Abstract
Nitrogen-containing porous carbons with numerous micropores were fabricated by pyrolysing polyvinylidene fluoride (PVDF) membrane following potassium hydroxide (KOH) activation at 600–900 °C for 2 h under N2 atmosphere. The fabricated nitrogen-containing porous carbons have a large specific surface area of approximately 2289 m2 g−1, a certain nitrogen content of 1.35 at.% and a favourable hierarchical porous structure possessing a great number of micropores and a part of mesopores. The carbon materials exhibit high specific capacitance of 338 F g−1 at 0.5 A g−1 in a 6 M KOH and outstanding cycling property of 91.6% maintenance at 2 A g−1 after 10000 cycles. An all-solid-state symmetrical supercapacitor with these as-prepared materials as electrodes can deliver energy densities of 21.9 to 10.4 W h kg−1 at power densities from 700.8 to 12910.3 W kg−1. To sum up, the as-obtained porous carbon is a type of potential electrode material for electrical double-layer capacitor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Electrical double-layer capacitors (EDLCs) have received much attention owing to their charge storage and release in electrical double layers across the electrode/electrolyte interface. EDLCs own various advantages such as high energy density, superior charge/discharge rate and good cycling stability [1,2,3,4,5]. Based on the functional mechanism of this supercapacitor, appropriate pore structure and high specific surface area for electrolyte ion diffusion are thus important [6,7,8]. Therefore, various materials and methods were developed to improve the performance of supercapacitors through adjusting these parameters. Porous carbon materials, as one of the electrode materials of EDLCs, possess unparalleled advantages [9]. They do not only have ultrahigh specific surface area and controllable pore structure but also have chemical stability, low-cost and favourable conductivity [10, 11]. The mesopores of porous carbon serve as special channels for faster electrolyte ion diffusion owing to their much larger bore diameter than solvated ions of electrolyte [6, 12]. Different from the main advantage of mesopore, the greatest contribution of micropore is to provide high specific surface area, which substantially expands the effective electrode/electrolyte contact area [13]. Additionally, porous carbonaceous electrode with rational micropore size can deliver an extraordinarily high capacitance at a suitable charge/discharge rate even though solvated ions are larger than the bore diameter of micropores and ion transport resistance exists [14]. According to some prior reports, subnanometer pores whose sizes are less than 1 nm and those that are located at (0.5-0.7) nm can enhance the specific capacitance, which is due to the fact that, when the pore diameter of the material is much smaller than the solvent ion diameter, a partial desolvation in the microporous structure carbon takes place [15,16,17,18,19].
To ulteriorly boost the property of EDLCs, introducing N heteroatom into the framework of porous carbon has been proven to be an efficient method [20]. Nitrogen-doped porous carbon can enhance the wettability of the electrode/electrolyte interface and improve the conductivity of electrode [21,22,23,24]. Currently, nitrogen-doped porous carbon has been obtained by various methods. Han et al. investigated high-nitrogen-content (approximately 8.25 wt%) porous carbon by using the mixture of block copolymer P123, dicyandiamide and TiO2 as precursor, showing a high specific capacitance of 537.3 F g−1 at 0.5 A g−1 in a three-electrode system [3]. Polypyrrole is also employed as a nitrogen source by direct synthesis in the experiment of Chen et al. [25] or carbonisation following activation in the study of Qie et al. [26]. Both processes showed excellent electrochemical performance. Apparently, direct pyrolysis of a nitrogen precursor is a relatively facile and efficient approach [27]. Considering the variety of nitrogen source and the unfavourable influence, which they create in porous carbon material, choosing rational nitrogen precursor is significant.
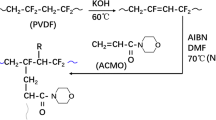
In this study, a new synthetic strategy was developed to fabricate nitrogen-containing porous carbon materials by choosing polyvinylidene fluoride (PVDF) as the carbon source and polyvinylpyrrolidone (PVP) and N,N-dimethylformamide (DMF) as the nitrogen sources (Scheme 1). The synthesis process includes two important parts: carbonising the PVDF membranes, which are prepared by mixing PVDF and PVP in DMF, and activating the previous carbon with several contents of potassium hydroxide (KOH). The obtained samples have a porous structure that provides accessible pathways for transport, amounts of rational micropores and high specific surface area for energy storage. In addition, doping nitrogen into porous carbon materials can improve the conductivity of electrodes, strengthen the wettability of the interface and decrease the ion transport resistance. All these features enhance the capacitance and performance of EDLCs.
