (SEM, Hitachi SU-8020) at an accelerating voltage of 10 kV and a high-resolution field-emission transmission electron microscope (HRTEM, JEOL JEM-2100F) at an accelerating voltage of 200 kV. The contents of titanium and indium in the catalysts were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES, IRIS Intrepid II XDL, Thermo Electron Corporation), and nitrogen and oxygen content were measured by a nitrogen/oxygen analyzer (TC-306, Baoying Technology). The Brunauer–Emmett–Teller (BET) surface areas of samples were analyzed by nitrogen adsorption by induced isotherms at 77 K with a Micromeritics ASAP 2020 instrument. The photoluminescence (PL) spectra were measured with a fluorescence spectrometer (F-4500, HITACHI). The ultraviolet–visible diffuse reflectance spectra (UV–Vis DRS) were probed by a spectrophotometer (Shimadzu, UV-2600).
Photodegradation of NOX
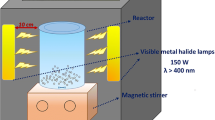
In2O3/TiO2(m), with 30 mg amount, and ITQ were utilized to catalyze the photodegradation of NOX (20 mg/L, 30 ml) in cuvettes under visible light from a 500-W Xeon lamp (wavelength below 420 nm cut off). After the treatment, ITQ was extracted and repeatedly washed by deionized water. Then, it was dried out at 100 °C and ready for recycle test. The photodegraded samples containing catalyst powders were all centrifuged, while the ones photocatalyzed by immobilized catalysts were separated without the presence of centrifugation. After the separation, the left solutions were analyzed by spectrophotometric method. The total organic carbon (TOC, Shimadzu, TOC-L) was adopted to measure the mineralization degree of NOX.
Results and discussion
Crystalline structure and morphology
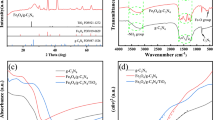
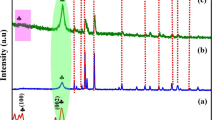
XRD patterns of In2O3/TiO2(m) with different indium contents are shown in Fig. 2. Accordingly, crystal phase of anatase TiO2 is mainly obtained (ICDD: 01-071-1166). Besides, a relatively small peak at ~ 30.2˚ is detected compared with the XRD result of the pristine TiO2 (Figure S1) and supposed to be ascribed to the overlapped Bragg diffraction peak of B2O3 and In2O3. A minor but nonnegligible shift toward lower 2Theta degree can also be detected as the mass ratio of indium increases (inset of Fig. 1), as is mainly caused by the TiO2 lattice disruption of In2O3/TiO2 heterostructure. Rietveld fittings were also executed by Xpert Highscore. The results show that the lattice parameters of sample IT(m)-3 (a = 3.811 Å, b = 3.811 Å, c = 9.511 Å) increase when compared with that of pristine TiO2 (a = 3.776 Å, b = 3.776 Å, c = 9.486 Å), indicating that nitrogen and boron are successfully introduced into the crystal lattice of TiO2 since the atomic radius of oxygen are smaller than those of nitrogen and boron [52].
The morphology was investigated by SEM and HRTEM. As shown in the SEM image for sample IT(m)-3 (Fig. 3a), the prepared photocatalysts are uniformly pseudo-spherical nanoparticles and stacked in a botryoidal structure with brain-textured sulci and gyri. The elemental mapping analysis result of IT(m)-3 (Figure S2) shows that the added elements are present in the sample and they are homogeneously dispersed on the TiO2. Also, the uniformity of particle size is favorable, ranging from 10 to 20 nm, and in conformity to the diameter of P25, the mainstream TiO2 commodity [28]. Interestingly, the BET surface area of IT(m)-3 is 206.89 m2/g which is 3.4 times larger than that of P25 (60.44 m2/g), the N2 adsorption–desorption isotherm of IT(m)-3 and its pore size distribution (Figure S3) indicate that mesoporous structure was formed during the calcination which is reported to be able to be beneficial for visible light absorption [53], additional active sites and higher electron transfer capacities [54]. HRTEM images (Fig. 3c, d) distinctly show the lattice fringes spacing of 0.35 nm, which is in correspondence with (101) facet of anatase [55]. Besides, the discernable lattice fringes spacing of 0.41 nm is in agreement with (211) facet of cubic In2O3 coupled with the (101) facet of anatase [56]. As a control, HRTEM image of IT-10 (Fig. 3b) shows distinct attachment of lattice fringes of anatase (0.35 nm) and cubic In2O3 (0.41 nm). Thus, the In2O3/TiO2 heterostructure is well established and it is reported that heterostructure is conducive to better charge separation via acting as trapping site and phase-junction [33]. The crystal phase structure obtained by HRTEM images is consistent with that obtained from XRD analysis.
