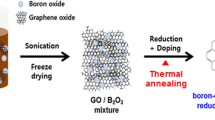
Abstract
Boron and nitrogen co-doped reduced graphene oxide (BN-rGO) materials were prepared via a facile dielectric barrier discharge plasma treatment method. X-ray photoelectron spectroscopy results demonstrated that the boron content in the boron-doped rGO (B-rGO) and BN-rGO is 1.21 at.% and 1.41 at.%, while the nitrogen content in the nitrogen-doped rGO (N-rGO) and BN-rGO is 2.12 at.% and 2.69 at.%, respectively. The doping of heteroatoms significantly improves the capacitance of the as-synthesized materials, giving BN-rGO a highly enhanced capacitance of 350 F g−1 at a current density of 0.5 A g−1, which is 2.36, 1.46 and 1.21 times higher than that of rGO, B-rGO or N-rGO, respectively.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past decades, graphene- and graphene oxide (GO)-based materials have attracted much attention due to their exceptional thermal, electrical properties and photocatalytic activity [1,2,3,4,5]. The doping of heteroatoms (B, N, P, S, etc.) into the graphene structure by chemical or physical methods has been demonstrated as an effective way to tailor its electronic properties by generating “N”- or “P”-type conductivity, significantly expanding the applications of GO-based materials in electrochemical devices [6], especially super-capacitors [2, 7, 8]. So far, although the doping mechanism and the applications of single-heteroatom-doped graphene-based materials have been well established [9,10,11,12,13], the investigations on the doping of dual or multiple atoms are still relatively scanty. Recently, Cai et al. [14] synthesized the nitrogen and sulfur co-doped graphene sheets by thermal treatment, which exhibit excellent lithium storage performance. Li et al. [15] prepared nitrogen and phosphorus co-doped porous graphene-like carbon with a high capacitance of 219 F g−1 through a one-step green route. Sun et al. [16] synthesized boron and nitrogen co-doped graphene by the two-step thermal annealing of graphene in the presence of melamine and boric acid, showing excellent activity and improved stability as the catalyst toward the methanol oxidation reduction. Among various doping atoms, boron and nitrogen have been considered as the most representative elements not only because they have the closest atomic radius to carbon, but also due to their unique electron deficiency or multiple electron nature [17,18,19].
To further understand the co-doping mechanism and the possible synergetic effect between the dopants, in this study, boron and nitrogen co-doped rGO (BN-rGO) materials have been successfully prepared via a facile and efficient H2-DBD (dielectric barrier discharge) plasma treatment route. The as-synthesized BN-rGO samples exhibit excellent electrochemical performance as electrode materials for supercapacitor. Compared with the single-doped rGO materials, i.e., boron-doped rGO (B-rGO) and nitrogen-doped rGO (B-rGO), the doping content of boron and nitrogen in BN-rGO is significantly increased, and its capacitance of 350 F g−1 at a current density of 0.5 A g−1 is 2.36, 1.46 and 1.21 times higher than that of rGO, B-rGO or N-rGO, respectively.
Experimental
Materials
All chemicals were used as received without further purification. Natural graphite powder (≤ 30 μm) was obtained from Huayi Chemical Co. Ltd. (Shanghai, China). H2SO4 (ACS grade, 95–98 wt%), H3PO4 (ACS grade, ≥ 85 wt%), KMnO4 (ACS grade, ≥ 99% wt%), NH4HCO3 (ACS grade, ≥ 99 wt%), HCl (ACS grade, 36–38 wt%), H2O2 (ACS grade, 30% aqueous solution) and ethanol (ACS grade, ≥ 99.8 wt%) were all purchased from Chuandong Chemical Co. Ltd. (Chongqing, China). The deionized (DI) water was obtained through a general pure water system manufactured by Qianyan Water Treatment Equipment Co. Ltd. (Chongqing, China).
