Abstract
In this study, a novel amphiphilic flocculant TP-ADL was synthesized using acrylamide, methacryloxyethyltrimethyl ammonium chloride and lauryl acrylate as monomers through UV-light-initiating template copolymerization technology. Copolymerization conditions were optimized towards higher intrinsic viscosity and conversion rate. The Fourier transform infrared spectroscopy and 1H nuclear magnetic resonance spectroscopy (NMR) analysis confirms the functional groups composition in TP-ADL, and the thermogravimetric (TGA) analysis indicates its good thermal stability. Furthermore, the detailed 1H NMR and TGA analysis confirms the microblock structure in polymer chain. The amphiphilic rheological characteristics of copolymer were detected according to apparent viscosity. TP-ADL displayed superior flocculation efficiency towards oily wastewater in terms of oil and turbidity removal rate, and zeta potential. The median floc size (d50) and fractal dimension (Df) results indicate a large and compact floc structure. The synergistic effect between cationic microblock structure and inter-molecular hydrophobic association is the main reason for the better treatment efficiency.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapid development of industries that produce significant amounts of oily wastewater discharge and leakage, including petrochemicals, leather, transportation, food and steel, is creating environmental problems in China [1]. The oily contaminants pollute not only the wastewater management system, including surface water and groundwater, but also affect the quality of the air environment due to their organic volatility [2] and will undoubtedly cause serious environmental and economic problems without proper disposal. Furthermore, the chemical composition of oil-containing wastewater can be complex due to the presence of a wide variety of chemical substances that are highly dispersed together with hydrophobic oil components and suspended colloidal particles [3].
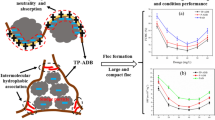
The separation of oil-based components from oily wastewater can be achieved using various technologies, including adsorption [4], air floatation [5], membrane filtration [6], biological technology [7], flocculation–coagulation [8] and electrochemical technology [9]. Among the listed technologies, flocculation is one of the most widely used methods in water treatment and exhibits unique superiorities for oil-containing wastewater treatment due to its easy operation, low cost, environmental friendliness, etc. [10]. The performance of flocculation methods is highly dependent on the physical and chemical characteristics of flocculants, and therefore, increasing attention has been paid to the design and development of flocculants that are tailored to the task of removing oil-based components from oily wastewater. One of the most promising flocculants for oily wastewater due to its high molecular weight (facilitating particle bridging) and charge density (facilitating charge neutralization) is the organic polymer cationic polyacrylamide (CPAM). Nevertheless, the treatment efficiency of traditional CPAMs is seriously limited when used alone in oily wastewater. Firstly, as shown in Fig. 1a, the cationic units are randomly distributed along the chain because of free radical copolymerization and the units situated on the loops and tails of the macromolecular chain cannot be fully utilized. Therefore, the theoretical charge neutralization capability according to the quantity of cationic units is less than the actual capability.
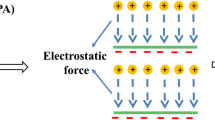
In recent years, template polymerization technology has been used to try to exert some degrees of control over the distribution of cationic units in CPAMs [11]. Like a genetic translation process, a low molecular weight polymer acts as an anionic template which can adsorb cationic monomers through electrostatic forces to realize an ordered arrangement of cationic units along with the anionic templates. Once the copolymerization reaction has been initialized, the pre-adsorbed cationic monomer tends to homopolymerize with one another next to it instead of other free acrylamide (AM) monomers in the reaction system. After separation of the anionic templates, a novel CPAM with cationic microblock structure can be obtained. As displayed in Fig. 1b, the cationic fragment structure can make full use of each cationic unit to provide stronger and less easily separated segments and therefore, a stronger and more compact floc structure can be obtained, which brings better flocculation and separation performance. Furthermore, the concentrated charge distribution also provides a more stretched linear configuration polymer chain in solution due to the stronger charge repulsion force, which is favourable for the bridging performance to obtain larger flocs [12]. Another main bottleneck in oily wastewater flocculation treatment is the weak interaction between the traditional hydrophilic CPAM polymer chain and the hydrophobic oily colloids [3]. The introduction of hydrophobic functional groups on the copolymer backbone can effectively enhance the interaction between the hydrophobic target contaminants and flocculants to achieve a better flocculation performance. Furthermore, the interaction between hydrophobic groups on the polymer chain can also lead to unique rheological characteristics, which is favourable for flocculation process [8]. As illustrated in Fig. 1c, the hydrophobic groups in aqueous solution can gather together to form both intra- and inter-molecular associations to minimize the exposure of hydrophobic units to the solvent [13]. This inter-molecular association effect gathers different polymer chains together to form a stretched net-like structure, and the adsorption bridging effect is further strengthened [13]. To the best of our knowledge, this is the first report that cationic microblock structure and hydrophobic units were combined for the better flocculation efficiency of oil-containing wastewater. As can be imagined, the stronger charge neutralization, adsorption bridging and hydrophobic association effects can work together to achieve higher flocculation performance. When compared to traditional heat initiation, UV initiation as a new initiation technology has many superiorities including lower reaction temperature, shorter initiation time, easy to operate, etc. [14].
In this study, a hydrophobically associating polyacrylamide with cationic microblock structure labelled as TP-ADL was synthesized through UV-initiating template copolymerization using cationic methacryloxyethyltrimethyl ammonium chloride (DMC), hydrophobic lauryl acrylate (LA) and acrylamide (AM) as monomers. Low molecular weight sodium polyacrylate (NaPAA) was introduced into the copolymerization system as an anionic template, so that the cationic units could be orderly distributed along with NaPAA to form cationic fragments in the copolymer chain. The basic principle for the UV-initiating template copolymerization technology is displayed in Fig. 2. The effects of related synthesis conditions including total monomers concentration, UV-light illumination time, initiator concentration, copolymerization system pH, cationic monomer DMC concentration and ratio of nNaPAA:nDMC towards intrinsic viscosity and monomers conversion percentage were optimized. The acquired representative microblocked TP-ADL was characterized using Fourier transform infrared spectroscopy (FTIR), 1H nuclear magnetic resonance (NMR), thermogravimetric analysis (TGA) and measurement of apparent viscosity. The flocculation efficiency towards oily wastewater was evaluated in terms of oil removal rate and turbidity removal rate. Its flocculation mechanism was further investigated and summarized based on the zeta potential results and flocs properties.