Chemical composition and structure
ICP-AES and nitrogen/oxygen analyzer were exerted to measure the metal and nonmetallic element contents in the catalysts, respectively. Based on the results shown in Table S1, the mass ratio of In/Ti for the sample IT(m)-3 is ~ 3% which is identical with the raw mass ratio of In/Ti in preparation, indicating that there is nearly no attrition of indium during the preparation.
X-ray photoelectron spectroscopy was used to investigate chemical structures of the sample IT(m)-3, and the chemical environments of N, B, In and Ti were obtained, as shown in Fig. 4. The N 1 s XPS spectrum (Fig. 5a) shows two peaks located at 399.34 eV and 397.49 eV after the deconvolution. The peak at 399.34 eV is ascribed to the structure of Ti-N-B [57] and independent interstitial N-doping [39, 58, 59] in the TiO2 crystal lattice, and the relatively smaller peak at 397.49 eV can be ascribed to the substitutional N-doping in the TiO2 crystal lattice [60]. The presence of above structures is reported to be conducive to the enhancement of photocatalytic activity of TiO2 under visible light. The B 1 s spectrum (Fig. 5b) is deconvoluted into two peaks corresponding to the binding energies of 192.04 eV and 193.99 eV which are attributed to the structure of Ti–O–B (interstitial B) [61] and B2O3 [62], respectively. The results from N 1 s and B 1 s XPS spectra indicate that the structure of Ti–N–B–O–Ti is generated during the doping, where N and B are co-interstitial in one lattice, and the independent interstitial B, interstitial and substitutional N are also introduced into the TiO2 crystal lattice. Moreover, the existence of B2O3 on the surface confirmed by B 1 s XPS spectrum result is in agreement with XRD results. As shown in Fig. 5C, the In 3d5/2 XPS spectrum can only be deconvoluted into one peak located at 444.39 eV belonging to In2O3 [63]. There are no peaks of In-N or In-B detected, indicating that indium is only present in the form of In2O3. According to the Ti 2p XPS spectrum displayed in Fig. 5D, characteristic peaks of Ti 2p1/2 and Ti 2p3/2 of the sample IT(m)-3 are located at 464.24 and 458.49 eV, respectively. Difference between the two characteristic peaks (5.75 eV) demonstrated that Ti is in the form of Ti4+ [46]. As a comparison, Ti 2p1/2 and Ti 2p3/2 peaks of the pristine TiO2 are located at 465.04 eV and 459.37 eV, respectively. Binding energies of Ti 2p1/2 and Ti 2p3/2 peaks of IT(m)-3 are 0.8 eV and 0.88 eV lowered compared with those of pristine TiO2, respectively; while the splitting to Ti 2p1/2 and Ti 2p3/2 is unchanged [28]. The shift can be ascribed to the generation of In2O3/TiO2 heterostructure which leads to the change of chemical environment.