Preparation of GO
Graphite oxide (GO) was synthesized by using the modified Hummers’ method [20]. In brief, in an ice water bath, 3.0 g of the natural graphite powders was added to a mixture of H2SO4 and H3PO4 (360:40 mL) under vigorous stirring. Then, 18.0 g of KMnO4 was slowly added to the mixture, which was then heated up to 323 K and maintained at the temperature for 60 h under continuous stirring. After the mixture was cooled to room temperature, 300 mL of DI water, 10 mL of H2O2 and 50 mL of HCl were added successively into the system to remove the impurities. The obtained bright yellow dispersion was then centrifuged, and the resulted brown precipitate was rinsed four times by using a large amount of DI water and then lyophilized to acquire the final product.
Preparation of rGO
Reduced graphene oxide (rGO) samples were prepared by the direct exfoliation and reduction of GO in a DBD plasma reactor (Figure S1 in the ESI†) [10] at room temperature and atmospheric pressure and under an AC input power of 80 V and 1.6 A with H2 as the working gas. The DBD plasma treatment can provide a reducing atmosphere as well as high electron temperature and density, leading to the convenient reduction, exfoliation and doping of GO with low costs. In brief, 200 mg of GO powders was placed in a quartz tube and held by quartz wools. Before discharging the plasma reactor, the working gas (H2, 99.99%) was introduced at a flow rate of 60 mL L−1 for 10 min to purge the quartz tube. After the plasma treatment for around 3 min, the obtained samples were rinsed by using DI water and ethanol successively for five times to remove the impurities and then dried in a vacuum oven for 8 h to acquire the final products.
Preparation of B-rGO, N-rGO, BN-rGO
For preparing the B-rGO, N-rGO and BN-rGO samples, 200 mg of GO samples and 20 mg of dopant (boric acid for B-rGO, ammonium bicarbonate for N-rGO, ammonium bicarbonate and boric acid (1:1) for BN-rGO) were added in 50 mL of DI water. After being ultrasonically dispersed for 30 min, the brown dispersion was lyophilized for 1 day. The resulted homogeneous mixtures were then input into the DBD reactor for plasma treatment. The operation condition is the same as that for the preparation of rGO. After plasma treatment, the obtained samples were rinsed by using DI water and ethanol successively for five times to remove the unreacted dopant and impurities and then dried in a vacuum oven for 8 h to acquire the final products.
As illustrated in Fig. 1, owing to the energetic nature of the H2-DBD plasma system, the free radicals of boron and nitrogen that resulted from the split of the dopants can be easily introduced in rGO at the sites of structural defects, completing the preparation of the heteroatoms-doped materials within minutes. Besides BN-rGO, the samples of rGO, B-rGO and N-rGO were also synthesized via the same process.
Characterization
The crystal structure of the samples was characterized by X-ray diffraction (XRD, Rigaku-D/max-3B) with Cu Kα radiation source (λ = 0.154056 nm), operating at 40 kV, 30 mA and a scanning rate of 2°/min. FT-IR spectra were recorded on a Nicolet 500 (Thermo Fisher Scientific Inc.) spectrophotometer using KBr pellet samples. Raman spectra were measured by a confocal Raman microscopy (LabRAM HR Evolution, Jobin-Yvon S.A.S, France, excited by 532 nm laser). N2 adsorption–desorption measurements were conducted on a Quadrasorb 2MP analyzer at 77 K. Brunauer–Emmett–Teller (BET) surface areas were determined by using the desorption data. Pore size distribution was obtained using the Barrett-Joyner-Halenda (BJH) method. X-ray photoelectron spectroscopy (XPS, Thermal ESCALAB 250 XI, USA) was used to confirm the elemental composition on the sample surface, in which the samples were fixed on a sample holder by tapes and all volatiles were removed before the vacuum test. The morphology of the synthesized samples was analyzed using field emission scanning electron microscopy (FESEM, JSM-7800F, Japan) and transmission electron microscopy (TEM, FEI Tecnai F20).