Materials and methods
Materials
The monomers employed in this work were all directly used without further purification. The monomer AM was purchased from Jiangxi Changjiu Biochemical Industry Co., Ltd. (Jiangxi, China); cationic monomer DMC (75 wt%) was from Guangdong Guanghua Technology Co., Ltd. (Guangdong, China), and hydrophobic monomer LA (98 wt%) was from Hubei Jusheng Technology Co., Ltd. (Hubei, China). Template reagent NaPAA (30 wt%) was from Shandong XinTai Water Treatment Technology Co., Ltd. (Shandong, China); initiator (VA-044) (industrial grade) was obtained from Eykits Research Biological Technology Co., Ltd., (Shanghai, China); surfactant Tween 80 (analytical grade) was obtained from Chengdu Chron Chemicals Co., Ltd. (Chengdu, China). Other reagents including urea, EDTA, absolute ethyl alcohol, acetone, sodium dodecyl sulphate (SDS), NaOH, HCl, NaCl, Na2CO3 were all analytical grade and purchased from Chongqing Chuandong Chemical Industry Co., Ltd.(Chongqing, China), and nitrogen gas (> 99.9%) was from Chongqing Shuangbei Liquefied Gas Plant (Chongqing, China). All aqueous solutions were prepared using deionized water.
Synthesis of copolymer
The amphiphilic flocculant TP-ADL with cationic microblock structure was synthesized using a template copolymerization technology through UV initiation. The detailed preparation steps are as follows: a predetermined amount of AM, DMC, LA, NaPAA, deionized water and other additive reagents were firstly added into a 100-mL highly transparent quartz jar, and the reaction system pH was then adjusted using 0.1 M HCl or NaOH solution. The additive reagents include polymer solubilizer urea, metal ion shielding agent EDTA-2Na and surfactant Tween-80, and their dosages were controlled at 0.3 wt%, 0.3 wt% and 1.5 wt%, respectively. After being purged by a nitrogen flow for 30 min to achieve an absolute N2 atmosphere, a certain dosage of VA-044 was added into the mixture. The reaction jar was then sealed up and exposed to UV irradiation sourced from a 1000 W high-pressure mercury lamp (Shanghai Jiguang Special Lighting Electrical Plant, China) for a certain time at room temperature. On completion of copolymerization, the reaction jar was aged for 2 h at ambient temperature. The jelly-like products were collected and dissolved into a pH < 2 aqueous solution, and absolute ethanol and acetone were then added into this acid solution to precipitate out the target copolymer products from NaPAA templates. Finally, the yielding samples were dried in a vacuum oven and labelled as TP-ADL. The non-template samples were fabricated through a same process without NaPAA addition and denoted as P-ADL. The proposed copolymerization route of TP-ALD is shown in Fig. 3. More details of the synthesis process can be seen in our previous works [13, 15, 16].
Characterization of copolymers
The molecular weight of copolymer is a key factor that directly affect its adsorption and bridging ability during flocculation process [17]. Gravimetric and one-point methods were employed to determine the monomer conversion rate and intrinsic viscosity of polymers (η), respectively, according to similar research works [18, 19]. The intrinsic viscosity (η) of polymers was determined using an Ubbelohde viscometer (Shanghai Shenyi Glass Instrument Co., Ltd., China) in 1 mol L−1 NaCl solution with the temperature maintained constant at 30 ± 0.5 °C. The (η) value was calculated through Eq. (1).
where ηr stands for the relative viscosity calculated from Eq. (2) and c stands for the concentration of polymer solution:
where t and t0 are the time that the polymer solution and the 1 mol·L−1 NaCl solution spent flowing through the upper and lower scale lines of the Ubbelohde viscometer.
The conversion rate used in this study is a mass yield ratio between the dosed monomers and the obtained polymer, which can be calculated from Eq. (3):
where m stands for the total mass of the dosed monomers, m0 is the total mass of the yielded gel-like product and m1 and m2 are the mass of sampled gel-like polymer before and after purification and drying process, respectively.
The FT-IR analysis of polymers was recorded on a 550 Series II infrared spectrometer (Mettler Toledo Instruments Co., Ltd., Switzerland). 1H NMR analysis was conducted with an Avance 500 nuclear magnetic resonance spectrometer (Bruker Company, Ettlingen, Germany) using D2O as solvent. The thermogravimetric analysis (TGA) was performed with a DTG-60H synchronal thermal analyser (Shimadzu, Kyoto, Japan) under a nitrogen flow atmosphere with a heating rate of 5 °C min−1 in the range of 25–600 °C. The rheological characteristics of copolymers were measured with an Anton Paar MCR302 rheometer (Anton Paar GmbH, Austria).
Flocculation experiments
Oily wastewater sample
The simulated oily wastewater samples used in the flocculation tests were prepared with 0# diesel, NaCl as an electrolyte, Na2CO3 as a stabilizer and SDS as a surfactant according to the previous work [20]. The composition of 1L simulated o/w emulsion was as follows: 0# diesel 5% (v/v), NaCl 1.79 g, Na2CO3 2.86 g, SDS 3.78 g. The emulsion pH was adjusted using 0.1 and 1 M HCl and NaOH solution. The basic characteristics of the raw wastewater are summarized in Table 1. The oil content of water sample was determined using a UV–visible spectrophotometer (TU-1901, Beijing Purkinje General Instrument Co., Ltd., China) according to the determination of oil content in oilfield-produced water spectrophotometry method (SY/T0530-2011). The turbidity and zeta potential of sample were determined using a 2100Q turbidimeter (HACH, USA) and Zetasizer Nano ZS90 (Malvern Instruments Ltd., Malvern, UK), respectively.