Photodegradation performance
The photocatalytic activities of as-prepared In2O3/TiO2(m) samples were tested by catalyzing the photodegradation of NOX. Figure S4A shows that the sample IT(m)-3 can achieve nearly complete decomposition of NOX after the illumination of visible light for 10 min which is higher than the photodegradation efficiencies of the sample IT(m)-1 and IT(m)-5, showing the marvelous photodegradation efficiency of IT(m)-3. As for the photodegradation results shown in Fig. 6a, about 68% NOX is photodegraded when catalyzed by TiO2(m); and less than 40% NOX can be photodegraded in the case of IT-10 and pristine TiO2. IT-10 and IT(m) both show inferior photocatalysis under visible light compared with that of IT(m)-3, indicating that the synergistic effect of ion doping and heterostructures is advantageous. Meanwhile, according to the results of pseudo first-order kinetic curves of NOX decomposition (Figure S4B), the calculated reaction rate constant (k) of IT(m)-3 is the highest, which is 2.46 and 6.52 times more than those of TiO2(m) and IT-10, respectively. Moreover, TOC was applied in this work to investigate the capacity of mineralization. As shown in Fig. 5B, under visible light, only 11%, 68% and 35% TOC are removed by the pristine TiO2, TiO2(m) and IT-10, respectively. 100% TOC removal rate is accomplished by the sample IT(m)-3 under the same condition, indicating its enhanced capacity of mineralization by the synergistic effect of ion doping and In2O3/TiO2 heterostructure. After loading the photocatalysts on quartz fabrics, as the sample ITQ prepared, photodegradation experiments can be carried out more conveniently, faster and more efficiently. In this case, photocatalytic activity and recyclability of ITQ were tested by cycling photodegradation of NOX. As the results shown in Fig. 7, in the 45 rounds of recycle experiments, nearly 100% NOX is decomposed at every turn. TOC result of ITQ (Fig. 6b) shows that 100% TOC is also removed in 25 min under visible light. Moreover, XRD pattern of ITQ (Figure S5) shows that anatase is still the dominate crystal phase of the supported photocatalysts and when compared to XRD pattern of pure quartz fabric (Figure S5 inset), the peak located at between 20° and 25° was belonging to quartz fabric, and the rest of it was overlapped by the main diffraction peak (2Theta = 25.2°) of the coated photocatalysts. SEM images of ITQ (Figure S6A, B) indicates that the photocatalysts were uniformly loaded on the quartz fabrics and maintained their crystal phase and morphology after coating process and after the recycling tests, ITQ retains its morphology (Figure S6C, D), indicating the existence of excellent binding force between the loaded IT(m)-3 and the substrates. The high photodegradation efficiency, robust recyclability, simple and convenient maneuverability endowed the ITQ with great potential in massive practical application.
Optical property
UV–Vis diffuse reflectance spectrometer was exerted to investigate photon harvest capacity of the as-prepared catalysts [52]. As the results shown in Fig. 8, all absorption edges of IT(m)-3, IT-10 and TiO2(m) display evident redshift from ~ 380 nm of the pristine TiO2 to ~ 435, ~ 455 and ~ 420 nm, respectively. At the wavelengths after the absorption edges, the three samples, IT(m)-3, IT-10 and TiO2(m), still possess light absorption, where optical absorption of pristine TiO2 can be negligible in fact. Among these three samples, TiO2(m) shows lowest light absorption in the whole visible-light region. From their absorption edges to 550 nm, IT-10 shows higher light absorption than IT(m)-3; while at longer wavelengths, absorbance of IT(m)-3 exceeds that of IT-10. It is notable that on the one hand, 10 wt% In was used in the sample IT-10, where absorption edge wavelength more than those of the other samples is realized; on the other hand, although only 3 wt% In was added in IT(m)-3, better absorbance stability is obtained over the visible light range, as mainly contributed from the ion doping.
Meanwhile, Tauc Eq. (1) was exerted to calculate the band gap of these samples [64].
where α is absorption coefficient, h is Plank constant, ν is photon frequency, k is a constant related to the type of transition and Eg is the optical band gap.
As demonstrated in Fig. 9, the band gaps of IT(m)-3, IT-10 and TiO2(m) are 2.37, 2.24 and 2.64 eV, respectively, which are all narrowed compared to the pristine TiO2. Although IT(m)-3 is not the best in each single contrast of absorption edge and band gap, it shows the best photocatalytic performance among these samples. Ion doping and heterostructure are, respectively, realized in the TiO2(m) and IT-10 samples, but synchronously established in the IT(m)-3 sample. It suggests that the ion doping and establishment of In2O3/TiO2 heterostructure are both conducive to the reduction in band gap and enhancement of visible light absorption simultaneously; combination of the two approaches can make a synergetic effect, resulting in the superior photon reaping capacity of IT(m)-3 under visible light.