Electrochemical measurements
The electrochemical performances of the as-prepared samples were evaluated on an electrochemical workstation (CHI 660E, China) using a standard three-electrode system. A Pt wire and an Ag/AgCl electrode (3.0 M KCl, 0.222 V vs. SHE at 298 K) were used as the counter electrode and reference electrode, respectively. The samples were dispersed in an ethanol solution via ultrasonication with 5% Nafion® as the binder for 30 min to obtain a homogeneous dispersion, which was then dropped onto a glassy carbon electrode (3 mm in diameter) to prepare the working electrode. All the experiments were performed in 6.0 M KOH aqueous electrolyte. The specific capacitance was calculated from the galvanostatic charge/discharge curves according to Eq. (1):
where I is the discharge current, \( \Delta t \) is the time elapsed for the discharge branch from 0 to 1 V, \( \Delta V \) is the voltage difference within the time \( \Delta t \) and m is the mass of carbon on an electrode.
Results and discussion
Morphology characterization
FESEM images in Fig. 2 show that the r-GO, B-rGO, N-rGO and BN-rGO samples exhibit typical laminated structures composed of planar sheets with wrinkles and corrugations. TEM images in Fig. 2e–h confirm the similar few-layer structures of the plasma-treated samples. Obviously, the exfoliation of GO layers has been successfully implemented by the plasma treatment [10]. The few-layer morphology of the plasma-treated samples is highly favorable for electron transport because it can provide an open pore structure for electrolyte solutions to easily access each individual sheet, thus making it possible to combine the ultra-high power density and high energy density in the super-capacitors based on the as-synthesized materials [21]. EDS analyses in Figure S2 verified the successful doping of B and N elements in the corresponding samples although the results are not very obvious due to the low doping content of B and N. For further confirming the heteroatoms doping, XPS analyses were carried out and the details are included in the later section.
To investigate the crystal structure and surface properties of the as-prepared samples, X-ray diffraction (XRD) and FT-IR analyses were carried out. As demonstrated in Fig. 3a, the characteristic diffraction peak at ca. 10.0° (001) in the XRD pattern of GO completely disappeared in that of r-GO and the corresponding plasma-treated samples, indicating that the oxygen-containing groups in GO have been effectively removed by the plasma-induced H2 reduction [19]. Meanwhile, the broad weak diffraction peak that emerged at ca. 25.0° (002) in the XRD patterns of r-GO, B-rGO, N-rGO and BN-rGO indicates the exfoliation and reduction of GO by the plasma treatment, as well as the amorphous nature of these samples [19]. The absence of the oxygen-containing functional groups in the plasma-treated samples can also be observed from their FT-IR spectra (Fig. 3b), in which the peaks attributed by the C–O (1070 cm−1), –OH (1430 cm−1) and C=O (1630 cm−1) groups are significantly weakened compared with those in the spectrum of GO. In the FT-IR spectra, the peaks corresponding to B–C (1044 cm−1), B–O (659 cm−1), C=N (1584 cm−1) and C–N (803 cm−1) cannot be observed, which is probably because the atomic diameter of boron, carbon and nitrogen is very close to each other, and the hybrid cannot be excited to generate significant dipole moment changes.
In the Raman spectra of the samples (Fig. 3c), the D band (at ca. 1343 cm−1) and G band (at ca. 1580 cm−1) reflect the disorder and graphitic symmetry of the GO structure, respectively [22, 23]. After the plasma treatment and heteroatom doping, both D and G bands for the modified samples exhibited insignificant shifts of binding energy, indicating the negligible effect on the inherent structure of the original GO samples. Meanwhile, the intensity ratio of D and G bands (ID/IG) of GO, r-GO, B-rGO, N-rGO and BN-rGO was calculated to be 1.07, 0.93, 0.91, 0.89 and 0.86, respectively, demonstrating that the defects in GO are decreased as the sp2 domains can be partially “healed” by the reduction and doping. Such “self-healing” phenomenon could be attributed to the introduction of N and B atoms in the crystal structure of r-GO, which replaced the sp3 C defect sites in the forms of pyridinic N, quaternary N and BC3 and subsequently restored them to the sp2 C form [12, 24, 25]. Notably, some of previous reports have demonstrated that the heteroatom doping would increase the structure defects in graphene [13, 26], and this conflict might be caused by the different pristine defects existing in the structure of the GO precursor and different treatment methods [21].