Flocculation performance
The detailed information of flocculants is shown in Table 2. For the comparative analysis of the function of cationic microblock structure and hydrophobic units, the flocculants employed in the flocculation experiments had a similar intrinsic viscosity and cationic degree. A program-controlled jar-test equipment (ZR4-6, Zhongrun Water Industry Technology Development Co. Ltd., China) was employed in the flocculation tests. The flocculants were prepared into 5 g/L stock solution in advance. Of the oil-containing wastewater, 700 mL was firstly transferred into a 1-L beaker, and a predetermined volume of flocculant stock solution was then pipetted into the wastewater system followed by rapid stirring at 200 rpm for 30 min to achieve a complete mixture between the oil and flocculant and then slow stirring at 30 rpm for 15 min for the growth of flocs. After 30 min of settling for the floating of flocs, the supernatant collected 3 cm below the surface was utilized for the oil content, turbidity and zeta potential determination. The oil and turbidity removal efficiencies were calculated according to Eqs. (4) and (5), respectively.
where the C0 and C stand for the oil content in the wastewater sample before and after flocculation, respectively, and T0 and T are the turbidity of sample before and after flocculation.
The flocs size distribution was measured using a laser diffraction analyser (Mastersizer 2000, Malvern, UK). The median diameter size d50 was used to represent the average floc size. The fractal dimension (Df) employed to estimate the floc compactness was determined using a light scattering method. The detailed analytical method of Df is displayed in Supporting Information Text S1 [21, 22].
Results and discussion
Optimization of TP-ADL copolymerization
Effect of total monomers concentration
The total monomers concentration directly determines the amount of free radicals, which plays a dominant role in free radical copolymerization system [23]. Hence, the effects of total monomers concentration on intrinsic viscosity and conversion rate were investigated at an initiation time period of 60 min, initiator concentration of 0.6 wt‰, pH = 4, DMC concentration of 25 wt% and nDMC:nNaPAA = 1:1. Other parameters including LA concentration, EDTA concentration, urea concentration and Tween 80 concentration were maintained at 2 wt%, 0.3 wt%, 0.3 wt% and 0.5 wt%, respectively, throughout the synthesis experiments. As can be seen from Fig. 4a, increasing the total monomers concentration from 20 to 40 wt% resulted in significant increases in intrinsic viscosity and copolymerization conversion rate with maximum values at 40 wt%. However, increasing beyond 40 wt% significantly decreased intrinsic viscosity but only slightly reduced the conversion rate. At low total monomers concentration, the initiated primary free radicals have fewer monomer units with which to collide and react and are surrounded by a large amount of solvent molecules, the so-called cage effect [24]. With fewer collision opportunities between monomers, chain termination occurs faster. Therefore, the copolymerization ended at low intrinsic viscosity and conversion rate. With increasing total monomers concentration, more free radical–monomer unit collisions occur and the copolymerization rate increases. On the contrary, when the total monomers concentration increased beyond 40 wt%, the collision of monomers rapidly increases and accelerates the copolymerization reaction generating a lot of reaction heat that cannot be dissipated, which may promote chain termination. Furthermore, the chain transfer process is more likely to cause cross-linking and leads to the generation of insoluble materials. Therefore, the total monomers concentration of 40 wt% was chosen as optimal in this study.
Effect of initiation time
Energy saving is one of the greatest advantages of UV initiation technology when compared to traditional thermal initiation due to its much shorter initiation time. The effects of UV initiation time on intrinsic viscosity and conversion rate were investigated with the total monomers concentration of 40 wt%, initiator concentration of 0.6 wt‰, pH = 4, DMC concentration of 25 wt% and nDMC:nNaPAA = 1:1. As displayed in Fig. 4b, both intrinsic viscosity and conversion rate rapidly increased along with the increasing initiation time and reached the maximum at 60 min. After that, the copolymerization entered a plateau stage where increasing UV illumination time did not change intrinsic viscosity or conversion rate any further. The copolymerization system was a highly transparent solution at the beginning, so that the UV beam could be transmitted into the solution and decompose the initiator into primary free radicals. Thus, the continuously generated primary free radicals would attack monomers and produce monomeric free radicals to promote the chain growth, increasing both intrinsic viscosity and conversion rate. However, with increasing UV illumination time, the initiator in the reaction system was gradually consumed up and the copolymerization was then mainly driven by monomeric free radicals. Therefore, the initiation time of 60 min was set as the optimal, which is much shorter than that of a traditional thermal initiation system and indicates the high efficiency and energy saving of UV initiation technology [8].
Effect of initiator concentration
The initiator VA-044 employed in this work is a type of water-soluble azo-initiator that can be homogeneously dispersed into the aqueous solution copolymerization system. Under UV light irradiation, the weak bonds in VA-044 molecules are broken and generate primary free radicals to initiate the copolymerization system. The effect of initiator concentration on copolymerization in this work was investigated with the other parameters held constant. As illustrated in Fig. 4c, increasing the initiator concentration resulted in the conversion rate reaching a plateau while the intrinsic viscosity reached a maximum at 0.6 wt‰ and then sharply decreased. The initiator is the active centre of copolymerization, and when its concentration is low, there are not enough active initiator sites available to receive enough energy from the UV irradiation to activate the whole copolymerization system, resulting in a small fraction of monomers joining the copolymerization process. Therefore, both intrinsic viscosity and conversion rate were restricted by the limited copolymerization activity. Increasing initiator concentration results in more active centres that can produce more primary radicals and thereby improve the active level of the copolymerization system. Once the amount of primary free radical is enough to get all monomers to join into the copolymerization reaction, both intrinsic viscosity and conversion rate reach the maximum. On the other hand, with the further increase in initiator concentration after 0.6 wt‰, the excessive amount of primary free radicals may produce too many monomeric free radicals, which are more likely to react with one another and lead to chain termination, but instead of with other monomers to promote chain growth. Therefore, although the conversion rate was maintained at a high level, the intrinsic viscosity was seriously affected. The initiator concentration of 0.6 wt‰ was chosen as optimal.
Effect of pH
The effects of pH on the copolymer intrinsic viscosity and conversion rate were investigated while the other parameters remained constant. From Fig. 4d, both intrinsic viscosity and conversion rate increased as pH value was changed from 1 to 4. With the further increase in pH to 7, the copolymerization efficiency decreased sharply. The low pH condition can promote the imidization reaction of amide groups in AM monomers and cross-linking between each AM group, which are unfavourable for the copolymerization process. In addition, a strong acid environment can acidize the –COO– group from NaPAA into –COOH, which reduced the solubility of NaPAA and hindered the copolymerization reaction, thereby leading to a lower intrinsic viscosity and conversion rate. In the meantime, the AM monomers would readily hydrolyze and generate free ammonia (NH3) at higher pH, which is not favourable for the template copolymerization neither [25]. Hence, the favourable pH value of 4 was chosen.