The photoluminescence (PL) spectrum indicates the recombination rate of photoexcited carriers by measuring the emission intensity from the secondary combination of these charge carriers [65, 66]. As shown in Fig. 10, the PL intensity of pristine TiO2 is the highest; it lowers much for IT-10 and TiO2(m). Moreover, it is notable that the PL intensity of IT(m)-3 is much lower than that of any other sample. For the pristine TiO2 sample, the fast recombination rate of photoexcited carriers naturally appears without obstruction. In the case of IT-10, In2O3/TiO2 heterostructure is established, hindering the recombination of photogenerated electron–hole pairs due to preferable separation and transfer efficiency on the In2O3/TiO2 interface. In the sample TiO2(m), N and B co-doping structure was constructed and band gap was narrowed. Recombination of charge carriers can also be effectually impeded by the augmented number and shortened migration time of the charge carriers. When the sample IT(m)-3 is used, the combination of ion doping and heterostructure construction generates synergistic positive effect on impeding the recombination of electron–hole pairs. Thus, from an optical point of view, the superior photocatalysis performance of IT(m)-3 is ascribed to the competent absorption in the whole visible-light area and substantially lowered recombination rate of photoexcited electron–hole pairs, both resulted from its narrowed band gap and incorporated In2O3/TiO2 heterostructure.
Photocatalytic reaction mechanism
Hydroxyl radicals (OH·), superoxide radicals (·O2−) and holes (h+) are the main reactive species when TiO2 photocatalyst is used to catalyze the degradation of NOX. Therefore, to fathom the photocatalytic mechanism of IT(m)-3, three different trapping agents, including EDTA-2Na as holes scavenger [67], tertiary butanol as OH· scavenger [68] and p-benzoquinone as ·O2− scavenger [69], were added to gauge their respective effects on the photocatalytic activity of IT(m)-3. According to the results shown in Figure S7, the photodegradation efficiencies of NOX are lowered down to 5%, 88% and 75% when t-butanol, EDTA-2Na and p-benzoquinone were implemented, respectively. The 95% photodegradation efficiency decrease indicates that hydroxyl radicals are the dominate reactive agents of IT(m)-3 when catalyzing the photodegradation of NOX under visible light.
Hence, based on the aforementioned results and band alignment of cubic In2O3 and anatase TiO2 [44, 47, 70, 71], the schematic photodegradation mechanism of IT(m)-3 is proposed and illustrated in Fig. 11. The ion doping narrows the band gap by generating impurity levels above the top of the valence band of TiO2, improving the visible-light absorption [46], and the In2O3/TiO2 heterostructure favors the separation and transfer of excited electrons of holes on the interface. Specifically, under the irradiation of visible light, modified TiO2 due to its narrowed band gap and In2O3 will generate charge carriers, and since the conduction band potential of TiO2 is more negative than that of In2O3, the generated electrons will transfer to the conduction band of TiO2. Also, the generated holes in the TiO2 will also migrate to the valence band of In2O3 because its lower valence band potential. The proposed photodegradation mechanism of IT(m)-3 under visible light may function as follows:
Thus, the facilitation of both the harvest of photons under visible light and the separation of charge carriers which further enhances the photocatalytic activity are realized simultaneously by the synergistic effect of the ion doping and the In2O3/TiO2 heterostructure.
Conclusion
In2O3/TiO2(m) photocatalyst was prepared by a novel precursor method and showed favorable photocatalytic activity. Under visible light, nearly 100% of NOX could be photodegraded by sample IT(m)-3 in 10 min and 100% TOC was removed in 25 min. The preferable photocatalytic activity of IT(m)-3 is due to the enhancement of specific surface areas, the narrowed band gap and the In2O3/TiO2 heterostructures whose synergistic effect improves the capacity of utilization of visible light and reduces the recombination rate of generated electron–hole pairs.
In2O3/TiO2(m) photocatalysts coated on the quartz fabrics are robust and can sustain superior photocatalytic activity after recycling and reusing for 45 times. It is noteworthy that the condition of the polymeric precursor method is moderate, and the centrifugation is not involved during the recycle process, as is a tremendous benefit for reducing cost of pollution treatment. Furthermore, the as-prepared immobilized catalysts could be prepared in a simple way and compatible with many substrates, owing to the favorable machinability of In2O3/TiO2(m) precursor. Thus, it shows great potential in large-scale treatment of industrial or domestic wastewater. The synergistically enhanced photocatalytic activity of IT(m)-3 through the co-modification was also readily achieved by this method, manifesting its proper compatibility with multiple modifications.