The nitrogen adsorption and desorption isotherms of the as-synthesized samples in Fig. 3d all demonstrated type IV curves with a distinct hysteresis loop. The Brunauer–Emmett–Teller (BET) specific surface area of r-GO, B-rGO, N-rGO and BN-rGO was measured to be 301.32, 208.96, 218.69, 269.48 m2 g−1, respectively. The pore distribution results (Table 1 and inset Fig. 3d) show that the pore diameter in these samples is mainly around 4 nm, indicating the mesoporous (the presence of pore diameters in between the range of 2–50 nm) structures of the as-synthesized materials, which is in accordance with the previous literature [10]. As has been reported, mesoporous structure is beneficial for the fast transport of charges since the pores of these sizes allow easy insertion–deinsertion of ions within the electrode during the charging–discharging process, which can enhance the electrochemical properties [27,28,29]. Although the lyophilization method was utilized during the drying process, the obtained materials exhibit much lower surface area than the theoretical value of a single-layer graphene (2600 m2 g−1) [30], possibly owing to the self-aggregation in the plasma reduction process [31]. After the heteroatom doping, there is no obvious change in the specific surface area of the material, or even a certain decrease. It may be because the addition of the dopant affects the process of reduction and stripping, but from the specific capacitance result, the effect is significantly less than the positive effect of the doping-induced increase in specific capacitance.
Electrochemical evaluation
The electrochemical performance of the samples was evaluated by cyclic voltammetry (CV) and GCD measurements (Fig. 4 and Figure S3, Figure S4 in the ESI†) as the electrode materials for super-capacitors. BN-rGO showed a typical mango-shaped CV curves with a pair of wide redox humps (Fig. 4a), which is considered a representative figure for an outstanding capacitive behavior and high charge mobility [9, 32]. The capacitance of the BN-rGO in the scan rate of 0.5 A g−1 calculated according to Eq. (1) is 350 F g−1 (Fig. 4b), which is much higher than that of r-GO (148 F g−1), B-rGO (240 F g−1) and N-rGO (288 F g−1) and also surpasses most of the reported graphene-based electrode materials for super-capacitors (Table S1, ESI†). Figure 4c shows the variation of specific capacitance at different current densities from 0.5 to 20 A g−1, indicating that all the prepared rGO materials have better specific capacitances at low current densities, since the fast charge–discharge rate will lower the rate of ion diffusion of electrolyte into the dense centers of the electrode materials. In addition, Fig. 4d demonstrates that all rGO materials prepared by the plasma route exhibit excellent cycling stability after 1,000 cycles at a current density of 10 A g−1 with only a slight decrease in the capacitance at the beginning of the cycles.