Effect of DMC concentration
The degree of the cationic monomer in CPAMs can significantly affect the copolymerization. The effects of DMC concentration on intrinsic viscosity and conversion rate were investigated with other conditions maintained constant. As shown in Fig. 4e, both intrinsic viscosity and conversion rate were slightly decreased from 5 to 25 wt%. but still maintained at a comparatively high level, but when increased above 25 wt%, the intrinsic viscosity and conversion rate rapidly decreased. According to the free radicals polymerization mechanism, the quantity of monomeric free radicals at the initial polymerization stage is essential to the intrinsic viscosity and conversion rate [26]. As the reactivity ratio of monomer AM is higher than that of cationic monomers, AM monomer may have more opportunities to be attacked by primary free radicals to produce more monomeric free radicals [27]. Hence, the higher AM concentration can improve the monomeric free radical concentration to promote the chain propagation process and obtain higher intrinsic viscosity, but increasing the dosage of the cationic monomer may decrease the reaction activity of copolymerization system. Furthermore, the electrostatic repulsion of the cationic functional group from DMC monomer would enhance the steric hindrance and reduce the probability of collision between monomers and monomeric free radicals. Therefore, the copolymerization process would be seriously restrained once the DMC concentration is increased beyond a critical point to play a dominant role in the copolymerization instead of AM monomers. Although the maximum intrinsic viscosity of copolymer was obtained without any DMC dosage, the flocculation performance is a synergistic effect of both adsorption bridging and charge neutralization. Therefore, DMC concentration, as the key factor of charge neutralization ability of polymer, is necessary to be taken into consideration. The DMC concentration of 25 wt% was finally chosen as optimal due to its high charge density, because it only slightly affected intrinsic viscosity.
Effect of template NaPAA concentration
The template reagent NaPAA plays an essential role in the formation of cationic microblock structure and also influences intrinsic viscosity and conversion rate during copolymerization. Thus, the effects of molar ratio between NaPAA and DMC on intrinsic viscosity and conversion rate were investigated with the other factors maintained constant. As shown in Fig. 4f, the intrinsic viscosity and conversion rate were both significantly influenced by NaPAA dosage. The intrinsic viscosity of the copolymer slightly increased with the increasing dosage of NaPAA and reached an optimal value at the ratio of nNaPAA:nDMC = 1:1. With further dosage of NaPAA, the intrinsic viscosity significantly decreased. The conversion rate exhibits a trend similar to that of the intrinsic viscosity, and the optimal conversion rate was also obtained at the ratio of nNaPAA:nDMC = 1:1. When the template NaPAA exists in the copolymerization system, the NaPAA can adsorb cationic monomer through electrostatic attraction force to arrange the DMC units orderly along with the NaPAA chain before the copolymerization was initiated. Once a cationic monomer sites at either end of the template chain is initiated by free radicals, it would be more likely to react with another which is nearby, instead of other free monomers in the copolymerization system. Therefore, the chain propagation process is promoted, and higher intrinsic viscosity and conversion rate can be obtained. On the other hand, a higher NaPAA dosage would prevent the copolymerization due to the high steric hindrance of –COO– group in NaPAA, and the DMC monomer would be wrapped and surrounded by the redundant NaPAA template. As a result, the so-called cage effect occurs, which will seriously impede free radicals from colliding with cationic DMC monomers [15]. Thus, the copolymerization reaction is restricted, and both intrinsic viscosity and conversion rate decreased. The ratio of nNaPAA:nDMC = 1:1 is optimal in this study.
Characterization of copolymers
FTIR spectra investigation
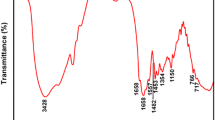
The FTIR spectra of P-ADL and TP-ADL can be seen from Fig. 5. The adsorption peaks at around 3434 cm−1 and 1162 cm−1 in the non-template P-ADL spectra are attributed to the stretching vibration of –NH2 in AM unit and the characteristic peak centred at 1662 cm−1 is assigned to the –C=O stretching vibration from AM and LA monomers, while the peak that appears around 954 cm−1 originates from the –CO–O groups of LA monomers [28]. The characteristic peaks belonging to AM and LA units indicate that both AM and LA monomers successfully joined into the copolymerization reaction and became part of the copolymer carbon main skeleton. The adsorption peak sited at around 2948 cm−1 is assigned to the asymmetric stretching vibration of C–H from –CH3 and –CH2– groups. The characteristic adsorption peak of bending vibration of –CH2– in –CH2–N+–(CH3)3 groups from DMC monomer can be observed in 1454 cm−1. As can be seen from Fig. 5b, the characteristic peaks belonging to the same functional groups also occurred in the TP-ADL spectra, accompanied by slight shifts in wave numbers. The FTIR results indicate that both template TP-ADL and non-template P-ADL samples are copolymerized from AM, DMC and LA monomers. No new characteristic peaks occurred in the spectra of template TP-ADL when compared to non-template P-ADL, which indicates that the introduction of template did not affect the chemical groups structure of the copolymer at all. The possible effect on the monomers ranking sequence was further analysed through 1H NMR.
1H NMR spectra investigation
1H NMR has been considered as an effective way to analyse the molecular structure of copolymers. Parallel to FTIR spectra, the 1H NMR studies of TP-ADL and P-ADL were conducted to investigate the effect of NaPAA on the molecular structure of copolymers. The data are plotted on the same intensity scale in Fig. 6, and the sharp signal displayed at around δ = 4.79 ppm indicates the solvent D2O. The signals centred at δ = 1.62 ppm (a) and 2.19 ppm (b) demonstrate the existence of the backbone methylene groups and methine groups (–CH2–CH–) from AM and LA monomers, respectively [29]. The signal at δ = 2.32 ppm (h) gives evidence to the protons in the methylene group connected to the ester function group in the LA monomer, while the signal of protons from the terminal methyl group in the LA units is identified at δ = 1.06 ppm (i), which confirms the existence of LA component in copolymer products [8, 30]. The signals at around δ = 1.74 ppm (c) and 1.15 ppm (d) belong to the protons from the CH2–C (c) and C–CH3 (d) groups of DMC monomer [31, 32]. The signals sited around δ = 4.48 ppm (e) and 3.72 ppm (f) are derived from the protons in the O–CH2 (e) and –CH2–N+ (f) groups of DMC units, respectively. The three equivalent methyl groups of the ammonium moiety –N+(CH3)3 in DMC monomers are confirmed at around δ = 3.20 ppm [27, 33]. The characteristic peaks belonging to the protons from specific functional groups of AM, DMC and LA monomers are observed in the 1H NMR spectra, which indicate that all these monomers have joined into the copolymerization and became the constituent units in the polymer chain. This result is also consistent with the FTIR analysis.