References
Zhang QQ, Ying GG, Pan CG, Liu YS, Zhao JL (2015) Comprehensive evaluation of antibiotics emission and fate in the river basins of China: source analysis, multimedia modeling, and linkage to bacterial resistance. Environ Sci Technol 49:6772–6782
Sarmah AK, Meyer MT, Boxall AB (2006) A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 65:725–759
Zhou LJ, Ying GG, Liu S, Zhao JL, Yang B, Chen ZF, Lai HJ (2013) Occurrence and fate of eleven classes of antibiotics in two typical wastewater treatment plants in South China. Sci Total Environ 452–453:365–376
Zhu YG, Johnson TA, Su JQ, Qiao M, Guo GX, Stedtfeld RD, Hashsham SA, Tiedje JM (2013) Diverse and abundant antibiotic resistance genes in Chinese swine farms. Proc Natl Acad Sci USA 110:3435–3440
Peng H, Feng S, Zhang X, Li Y, Zhang X (2012) Adsorption of norfloxacin onto titanium oxide: effect of drug carrier and dissolved humic acid. Sci Total Environ 438:66–71
Tan F, Sun D, Gao J, Zhao Q, Wang X, Teng F, Quan X, Chen J (2013) Preparation of molecularly imprinted polymer nanoparticles for selective removal of fluoroquinolone antibiotics in aqueous solution. J Hazard Mater 244–245:750–757
Pruden A (2014) Balancing water sustainability and public health goals in the face of growing concerns about antibiotic resistance. Environ Sci Technol 48:5–14
Wammer KH, Korte AR, Lundeen RA, Sundberg JE, McNeill K, Arnold WA (2013) Direct photochemistry of three fluoroquinolone antibacterials: norfloxacin, ofloxacin, and enrofloxacin. Water Res 47:439–448
Dinh QT, Moreau-Guigon E, Labadie P, Alliot F, Teil MJ, Blanchard M, Chevreuil M (2017) Occurrence of antibiotics in rural catchments. Chemosphere 168:483–490
Sharma VK, Johnson N, Cizmas L, McDonald TJ, Kim H (2016) A review of the influence of treatment strategies on antibiotic resistant bacteria and antibiotic resistance genes. Chemosphere 150:702–714
Chen QL, Li H, Zhou XY, Zhao Y, Su JQ, Zhang X, Huang FY (2017) An underappreciated hotspot of antibiotic resistance: the groundwater near the municipal solid waste landfill. Sci Total Environ 609:966–973
Liao P, Zhan Z, Dai J, Wu X, Zhang W, Wang K, Yuan S (2013) Adsorption of tetracycline and chloramphenicol in aqueous solutions by bamboo charcoal: a batch and fixed-bed column study. Chem Eng J 228:496–505
Kümmerer K, Al-Ahmad A, Mersch-Sundermann V (2000) Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere 40:701–710
Liu W, Zhang J, Zhang C, Ren L (2011) Sorption of norfloxacin by lotus stalk-based activated carbon and iron-doped activated alumina: mechanisms, isotherms and kinetics. Chem Eng J 171:431–438
Sassman SA, Sarmah AK, Lee LS (2007) Sorption of tylosin A, D, and A-aldol and degradation of tylosin A in soils. Environ Toxicol Chem 26:1629–1635
Zhang W, Gao H, He J, Yang P, Wang D, Ma T, Xia H, Xu X (2017) Removal of norfloxacin using coupled synthesized nanoscale zero-valent iron (nZVI) with H2O2 system: optimization of operating conditions and degradation pathway. Sep Purif Technol 172:158–167
Liao W, Sharma VK, Xu S, Li Q, Wang L (2017) Microwave-enhanced photolysis of norfloxacin: kinetics, matrix effects, and degradation pathways. Int J Environ Res Public Health 14:1564
Pignatello JJ, Oliveros E, MacKay A (2006) Advanced oxidation processes for organic contaminant destruction based on the fenton reaction and related chemistry. Crit Rev Env Sci Tec 36:1–84
Kumar A, Sharma SK, Sharma G, AaH Al-Muhtaseb, Naushad M, Ghfar AA, Stadler FJ (2019) Wide spectral degradation of Norfloxacin by Ag@BiPO4/BiOBr/BiFeO3 nano-assembly: elucidating the photocatalytic mechanism under different light sources. J Hazard Mater 364:429–440