a CV curves at a scanning rate of 10 mV S−1, b GCD curves at a current density of 0.5 A g−1, c specific capacitance as a function of discharge current density, d cycling stability of rGO, B-rGO, N-rGO and BN-rGO at a current density of 10 A g−1, e EIS (Nyquist plots) and f local enlargement of the high-frequency region of rGO, B-rGO, N-rGO and BN-rGO from 10 kHz to 10 mHz
Furthermore, the as-prepared materials were evaluated by using electrochemical impedance spectroscopy (EIS). As shown in Fig. 4e, f, the Nyquist plots for the four samples exhibit similar profiles, with the quasi-semicircles at the high-frequency region representing the charge transfer resistance (Rct) in the equivalent circuit (inset in Fig. 4e) and the linear tail in the low-frequency region indicating the characteristic Warburg impedance (Rw) [33, 34]. According to the data in Table 2, the BN-rGO sample exhibits the smaller charge transfer resistance (Rct) of 20.91 Ω, correlating well the smallest radius of the quasi-semicircle in its Nyquist plot. In addition, compared with that of rGO without doping, the charge transfer resistance of N-rGO and B-rGO is also significantly decreased. According to previous studies [7, 13, 35,36,37], N-doping could increase the hydrophilicity, polarity and electron transfer of the rGO samples [33, 34] while B-doping could enhance the surface wettability of the rGO samples toward electrolyte, thus lowering the hostility of the electrode [7, 13]. Therefore, it is rational that the co-effect of the two dopants leads to the lowest charge transfer resistance for BN-rGO by increasing its surface polarity [37]. On the other hand, the characteristic Warburg impedance (Rw), corresponding to the linear region in the Nyquist plots, is related to the kinetics of the diffusion/transport processes of electrolyte ions in the electrodes. As shown in Fig. 4f, the samples of N-rGO and BN-rGO exhibit obviously larger slope in the low-frequency region than B-rGO and rGO, suggesting the faster kinetics of ion diffusion processes, which is also demonstrated by the lower Rw values for N-rGO and BN-rGO in Table 2. In general, the fitting results of the charge transfer and diffusion impedances in Table 2 show that BN-rGO exhibits the best overall impedance performance among all samples although it has a slightly larger Rw than BN-rGO.
X-ray photoelectron spectroscopy analysis
The better performance of BN-rGO as electrode materials of super-capacitors might be relevant to the synergistic effect between the doped boron and nitrogen atoms [17, 38, 39]. To verify this hypothesis, X-ray photoelectron spectroscopy (XPS) analyses of the as-prepared samples were carried out. In the XPS survey spectra (Fig. 5a), the existence of two weak peaks at the binding energy of 400.0 eV (N 1s) and 191.0 eV (B 1s) confirms the successful doping of boron and nitrogen elements in the corresponding samples. According to the peak fitting of the high-resolution spectra of B 1s, N 1s and C 1s in Fig. 5b-d, the doping content of boron in B-rGO and BN-rGO is determined to be 1.21 at.% and 1.47 at.%, while the doping content of nitrogen in N-rGO and BN-rGO is estimated to be 2.12 at.% and 2.69 at.%, respectively (Table 1). Clearly, the doping content of both boron and nitrogen in BN-rGO is higher than that in the corresponding single-doped samples of N-rGO and B- rGO, indicating the mutual promotion of nitrogen and boron doping in the co-doping process.
The variation of the ratio of different doping forms of nitrogen and boron in the as-prepared materials might be able to give further information on such mutual promotion effect. Figure 5 reveals that two forms of boron exist in the samples of B-rGO and BN-rGO: BC3 (sp2 hybridized, 191.3 eV) and BC2O (sp3 hybridized, 192.13 eV) [40,41,42]. On the other hand, there are four forms of nitrogen in N-rGO and BN-rGO: C=N (398.43 eV), pyridinic N (sp2 hybridized, 398.88 eV), pyrrolic N (sp3 hybridized, 400.2 eV) and graphitic N (sp2 hybridized, 401.3 eV) [43, 44]. The ratio between BC3 with BC2O is rather close in BN-rGO (0.94:1) and B-rGO (0.86:1), while the total ratio of sp2 N (pyridine N and graphitic N) to sp3 N (pyrrolic N) increases from 2.5 in N-rGO to 3.0 in BN-rGO, indicating that the amount of sp2 N in the BN-rGO is significantly enhanced (Table 3). A reasonable explanation of this observation is that as an electron acceptor, the introduction of boron atoms in graphene can enhance the electron-deficient holes around the nearby carbon atoms, making it easier for carbon atoms to further combine with electron-donating nitrogen atoms. Moreover, such effect is mainly distributed within the conjugated area [20, 32, 45]. Therefore, the increased N-doping content from the B–N co-doping effect was mostly contributed from the enhancement of sp2 N, which is suggested to be the crucial factor that is positively related to the performance of super-capacitors [12] and thus resulted in the relatively high capacitance of BN-rGO.