As shown in Fig. 6b, the template copolymer TP-ADL exhibits very similar 1H NMR spectra when compared to that of the non-template P-ADL in Fig. 6a, which confirms the same constituent between TP-ADL and P-ADL. Nevertheless, the tiny differences between them should not be ignored, which are essential for the detailed analysis of the cationic microblock structure in TP-ADL polymer chain. There are four adsorption peaks at δ = 3.64, 3.63, 3.62 and 3.60 ppm identified in the P-ADL spectra that disappeared in the TP-ADL spectra. This phenomenon is assigned to the different cationic unit ranking sequences between P-ADL and TP-ADL. In P-ADL, cationic units randomly distribute in the polymer chain, the related functional groups in DMC are readily affected by other functional groups from an adjacent AM monomer and thereby the corresponding interference peaks occurred [32]. But for template copolymer TP-ADL, a regular long DMC microblock structure formed through a template copolymerization technology and the DMC units in the copolymer chain were in a chemical environment similar to its homopolymer [15]. Therefore, the interference from the adjacent unit is far less significant; negligible splitting peaks can be identified from 1H NMR spectra of TP-ADL. The 1H NMR characterization also proves the concentrated distribution of cationic units in the main chain of TP-ADL.
Thermogravimetric analysis
Usually, thermogravimetric analysis is utilized in materials thermal stability analysis, but there are also researches using thermogravimetric curves to explore the microblock structure in the macrochain [13, 34]. In Fig. 7, it can be clearly seen that both P-ADL and TP-ADL exhibit four stages of weight loss. At the first stage, P-ADL loses 3.9 wt% of its total weight in a temperature range of 30–190 °C, while the weight loss of TP-ADL is 5.9 wt% in the range of 30–195 °C. The weight loss in this stage is mainly attributed to the moisture evaporation of copolymers. Both amido and trimethylammonium chloride groups in the copolymer are hydrophilic and readily capture H2O molecules in an air atmosphere, and this combined moisture component easily evaporates in heating [35]. At the second stage, the weight loss of P-ADL is 14.6 wt% in the temperature range of 190–265 °C, while that of TP-ADL is 14.4 wt% within 195–265 °C. The thermal decomposition of the copolymer side chains and the methyl thermal decomposition of quaternary amine group from DMC would be the main reason for the second stage of weight loss [8]. The copolymer weight loss further increased during 265–350 °C and P-ADL has third stage weight loss of 18.0 wt% in this temperature range, while that of the TP-ADL is 17.4 wt%. The ammonium formation of the side chain amide group (-CO-NH2) from AM unit through imine reaction is mainly responsible for the third stage of weight loss [36]. When the temperature rose above 350 °C, the fourth stage weight loss of both P-ADL and TP-ADL occurred. The weight loss of this stage is 41.4 wt% and 44.1 wt% for P-ADL and TP-ADL, respectively. The C–C bonds broke under high-temperature condition, and the copolymer main chain decomposed and generated NH3, H2O and CO2 [37]. According to the thermogravimetric results, both non-template P-ADL and template TP-ADL displayed good thermal stability. Notably, three obvious heat adsorption peaks at 347.8 °C, 378.5 °C and 406.3 °C can be observed from the DSC curve of TP-ADL, while only two heat adsorption peaks at 338.3 °C and 379.7 °C are identified from those of the non-template P-ADL during the last stage of weight loss. Presumably, this phenomenon is due to the microblock structure in the TP-ADL polymer chain. In template copolymer TP-ADL, both AM units and DMC units are uniformly distributed in the main chain in a fragment form, respectively. Therefore, the AM fragments and DMC fragments have distinct thermal properties with two separate heat adsorption peaks at 347.8 °C and 406.3 °C belonging to different monomer unit sequences. In contrast, the AM and DMC units are randomly distributed in the non-template P-ADL copolymer chain, and the decomposition of its backbone can be regarded as a combination of different units and showed only one heat adsorption peak at 338.3 °C [27]. Furthermore, the weak heat adsorption peak at around 379 °C in both P-ADL and TP-ADL can be attributed to the decomposition of formed LA monomer short fragments as aforementioned in Fig. 2. The thermogravimetric analysis of copolymers further confirms the formation of microblock structure in TP-ADL, which is in good agreement with the NMR results.
Apparent viscosity analysis
Apparent viscosity is generally employed to evaluate the associative performance of amphiphilic copolymers in aqueous solutions [38]. The apparent viscosity was determined as a function of the copolymer concentration including TP-ADL, P-ADL and CP-AD. As illustrated in Fig. 8, the apparent viscosity of all the three copolymers obviously increased with the increasing copolymer concentration. The apparent viscosities of the amphiphilic copolymers TP-ADL and P-ADL are almost the same but slightly lower than those of the single hydrophilic copolymer CP-AD at low concentration. However, along with the continuous increasing copolymer concentration beyond 0.3 g dL−1, the apparent viscosities of the hydrophobically modified copolymer increased nonlinearly, while those of the CP-AD still maintained a linear increase as before. This phenomenon is due to the phase conversion of hydrophobic units from intra-molecular association to inter-molecular association [13]. The hydrophobic units would preferentially interact with other hydrophobic units from the same polymer chain when the polymer concentration is below the critical association concentration (CAC). Therefore, the intra-molecular association plays the dominant role in solution and results in curled configuration of the polymer chain, which leads to a reduction of its hydrodynamic volume. Hence, the amphiphilic copolymers have comparatively lower apparent viscosities than the hydrophilic copolymer at low concentration. Conversely, once the polymer concentration increases beyond CAC, inter-molecular association becomes the driving interaction between hydrophobic units and forms a physical network structure with the polymer chains, which significantly increases the hydrodynamic volume of polymers. Therefore, the apparent viscosities rapidly increased. In this study, the CACs of TP-ADL and P-ADL were calculated to be around 0.34 g dL−1 according to the change in apparent viscosities. Their apparent viscosities finally achieved 48.796 and 45.668 mPa s−1, respectively, within the examined concentration range. Nevertheless, the corresponding apparent viscosity of CP-AD achieved only 30.189 mPa s−1 at the same concentration. It is also worth noting that the apparent viscosity of the template copolymer TP-ADL is slightly higher than that of the non-template copolymer P-ADL, which can be ascribed to the formation of cationic microblock structure. The concentrated distribution of positive charges on polymer chain made it a more stretched linear configuration in solution, which is preferable for the occurrence of the inter-molecular association. This result also proves the formation of cationic microblock structure in the main chain of template copolymer. Furthermore, this reversible physical network structure generated through inter-molecular association is also favourable for the formation of large flocs and its recovery ability.