Kumar A, Rana A, Sharma G, Naushad M, AaH Al-Muhtaseb, Guo C, Iglesias-Juez A, Stadler FJ (2018) high-performance photocatalytic hydrogen production and degradation of levofloxacin by wide spectrum-responsive Ag/Fe3O4 Bridged SrTiO3/g-C3N4 plasmonic nanojunctions: joint effect of Ag and Fe3O4. ACS Appl Mater Interfaces 10:40474–40490
Sharma G, Kumar A, Naushad M, Kumar A, AaH Al-Muhtaseb, Dhiman P, Ghfar AA, Stadler FJ, Khan MR (2018) Photoremediation of toxic dye from aqueous environment using monometallic and bimetallic quantum dots based nanocomposites. J Cleaner Prod 172:2919–2930
Dhiman P, Chand J, Kumar A, Kotnala RK, Batoo KM, Singh M (2013) Synthesis and characterization of novel Fe@ZnO nanosystem. J Alloys Compd 578:235–241
Kumar A, Kumar A, Sharma G, Naushad M, Veses RC, Ghfar AA, Stadler FJ, Khan MR (2017) Solar-driven photodegradation of 17-β-estradiol and ciprofloxacin from waste water and CO2 conversion using sustainable coal-char/polymeric-g-C3N4/RGO metal-free nano-hybrids. New J Chem 41:10208–10224
Dong H, Guo X, Yang C, Ouyang Z (2018) Synthesis of g-C3N4 by different precursors under burning explosion effect and its photocatalytic degradation for tylosin. Appl Catal B 230:65–76
Wang J, Tang L, Zeng G, Deng Y, Liu Y, Wang L, Zhou Y, Guo Z, Wang J, Zhang C (2017) Atomic scale g-C3N4/Bi2WO6 2D/2D heterojunction with enhanced photocatalytic degradation of ibuprofen under visible light irradiation. Appl Catal B 209:285–294
Dong S, Ding X, Guo T, Yue X, Han X, Sun J (2017) Self-assembled hollow sphere shaped Bi2WO6/RGO composites for efficient sunlight-driven photocatalytic degradation of organic pollutants. Chem Eng J 316:778–789
Schneider J, Matsuoka M, Takeuchi M, Zhang J, Horiuchi Y, Anpo M, Bahnemann DW (2014) Understanding TiO2 photocatalysis: mechanisms and materials. Chem Rev 114:9919–9986
Tan Y, Shu Z, Zhou J, Li T, Wang W, Zhao Z (2018) One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl Catal B 230:260–268
Zuo F, Wang L, Wu T, Zhang Z, Borchardt D, Feng P (2010) Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J Am Chem Soc 132:11856–11857
Cheng Y, Zhang M, Yao G, Yang L, Tao J, Gong Z, He G, Sun Z (2016) Band gap manipulation of cerium doping TiO2 nanopowders by hydrothermal method. J Alloys Compd 662:179–184
Hwang YJ, Yang S, Lee H (2017) Surface analysis of N-doped TiO2 nanorods and their enhanced photocatalytic oxidation activity. Appl Catal B 204:209–215
Yan X, Xing Z, Cao Y, Hu M, Li Z, Wu X, Zhu Q, Yang S, Zhou W (2017) In-situ C-N-S-tridoped single crystal black TiO2 nanosheets with exposed 001 facets as efficient visible-light-driven photocatalysts. Appl Catal B 219:572–579
Zhang X, Wang Y, Liu B, Sang Y, Liu H (2017) Heterostructures construction on TiO2 nanobelts: a powerful tool for building high-performance photocatalysts. Appl Catal B 202:620–641
Kwon Kaang B, Han N, Jang W, Young Koo H, Boo Lee Y, San Choi W (2018) Crossover magnetic amphiprotic catalysts for oil/water separation, the purification of aqueous and non-aqueous pollutants, and organic synthesis. Chem Eng J 331:290–299
Kumar A, Kumar A, Sharma G, AaH Al-Muhtaseb, Naushad M, Ghfar AA, Stadler FJ (2018) Quaternary magnetic BiOCl/g-C3N4/Cu2O/Fe3O4 nano-junction for visible light and solar powered degradation of sulfamethoxazole from aqueous environment. Chem Eng J 334:462–478
Kumar A, Kumar A, Sharma G, Naushad M, Stadler FJ, Ghfar AA, Dhiman P, Saini RV (2017) Sustainable nano-hybrids of magnetic biochar supported g-C3N4/FeVO4 for solar powered degradation of noxious pollutants- Synergism of adsorption, photocatalysis & photo-ozonation. J Cleaner Prod 165:431–451