Experimental section
Materials
KOH and concentrated hydrochloric acid (HCl, 36–38 wt%.) were obtained from Adamas-Beta Inc., Shanghai, China. PVDF, PVP and DMF were purchased from Sinopharm Chemical Reagent Co., Ltd. All chemicals and reagents are of analytical grade and were used without any further treatment.
Synthesis
About 7 g PVDF and 2 g PVP were added into 41 g DMF. Then, the mixture was heated at 70 °C in oil bath and stirred simultaneously for 6 h. After that, the mixture was placed in an air-drying oven (60 °C) for 4 h to remove air bubbles. Subsequently, the mixture was prepared into membranes with a thickness of 250 nm by phase inversion technique [28]. The membrane was carbonised at 600 °C for 2 h in a tubular furnace under N2 atmosphere with a heating rate of 5 °C min−1 to obtain a carbon material (C-600). In this work, the activation treatment is a chemical process, and the C-600 material and KOH were mixed together and the weight ratio values of C-600 to KOH are 1:2 and 1:4, respectively. Before mixing, the KOH was dissolved in a little amount of deionised water for intensive mixing. To evenly mix and quickly evaporate the water, a little absolute ethyl alcohol was added into above mixture and the mixture was placed in air-drying oven at 60 °C. The dried products were thermally treated at 600, 700, 800, 900 and 1000 °C for 2 h under N2 protection. Then, the activated carbon was treated with HCl solution (1 M) and then washed repeatedly with deionised water until the pH value reached neutral. In the end, the product was dried at 60 °C overnight in vacuum-drying oven. For simplicity, these products were abbreviated as CK-x–y, where x represents the mass ratio of KOH to C-600 and y represents the activation temperature.
Physical characterisation
The mass change of sample during carbonisation process under N2 atmosphere from room temperature to 900 °C (10 °C min−1) was determined by thermogravimetric analyser (DTG-60AH) (Fig. S1). Scanning electron microscopy (SEM, Hitachi S-4800) and transmission electron microscopy (TEM, JEM-2100, JEOL) were operated for surface morphology and structure investigation of porous carbon. X-ray diffraction (XRD) experiments were performed using a Bruker Focus D8 diffractometer with Cu Kα radiation (40 kV, 40 mA, k = 1.5418 Å) between 10° and 80°. Raman spectra were collected from 800 to 2000 cm−1 on a LabRAM HR800 Raman spectrometer. X-ray photoemission spectroscopy (XPS) analyses were carried out on a SPECS spectrometer with Al Kα radiation. The surface areas were calculated by using 3H-2000PM surface and pore size analysis instrument (Beishide Instrument-S&T Co., Ltd., China) at − 196 °C.
Electrochemical characterisation
The electrochemical measurements were performed by using a three-electrode system in 6 M KOH aqueous electrolyte at room temperature. Working electrodes were fabricated by mixing activated carbon material, carbon black and polytetrafluoroethylene with a mass ratio of 8:1:1. After stirring the above mixture, the homogeneous slurry was obtained and was coated onto a nickel foam, pressed at 10 Mpa and dried at 80 °C overnight in vacuum-drying oven. The reference and counter electrode are Ag/AgCl and Ni foam, respectively. The cycling performance was studied by using CT2001A Land Electronic testing equipment (Wuhan Land Electronic Co., Ltd., China). The electrochemical performance of CK-4-y was investigated by employing galvanostatic charge/discharge (GCD), cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) techniques on an electrochemical workstation (CHI760D, Chenhua Instruments Co. Ltd., Shanghai, China). The gravimetric specific capacitances can be calculated from the charge/discharge curves according to Eq. (1): [2].
where I (A) is the charge/discharge current, ∆t (s) is the discharge time, m (g) is the mass of activated materials, and V (v) is the working voltage window.
Electrochemical tests were also conducted by a two-electrode system, which consisted of two symmetrical activated material electrodes as preceding electrodes, carbon paper as collectors and H2SO4/PVA as solid electrolyte. The mass of active material coated on each electrode is appropriately 1.5 mg. The gravimetric specific capacitances can be calculated from the charge/discharge curves according to Eq. (2): [29, 30].
where I (A) is the charge/discharge current, ∆t (s) is the discharge time, m (g) is the total mass of activated materials, and V (v) is the working voltage window. The energy density (E, W h kg−1) was calculated by Eq. (3): [29, 30].
where C (F g−1) is the gravimetric specific capacitance and V represents the working voltage window. The specific power (P, W kg−1) can be calculated by Eq. (4): [29, 30].
where E and ∆t are the energy density and discharge time, respectively.