Flocculation properties
Effect of copolymer dosage on flocculation efficiency
The flocculation efficiency of copolymers towards oily wastewater was evaluated in terms of oil removal rate (%), turbidity removal rate (%) and zeta potential. As can be seen in Fig. 9a, b, all of the copolymers displayed a similar trend at different dosages towards oil and turbidity removal rate, both of the removal rates firstly significantly increased along with increasing flocculant dosage, but then slightly deteriorated with the further dosage after the optimal treatment efficiency was obtained. The optimal oil and turbidity removal rates of each copolymer were as follows: TP-ADL (97.7% and 99.9% at 160 mg/L) > P-ADL (94.5% and 98.8% at 180 mg/L) > CP-AD (88.0% and 94.9% at 180 mg/L). The amphiphilic template copolymer TP-ADL yielded the best treatment efficiency. This result is closely related to the copolymer functional groups and their distribution on the macrochain.
The superiority of this TP-ADL in oily wastewater treatment reveals that the synergistic effect between hydrophobic association and cationic microblock structure can effectively facilitate the oily wastewater flocculation process. As illustrated in Fig. 9c, the zeta potential rapidly increased from the negative value (− 51.0 mV) to the isoelectric point along with the increasing flocculant dosage. The isoelectric point was achieved at the dosage around 160 mg/L by the template copolymer TP-ADL and around 180 mg/L by the non-template copolymer P-ADL and CP-AD. The highest zeta potential of TP-ADL indicates that the concentrated distribution of cationic units on the polymer chain can make the best use of every single cationic unit to have stronger charge neutralization ability than that of the random distribution in P-ADL and CP-AD which have the same cationic degree. The cationic segments of the template copolymer provide strong local positive charge on the polymer chain, which can tightly adsorb on the negatively charged surface of oil droplets [39]. Therefore, the negatively charged oil colloidal system is effectively destabilized and the oil droplets can gather together to form compact primary flocs. The more detailed floc properties are discussed based on d50 and Df analysis in “Flocs properties investigation” section. Notably, the optimal dosage of each copolymer occurred near its corresponding isoelectric point, which indicates that charge neutralization mechanism played the dominant role in the oily wastewater flocculation process. Another phenomenon worth noting is that the treatment efficiency of the amphiphilic samples TP-ADL and P-ADL is significantly higher than that of the hydrophilic CP-AD in almost the whole investigated dosage range, which confirms the superiority of hydrophobic modification. The hydrophobic units distributed on the linear polymer chain merge together with each other to form a network structure through inter-molecular hydrophobic association, which significantly increased the opportunities of collisions between polymer chain and oil droplets, so that the bridging effect could play the best role in promoting the aggregation of primary flocs into large flocs [40]. The possible flocculation mechanism of TP-ADL towards oily wastewater is summarized in Fig. 9d. It can be confirmed that the synergistic effect between hydrophobic association and cationic microblock structure is the main reason for the superior flocculation efficiency of TP-ADL towards oily wastewater treatment. Furthermore, the introduced hydrophobic units can also enhance the interaction between the flocculant molecular chain and the hydrophobic oil droplets.
Flocs properties investigation
The flocs properties directly reflect the flocculation effect. Generally, large and compact flocs are favourable for the flocs separation from water. Herein, d50 and Df were used to evaluate the average floc size and floc compactness; the higher Df value is believed to indicate a compacter floc inner structure [41, 42]. The d50 and Df results of samples conditioned by series of copolymers at the corresponding optimal dosage are shown in Fig. 10. The floc sizes (d50) are 307.023 µm, 336.767 µm and 229.261 µm after being conditioned by TP-ADL, P-ADL and CP-AD, respectively. Obviously, the introduction of hydrophobic association significantly enhanced the floc average diameter of oily colloidal system. This phenomenon could be attributed to the reinforcement of the bridging adsorption effect. The inter-molecular hydrophobic association connected different copolymer chains together to form a network structure, which significantly enhanced the opportunities for the collision between polymer chains and small primary flocs, so that the dissociative primary oily flocs in emulsion could be effectively captured by the polymer nets and gathered into giant flocs. In this way, the bridging effect was significantly strengthened. A number of previous studies also reported that the stronger bridging effect is the main reason for the increase in floc size [43, 44]. It is worth noting that the formed cationic microblock structure through template copolymerization has a negative effect towards the floc size growth, which may be attributed to the decrease in the repulsive force between oil droplets. The concentrated distribution of cationic units significantly enhanced the charge neutralization ability of copolymer to effectively reduce the charge repulsion and compress the electrical double layer of oily colloidal particles. Therefore, the stronger charge neutralization ability enables the flocs to form a compacter structure. The Df results shown in Fig. 10b, d also confirm the compacter floc structure of sample conditioned by TP-ADL (Df = 0.686) than that conditioned by P-ADL (Df = 0.677). The stronger charge neutralization ability of the cationic microblock structure makes the flocs compacter, while the strengthened bridging adsorption ability through inter-molecular hydrophobic association assembles these compact primary flocs into giant flocs. Therefore, the better flocculation and flocs separation performance can be obtained.