Conclusions
In this study, boron and nitrogen co-doped rGO materials were prepared by a facile DBD plasma treatment method. Compared with the single-doped rGO materials of B-rGO and N-rGO, BN-rGO samples exhibit not only the highest doping content of nitrogen (2.69 at.%) and boron (1.47 at.%), but also the highest capacitance of 350 F g−1. The mutual promotion is possibly facilitated by a synergetic effect of the two dopants in the doping process due to the electron-accepting nature of boron and electron-donating nature of nitrogen. This work can shed significant light on understanding the doping mechanism of duel elements in the graphene-based materials and hopefully provide new prospects in the design and synthesis of graphene-based electrochemical materials.
References
Balandin AA, Ghosh S, Bao WZ, Calizo I, Teweldebrhan D, Miao F, Lau CN (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8:902–907
Huang Y, Liang JJ, Chen YS (2012) An overview of the applications of graphene-based materials in supercapacitors. Small 8:1805–1834
Stoller MD, Park SJ, Zhu YW, An JH, Ruoff RS (2008) Graphene-based ultracapacitors. Nano Lett 8:3498–3502
Du X, Skachko I, Barker A, Andrei EY (2008) Approaching ballistic transport in suspended graphene. Nat Nanotechnol 3:491–495
Zhang ZY, Xu RD, Wang ZG, Dong MD, Cui BX, Chen ML (2017) Visible-light neural stimulation on graphitic-carbon nitride/graphene photocatalytic fibers. ACS Appl Mater Interfaces 9:34736–34743
Liu HT, Liu YQ, Zhu DB (2011) Chemical doping of graphene. J Mater Chem 21:3335–3345
Niu LY, Li ZP, Hong W, Sun JF, Wang ZF, Ma LM, Wang JQ, Yang SR (2013) Pyrolytic synthesis of boron-doped graphene and its application as electrode material for supercapacitors. Electrochim Acta 108:666–673
Wang K, Li LW, Zhang TZ, Liu ZF (2014) Nitrogen-doped graphene for supercapacitor with long-term electrochemical stability. Energy 70:612–617
Li SM, Yang SY, Wang YS, Tsai HP, Tien HW, Hsiao ST, Liao WH, Chang CL, Ma CCM, Hu CC (2015) N-doped structures and surface functional groups of reduced graphene oxide and their effect on the electrochemical performance of supercapacitor with organic electrolyte. J Power Sources 278:218–229
Li SB, Wang ZF, Jiang HM, Zhang LM, Ren JZ, Zheng MT, Dong LC, Sun RY (2016) Plasma-induced high-efficient synthesis of boron doped reduced graphene oxide for supercapacitors. Chem Commun 52:10988–10991
Wang YQ, Yu F, Zhu MY, Ma CH, Zhao D, Wang C, Zhou A, Dai B, Ji JY, Guo XH (2018) N-Doping of plasma exfoliated graphene oxidevia dielectric barrier discharge plasma treatment for the oxygen reduction reaction. J Mater Chem A 6:2011–2017
Wang HB, Maiyalagan T, Wang X (2012) Review on recent progress in nitrogen-doped graphene: synthesis, characterization, and its potential applications. ACS Catal 2:781–794
Sahoo M, Sreena KP, Vinayan BP, Ramaprabhu S (2015) Green synthesis of boron doped graphene and its application as high performance anode material in Li ion battery. Mater Res Bull 61:383–390
Cai DD, Wang CS, Shi CY, Tan N (2018) Facile synthesis of N and S co-doped graphene sheets as anode materials for high-performance lithium-ion batteries. J Alloy Compd 731:235–242