Liu G, Wang L, Yang HG, Cheng HM, Lu GQ (2010) Titania-based photocatalysts—crystal growth, doping and heterostructuring. J Mater Chem 20:831–843
Giannakas AE, Antonopoulou M, Daikopoulos C, Deligiannakis Y, Konstantinou I (2016) Characterization and catalytic performance of B-doped, B-N co-doped and B–N–F tri-doped TiO2 towards simultaneous Cr(VI) reduction and benzoic acid oxidation. Appl Catal B 184:44–54
Teh CY, Wu TY, Juan JC (2015) Facile sonochemical synthesis of N, Cl-codoped TiO2: synthesis effects, mechanism and photocatalytic performance. Catal Today 256:365–374
Kuvarega AT, Krause RWM, Mamba BB (2015) Evaluation of the simulated solar light photocatalytic activity of N, Ir co-doped TiO2 for organic dye removal from water. Appl Surf Sci 329:127–136
Wu Y, Dong Y, Xia X, Liu X, Li H (2016) Facile synthesis of N-F codoped and molecularly imprinted TiO2 for enhancing photocatalytic degradation of target contaminants. Appl Surf Sci 364:829–836
Zhang J, Xiao G, Xiao FX, Liu B (2017) Revisiting one-dimensional TiO2 based hybrid heterostructures for heterogeneous photocatalysis: a critical review. Mater Chem Front 1:231–250
Quarto FD, Sunseri C, Piazza S, Romano MC (1997) Semiempirical correlation between optical band gap values of oxides and the difference of electronegativity of the elements. Its importance for a quantitative use of photocurrent spectroscopy in corrosion studies. J Phys Chem B 101:333–340
Lv J, Kako T, Li Z, Zou Z, Ye J (2010) Synthesis and photocatalytic activities of NaNbO3 rods modified by In2O3 nanoparticles. J Phys Chem C 114:6157–6162
Wang Z, Huang B, Dai Y, Qin X, Zhang X, Wang P, Liu H, Yu J (2009) Highly photocatalytic ZnO/In2O3 heteronanostructures synthesized by a coprecipitation method. J Phys Chem C 113:4612–4617
Mu J, Chen B, Zhang M, Guo Z, Zhang P, Zhang Z, Sun Y, Shao C, Liu Y (2012) Enhancement of the visible-light photocatalytic activity of In2O3-TiO2 nanofiber heteroarchitectures. ACS Appl Mater Interfaces 4:424–430
Kadi MW, Ismail AA, Mohamed RM, Bahnemann DW (2018) Photodegradation of the herbicide imazapyr over mesoporous In2O3-TiO2 nanocomposites with enhanced photonic efficiency. Sep Purif Technol 205:66–73
Du Q, Ma J, Shao X, Wang W, Tian G (2019) Core-shell structured TiO2@In2O3 for highly active visible-light photocatalysis. Chem Phys Lett 714:208–212
Singh S, Mahalingam H, Singh PK (2013) Polymer-supported titanium dioxide photocatalysts for environmental remediation: a review. Appl Catal A 462–463:178–195
An GW, Mahadik MA, Piao G, Chae WS, Park H, Cho M, Chung HS, Jang JS (2019) Hierarchical TiO2@In2O3 heteroarchitecture photoanodes: mechanistic study on interfacial charge carrier dynamics through water splitting and organic decomposition. Appl Surf Sci 480:1–12
Yu H, Ye L, Zhang T, Zhou H, Zhao T (2017) Synthesis, characterization and immobilization of N-doped TiO2 catalysts by a reformed polymeric precursor method. RSC Adv 7:15265–15271
Cong Y, Zhang J, Chen F, Anpo M (2007) Synthesis and characterization of nitrogen-doped TiO2 nanophotocatalyst with high visible light activity. J Phys Chem C 111:6976–6982
Yu S, Wu X, Wang Y, Guo X, Tong L (2017) 2D Materials for optical modulation: challenges and opportunities. Adv Mater 29:1606128
Wang F, Feng Y, Chen P, Wang Y, Su Y, Zhang Q, Zeng Y, Xie Z, Liu H, Liu Y, Lv W, Liu G (2018) Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: kinetics, mechanism, and antibacterial activity elimination. Appl Catal B 227:114–122
Xu M, Wang Y, Geng J, Jing D (2017) Photodecomposition of NOx on Ag/TiO2 composite catalysts in a gas phase reactor. Chem Eng J 307:181–188