Conclusion
This work develops a novel amphiphilic polyacrylamide TP-ADL with cationic microblock structure through a UV-light-initiating template copolymerization technology. The related copolymerization factors were well optimized towards a higher intrinsic viscosity and conversion rate. The copolymer maximum intrinsic viscosity of 15.7 dL g−1 and conversion rate of 99.7% were obtained at the total monomers concentration of 40 wt%, UV light illumination time of 60 min, photo-initiator concentration of 0.6 wt‰, pH = 4, DMC concentration of 25 wt% and nNaPAA:nDMC = 1:1. Moreover, the characterization analysis of FT-IR, 1H NMR indicates the successful synthesis of this amphiphilic cationic flocculant TP-ADL, while the TGA analysis confirms its good thermal stability. The 1H NMR and TGA results also confirm the special cationic microblock structure. Furthermore, the apparent viscosity investigation reveals the inter-molecular hydrophobic association of this amphiphilic TP-ADL. The formed cationic microblock structure significantly enhanced the charge neutralization ability of copolymer, and the introduced hydrophobic units improved its bridging performance. The synergistic effect between cationic microblock structure and hydrophobic association makes the flocs compacter and larger to obtain a better flocculation efficiency. The amphiphilic template copolymer TP-ADL displayed superior flocculation performance with the optimal oil and turbidity removal rates of 97.7% and 99.9%, respectively, at the optimal dosage of 160 mg L−1. The charge neutralization, adsorption bridging and inter-molecular hydrophobic association mechanism all played important roles during the flocculation process. Thus, this work provides an effective and suitable candidate for the oily wastewater purification and also a new idea for the development of high-performance flocculants for water treatment.
References
Yang T, Qiao B, Li GC, Yang QY (2015) Improving performance of dynamic membrane assisted by electrocoagulation for treatment of oily wastewater: effect of electrolytic conditions. Desalination 363:134–143
Sun YJ, Zhu CY, Zheng HL, Sun WQ, Xu YH, Xiao XF, You ZY, Liu CY (2017) Characterization and coagulation behavior of polymeric aluminum ferric silicate for high-concentration oily wastewater treatment. Chem Eng Res Des 119:23–32
Zhao CL, Zheng HL, Gao BY, Liu YZ, Zhai J, Zhang SX, Xu BC (2018) Ultrasound-initiated synthesis of cationic polyacrylamide for oily wastewater treatment: enhanced interaction between the flocculant and contaminants. Ultrason Sonochem 42:31–41
Liu XJ, Ge L, Li W, Wang XZ, Li F (2015) Layered double hydroxide functionalized textile for effective oil/water separation and selective oil adsorption. ACS Appl Mater Interfaces 7(1):791–800
Painmanakul P, Sastaravet P, Lersjintanakarn S, Khaodhiar S (2010) Effect of bubble hydrodynamic and chemical dosage on treatment of oily wastewater by Induced Air Flotation (IAF) process. Chem Eng Res Des 88(5-6A):693–702
Zhu XB, Dudchenko A, Gu XT, Jassby D (2017) Surfactant-stabilized oil separation from water using ultrafiltration and nanofiltration. J Membr Sci 529:159–169
Sarac N, Ugur A (2016) A green alternative for oily wastewater treatment: lipase from Acinetobacter haemolyticus NS02-30. Desalin Water Treat 57(42):19750–19759
Yang ZL, Gao BY, Li CX, Yue QY, Liu B (2010) Synthesis and characterization of hydrophobically associating cationic polyacrylamide. Chem Eng J 161(1–2):27–33
An CJ, Huang G, Yao Y, Zhao S (2017) Emerging usage of electrocoagulation technology for oil removal from wastewater: a review. Sci Total Environ 579:537–556
Ma JY, Shi J, Ding L, Zhang HW, Zhou S, Wang QJ, Fu X, Jiang LY, Fu K (2018) Removal of emulsified oil from water using hydrophobic modified cationic polyacrylamide flocculants synthesized from low-pressure UV initiation. Sep Purif Technol 197:407–417
Zhang YX, Wu FP, Li MZ, Wang EJ (2005) Novel approach to synthesizing hydrophobically associating copolymer using template copolymerization: the synthesis and behaviors of acrylamide and 4-((9-propenoyloxyethoxy) benzoic acid copolymer. J Phys Chem B 109(47):22250–22255
Popa I, Gillies G, Papastavrou G, Borkovec M (2009) Attractive electrostatic forces between identical colloidal particles induced by adsorbed polyelectrolytes. J Phys Chem 113(25):8458–8561
Zhou YH, Zheng HL, Gao BY, Gu YP, Li X, Liu BZ, Jimenez AM (2017) Waste activated sludge (WAS) dewatering properties of an original hydrophobically modified polyacrylamide containing a cationic microblock structure. RSC Adv 7(46):28733–28745
Li X, Zheng HL, Gao BY, Zhao C, Sun YJ (2017) UV-initiated polymerization of acid-and alkali-resistant cationic flocculant P(AM-MAPTAC): synthesis, characterization, and application in sludge dewatering. Sep Purif Technol 187:244–254
Li X, Zheng HL, Gao BY, Sun YJ, Liu BZ, Zhao CL (2017) UV-initiated template copolymerization of AM and MAPTAC: microblock structure, copolymerization mechanism, and flocculation performance. Chemosphere 167:71–81
Zhang ZA, Zheng HL, Huang F, Li X, He SY, Zhao C (2016) Template polymerization of a novel cationic polyacrylamide: sequence distribution, characterization, and flocculation performance. Ind Eng Chem Res 55(37):9819–9828
Guan QQ, Zheng HL, Zhai J, Zhao C, Zheng XK, Tang XM, Chen W, Sun YJ (2014) Effect of template on structure and properties of cationic polyacrylamide: characterization and mechanism. Ind Eng Chem Res 53(14):5624–5635
Shang HZ, Liu JP, Zheng YB, Wang LG (2010) Synthesis, characterization, and flocculation properties of poly(acrylamide-methacryloxyethyltrimethyl ammonium chloride-methacryloxypropyltrimethoxy silane). J Appl Polym Sci 111(3):1594–1599