Li G, Sun ZG, Zhang YY, Huang LL, Tian ZX, Wang G, Ling LC, Liu ZY (2017) One-step green synthesis of nitrogen and phosphorus co-doped pitch-based porous graphene-like carbon for supercapacitors. J Porous Mater 24:1689–1696
Sun YR, Du CY, Han GK, Qu YT, Du L, Wang YJ, Chen GY, Gao YZ, Yin GP (2016) Boron, nitrogen co-doped graphene: a superior electrocatalyst support and enhancing mechanism for methanol electrooxidation. Electrochim Acta 212:313–321
Umrao S, Gupta TK, Kumar S, Singh VK, Sultania MK, Jung JH, Oh IK, Srivastava A (2015) Microwave-assisted synthesis of boron and nitrogen co-doped reduced graphene oxide for the protection of electromagnetic radiation in Ku-Band. ACS Appl Mater Interfaces 7:19831–19842
Zheng WT, Sun CQ (2006) Electronic process of nitriding: mechanism and applications. Prog Solid State Chem 34:1–20
Allen BL, Keddie MB, Star A (2010) Controlling the volumetric parameters of nitrogen-doped carbon nanotube cups. Nanoscale 2:1105–1108
Hummers WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80:1339
Zhou Q, Zhao ZB, Chen YS, Hu H, Qiu JS (2012) Low temperature plasma-mediated synthesis of graphene nanosheets for supercapacitor electrodes. J Mater Chem 22:6061–6066
Wang ZF, Wang JQ, Li ZP, Gong PW, Liu XH, Zhang LB, Ren JF, Wang HG, Yang SR (2012) Synthesis of fluorinated graphene with tunable degree of fluorination. Carbon 50:5403–5410
Wang ZF, Wang JQ, Li ZP, Gong PW, Liu XH, Zhang LB, Ren JF, Wang HG, Yang SR (2012) Cooperatively exfoliated fluorinated graphene with full-color emission. RSC Adv 2:11681–11686
Yazdi AZ, Fei HL, Ye RQ, Wang G, Tour J, Sundararaj U (2015) Boron/nitrogen co-doped helically unzipped multiwalled carbon nanotubes as efficient electrocatalyst for oxygen reduction. ACS Appl Mater Interfaces 7:7786–7794
Guo DH, Shibuya R, Akiba C, Saji S, Kondo T, Nakamura J (2016) Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science 351:361–365
Yu C, Fang HQ, Liu ZQ, Hu H, Meng XT, Qiu JS (2016) Chemically grafting graphene oxide to B, N co-doped graphene via ionic liquid and their superior performance for triiodide reduction. Nano Energy 25:184–192
Liu L, Wang Y, Meng QH, Cao B (2017) A novel hierarchical graphene/polyaniline hollow microsphere as electrode material for supercapacitor applications. J Mater Sci 52:7969–7983. https://doi.org/10.1007/s10853-017-1000-2
Tong J, Zhang HH, Gu JN, Li L, Ma C, Zhao J, Wang CY (2016) Poly(ethylene glycol)-block-poly(propylene glycol)-block-poly(ethylene glycol)-assisted synthesis of graphene/polyaniline composites as high-performance supercapacitor electrodes. J Mater Sci 51:1966–1977. https://doi.org/10.1007/s10853-015-9506-y
Kim M, Oh L, Kim J (2015) Supercapacitive behavior depending on the mesopore size of three-dimensional micro-, meso- and macroporous silicon carbide for supercapacitors. Phys Chem Chem Phys 17:4424–4433
Ning GQ, Fan ZJ, Wang G, Gao JS, Qian WZ, Wei F (2011) Gram-scale synthesis of nanomesh graphene with high surface area and its application in supercapacitor electrodes. Chem Commun 47:5976–5978
Yang XW, Zhu JW, Qiu L, Li D (2011) Bioinspired effective prevention of restacking in multilayered graphene films: towards the next generation of high-performance supercapacitors. Adv Mater 23:2833–2838