Li Z, Zhang P, Li J, Shao T, Wang J, Jin L (2014) Synthesis of In2O3 porous nanoplates for photocatalytic decomposition of perfluorooctanoic acid (PFOA). Catal Commun 43:42–46
Xing MY, Li WK, Wu YM, Zhang JL, Gong XQ (2011) Formation of new structures and their synergistic effects in boron and nitrogen codoped TiO2 for enhancement of photocatalytic performance. J Phys Chem C 115:7858–7865
Patel N, Jaiswal R, Warang T, Scarduelli G, Dashora A, Ahuja BL, Kothari DC, Miotello A (2014) Efficient photocatalytic degradation of organic water pollutants using V-N-codoped TiO2 thin films. Appl Catal B 150–151:74–81
Umare SS, Charanpahari A, Sasikala R (2013) Enhanced visible light photocatalytic activity of Ga, N and S codoped TiO2 for degradation of azo dye. Mater Chem Phys 140:529–534
Zeng L, Lu Z, Li M, Yang J, Song W, Zeng D, Xie C (2016) A modular calcination method to prepare modified N-doped TiO2 nanoparticle with high photocatalytic activity. Appl Catal B 183:308–316
Ling Q, Sun J, Zhou Q (2008) Preparation and characterization of visible-light-driven titania photocatalyst co-doped with boron and nitrogen. Appl Surf Sci 254:3236–3241
Feng N, Zheng A, Wang Q, Ren P, Gao X, Liu S-B, Shen Z, Chen T, Deng F (2011) Boron environments in B-doped and (B, N)-codoped TiO2 photocatalysts: a combined solid-state NMR and theoretical calculation study. J Phys Chem C 115:2709–2719
Li Z, Zhang P, Shao T, Wang J, Jin L, Li X (2013) Different nanostructured In2O3 for photocatalytic decomposition of perfluorooctanoic acid (PFOA). J Hazard Mater 260:40–46
Zhou W, Du G, Hu P, Li G, Wang D, Liu H, Wang J, Boughton RI, Liu D, Jiang H (2011) Nanoheterostructures on TiO2 nanobelts achieved by acid hydrothermal method with enhanced photocatalytic and gas sensitive performance. J Mater Chem 21:7937–7945
Zhang W, Xiao X, Li Y, Zeng X, Zheng L, Wan C (2016) Liquid-exfoliation of layered MoS2 for enhancing photocatalytic activity of TiO2/g-C3N4 photocatalyst and DFT study. Appl Surf Sci 389:496–506
Wei Z, Liang F, Liu Y, Luo W, Wang J, Yao W, Zhu Y (2017) Photoelectrocatalytic degradation of phenol-containing wastewater by TiO2/g-C3N4 hybrid heterostructure thin film. Appl Catal B 201:600–606
Wei Z, Liu Y, Wang J, Zong R, Yao W, Wang J, Zhu Y (2015) Controlled synthesis of a highly dispersed BiPO4 photocatalyst with surface oxygen vacancies. Nanoscale 7:13943–13950
Lee H, Choi W (2002) Photocatalytic oxidation of arsenite in TiO2 suspension: kinetics and mechanisms. Environ Sci Technol 36:3872–3878
Liu W, Wang M, Xu C, Chen S, Fu X (2013) Significantly enhanced visible-light photocatalytic activity of g-C3N4 via ZnO modification and the mechanism study. J Mol Catal A Chem 368–369:9–15
Scanlon DO, Dunnill CW, Buckeridge J, Shevlin SA, Logsdail AJ, Woodley SM, Catlow CR, Powell MJ, Palgrave RG, Parkin IP, Watson GW, Keal TW, Sherwood P, Walsh A, Sokol AA (2013) Band alignment of rutile and anatase TiO2. Nat Mater 12:798–801
Xu Y, Schoonen MAA (2000) The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am Mineral 85:543–556
Acknowledgements
This work was financially supported by National Science Foundation of China (Nos. 21604090 and 51403218).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, H., Chen, F., Ye, L. et al. Enhanced photocatalytic degradation of norfloxacin under visible light by immobilized and modified In2O3/TiO2 photocatalyst facilely synthesized by a novel polymeric precursor method. J Mater Sci 54, 10191–10203 (2019). https://doi.org/10.1007/s10853-019-03636-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03636-z