Niranjan PS, Tiwari AK, Upadhyay SK (2011) Polymerization of acrylamide in a micellar medium: inhibition effect of surfactants. J Appl Polym Sci 122(2):981–986
Kundu P, Mishra IM (2013) Removal of emulsified oil from oily wastewater (oil-in-water emulsion) using packed bed of polymeric resin beads. Sep Purif Technol 118:519–529
Jarvis P, Jefferson A, Parsons SA (2005) Breakage, regrowth, and fractal nature of natural organic matter flocs. Environ Sci Technol 39(7):2307–2314
Greenwood J, Rainey T, Doherty WOS (2007) Light scattering study on the size and structure of calcium phosphate/hydroxyapatite flocs formed in sugar solutions. J Colloid Interface Sci 306(1):66–71
Wu YM, Zhang N (2009) Aqueous photo-polymerization of cationic polyacrylamide with hybrid photo-initiators. J Polym Res 16(6):647–653
Wang XN, Yue QY, Gao BY, Si XH, Sun X, Zhang SX (2011) Dispersion copolymerization of acrylamide and dimethyl diallyl ammonium chloride in ethanol-water solution. J Appl Polym Sci 120(3):1496–1502
Bao Y, Ma JZ, Li N (2011) Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohyd Polym 84(1):76–82
Zhu JR, Zheng HL, Jiang ZZ, Zhang Z, Liu LW, Sun YJ (2013) Synthesis and characterization of a dewatering reagent: cationic polyacrylamide (P(AM–DMC–DAC)) for activated sludge dewatering treatment. Desalin Water Treat 51(13–15):2791–2801
Chen W, Zheng HL, Guan QQ, Teng HK, Zhao CL, Zhao C (2016) Fabricating a flocculant with controllable cationic microblock structure: characterization and sludge conditioning behavior evaluation. Ind Eng Chem Res 55(10):2892–2902
Zheng HL, Sun YJ, Guo JS, Li FT, Fan W, Liao Y, Guan QQ (2014) Characterization and evaluation of dewatering properties of PADB, a highly efficient cationic flocculant. Ind Eng Chem Res 53(7):2572–2582
Gou SH, Li SW, Feng MM, Zhang Q, Pan QL, Wen J, Wu YP, Guo QP (2017) Novel biodegradable water-soluble graft-modified copolymer using acrylamide and konjac glucomannan for enhanced oil recovery. Ind Eng Chem Res 56(4):942–951
Yahaya GO, Ahdab AA, Ali SA, Abu-Sharkh BF, Hamad EZ (2001) Solution behavior of hydrophobically associating water-soluble block copolymers of acrylamide and N -benzylacrylamide. Polymer 42(8):3363–3372
Chen DN, Liu XG, Yue YM, Zhang WD, Wang PX (2006) Dispersion copolymerization of acrylamide with quaternary ammonium cationic monomer in aqueous salts solution. Eur Polym J 42(6):1284–1297
Abdollahi Z, Frounchi M, Dadbin S (2011) Synthesis, characterization and comparison of PAM, cationic PDMC and P(AM–DMC) based on solution polymerization. J Ind Eng Chem 17(3):580–586
Zheng HL, Sun YJ, Zhu CJ, Guo JS, Zhao C, Liao Y, Guan QQ (2013) UV-initiated polymerization of hydrophobically associating cationic flocculants: synthesis, characterization, and dewatering properties. Chem Eng J 234:318–326
Feng L, Zheng HL, Gao BY, Zhao CL, Zhang SX, Chen N (2017) Enhancement of textile-dyeing sludge dewaterability using a novel cationic polyacrylamide: role of cationic block structures. RSC Adv 7(19):11626–11635
Li JM, Hu CS, Shao JM, Li HJ, Li PY, Li XC, He WD (2017) Fabricating ternary hydrogels of P(AM-co-DMAEMA)/PVA/beta-CD based on multiple physical crosslinkage. Polymer 119:152–159
Wan XF, Li YM, Wang XJ, Chen SL, Gu XY (2007) Synthesis of cationic guar gum-graft-polyacrylamide at low temperature and its flocculating properties. Eur Polym J 43(8):3655–3661
Zheng HL, Ma JY, Zhu CJ, Zhang Z, Liu LW, Sun YJ, Tang XM (2014) Synthesis of anion polyacrylamide under UV initiation and its application in removing dioctyl phthalate from water through flocculation process. Sep Purif Technol 123:35–44
Liao Y, Zheng HL, Qian L, Sun YJ, Dai L, Xue WW (2014) UV-initiated polymerization of hydrophobically associating cationic polyacrylamide modified by a surface-active monomer: a comparative study of synthesis, characterization, and sludge dewatering performance. Ind Eng Chem Res 53(27):11193–11203
Zheng HL, Feng L, Gao BY, Zhou YH, Zhang SX, Xu BC (2017) Effect of the cationic block structure on the characteristics of sludge flocs formed by charge neutralization and patching. Materials 10(5):487
Niu MQ, Zhang WJ, Wang DS, Chen Y, Chen RL (2013) Correlation of physicochemical properties and sludge dewaterability under chemical conditioning using inorganic coagulants. Bioresour Technol 144:337–343
Chen Q, Wang YL (2015) Influence of single- and dual-flocculant conditioning on the geometric morphology and internal structure of activated sludge. Powder Technol 270:1–9
Chakraborti RK, Atkinson JF, Van Benschoten JE (2000) Characterization of alum floc by image analysis. Environ Sci Technol 34(18):3969–3976
Jarvis P, Jefferson B, Gregory J, Parsons SA (2005) A review of floc strength and breakage. Water Res 39(14):3121–3137
Zhao YX, Gao BY, Qi QB, Wang Y, Phuntsho S, Kim JH, Yue QY, Li Q, Shon HK (2013) Cationic polyacrylamide as coagulant aid with titanium tetrachloride for low molecule organic matter removal. J Hazard Mater 258(6):84–92
Acknowledgement
We gratefully acknowledge financial support from National Natural Science Foundation of China (Project No. 21477010), Key Research and Development Project of Chongqing Special Industry Technological Innovation and Application Demonstration (Project No. cstc2018jszx-cyzdX0035), Chongqing Special Postdoctoral Science Foundation (Project No. Xm2017035).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhou, Y., Zheng, H., Huang, Y. et al. Hydrophobic modification of cationic microblocked polyacrylamide and its enhanced flocculation performance for oily wastewater treatment. J Mater Sci 54, 10024–10040 (2019). https://doi.org/10.1007/s10853-019-03601-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03601-w