Chen H, Xiong YC, Yu T, Zhu PF, Yan XZ, Wang Z, Guan SY (2017) Boron and nitrogen co-doped porous carbon with a high concentration of boron and its superior capacitive behaviour. Carbon 113:266–273
Wu JQ, Sun Y, Yang X, Long GK, Zong Y, Li XH, Zheng XL (2018) Effect of graphene thickness on the morphology evolution of hierarchical NiCoO2 architectures and their superior supercapacitance performance. Ceram Int 44:4875–4882
Jiang HM, Wang ZG, Yang Q, Hanif Q, Wang ZM, Dong LC, Dong MD (2018) A novel MnO2/Ti3C2Tx MXene nanocomposite as high performance electrode materials for flexible supercapacitors. Electrochim Acta 290:695–703
Shao CF, Qiu SJ, Chu HL, Zou YJ, Xiang CL, Xu F, Sun LX (2017) Nitrogen-doped porous microsphere carbons derived from glucose and aminourea for high-performance super capacitors. Catal Today 318:150–156
Wang Q, Qin B, Zhang XH, Xie XL, Jin LE, Cao Q (2018) Synthesis of N-doped carbon nanosheets with controllable porosity derived from bio-oil for high-performance super capacitors. J Mater Chem A 6:19653–19663
Gopalsamy K, Balamurugan J, Thanh TD, Kim NH, Lee JH (2017) Fabrication of nitrogen and sulfur co-doped graphene nanoribbons with porous architecture for high-performance super capacitors. Chem Eng J 312:180–190
Long D, Li W, Ling L, Miyawaki J, Mochida I, Yoon S (2010) Preparation of nitrogen-doped graphene sheets by a combined chemical and hydrothermal reduction of graphene oxide. Langmuir 26:16096–16102
Jiang ZQ, Zhao XS, Tian XN, Luo LJ, Fang JH, Gao HQ, Jiang ZJ (2015) Hydrothermal synthesis of boron and nitrogen co-doped hollow graphene microspheres with enhanced electrocatalytic activity for oxygen reduction reaction. ACS Appl Mater Interfaces 7:19398–19407
Jansen RJJ, Vanbekkum H (1995) Xps of nitrogen-containing functional-groups on activated carbon. Carbon 33:1021–1027
Nakayama Y, Soeda F, Ishitani A (1990) XPS study of the carbon-fiber matrix interface. Carbon 28:21–26
Ferrighi L, Trioni MI, Di CV (2015) Boron-doped, nitrogen-doped, and co-doped graphene on Cu(111): a DFT + vdW study. J Phys Chem C 119:6056–6064
Pech D, Brunet M, Durou H, Huang P, Mochalin V, Gogotsi Y, Taberna P, Simon P (2010) Ultrahigh-power micrometre-sized supercapacitors based on onion-like carbon. Nat Nanotechnol 5:651–654
Schiros T, Nordlund D, Palova L, Zhao L, Levendorf M, Jaye C, Reichman D, Park J, Hybertsen M, Pasupathy A (2016) Atomistic interrogation of B–N co-dopant structures and their electronic effects in graphene. ACS Nano 10:6574–6584
Mannan MA, Noguchi H, Kida T, Nagano M, Hirao N, Baba Y (2010) Growth and characterization of stoichiometric BCN films on highly oriented pyrolytic graphite by radiofrequency plasma enhanced chemical vapor deposition. Thin Solid Films 518:4163–4169
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21606026, 21776025) and partially supported by the Fundamental Research Funds for the Central Universities (106112017CDJXY220005, 106112017CDJXF220009, 106112017CDJPT220001, 106112017CDJQJ228809, 106112017CDJXFLX0014).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhu, T., Li, S., Ren, B. et al. Plasma-induced synthesis of boron and nitrogen co-doped reduced graphene oxide for super-capacitors. J Mater Sci 54, 9632–9642 (2019). https://doi.org/10.1007/s10853-019-03552-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-019-03552-2