Results and discussion
Textural and structural properties
The morphology and microstructure of the CK-4-900 sample were studied by SEM and TEM. From the low-magnification SEM image (Fig. 1a), a cross-linked wrinkle architecture can be observed. Apparently, the high-resolution SEM image (Fig. 1b) displays the ultrathin nanosheets construction and some visible pores, which are interconnected and interweaved in nanosheets. The observed interconnected microstructure of the CK-4-900 serves as a basis for high surface area. In addition, many pores can be distinctly observed in the TEM image (Fig. 1c). Undoubtedly, porous structure exists in the CK-4-900. To further clarify the porous structure in the high-resolution TEM image (Fig. 1d), a great number of narrow micropores were exhibited, which can provide high surface area and deliver an extraordinarily high capacitance [14].
To further investigate the porosity structure of CK-x–y, the N2 adsorption–desorption measurements were conducted. The isotherm plots of CK-x–y samples are shown in Figs. 2a and S2a. Notably, all samples exhibit I-type adsorption–desorption isotherm, which indicate the existence of numerous micropores. The pore size distribution determined by density functional theory is shown in Figs. 2b and S2b. All samples show a narrow micropore size distribution from 0.5 nm to 1.95 nm. The textural parameters of as-prepared samples are summarised in Table 1. As shown in the table, C-600 has a low N2 adsorption capacity and a small BET specific surface area (749 m2·g−1). After the activation of KOH, all of the sample adsorption capacities and the specific surface area dramatically increased. This result indicates that the activation process produces samples with more micropore structures. In addition, with the increase in KOH (KOH/C-600 value from 2 to 4), the activation degree was enhanced and the BET specific surface area apparently increased. Moreover, the specific surface area increases first (up to 2289 m2·g−1) and then decreases with the increase in activation temperature. When the temperature reached to 1000 °C, the sample of CK-4-1000 average pore diameter is up to 3.49 nm and the specific surface area only is 1194 m2·g−1. This finding indicates that as the temperature rises, the KOH activation degree strengthens, so that the material structure was destroyed seriously, resulting in an increase in average pore size (Table 1 and Fig. S3) and a decrease in specific surface area.
The crystallographic structure of CK-4-y samples was analysed by XRD and Raman spectroscopy. Every sample displayed two diffraction peaks at 2θ = 23° and 44° (Fig. 3a), which can correspond to the (002) plane of amorphous character and the (100) plane of graphitic layer, respectively [31, 32]. Obviously, the intensity of two peaks weakened with the activation temperature increase, suggesting that the graphitic architectures are destructed severely through the KOH activation at high temperature. Meanwhile, the Raman spectra further confirmed the graphitic structure presented in Fig. 3b. The peaks were observed at 1345 (D band) and 1590 cm−1 (G band), representing the disordered structure and graphitic structure of carbon materials [33, 34]. As a rule, the intensity ratio of the D band to G band (ID/IG) was used to evaluate the degree of carbon graphitisation, defects or the domain size of graphitisation [35]. A smaller ID/IG ratio represents higher graphitisation. Evidently, the ID/IG value (0.966, 0.999, 1.029 and 1.038 for CK-4-600, CK-4-700, CK-4-800 and CK-4-900, respectively) keeps increasing as the activation temperature rises. Consequently, the result indicates that the degree of graphitisation decreased with the increasing temperature owing to the harsh KOH activation. It will improve carbon material pore structure and benefit the electrochemical performance of porous carbon.
XPS analysis was employed to study the surface functionalities and elemental composition of CK-4-y samples. Table S1 is the elemental content (C, N, O, F) of porous carbon. We can see that the material contain N elemental and a small amount of F elemental, indicating that N elemental was successfully introduced into the porous carbon material. As shown in Fig. 4, the predominant N 1s signal was differentiated into three peaks, which correspond to pyridinic N (N-6, 398.7 eV), pyrrolic N (N-5, 399.5 eV) and graphitic N (N-Q, 401.5 eV) [13, 36, 37]. Therefore, the existence of various N species indicates successful N doping in the porous carbon materials. Furthermore, the N content of the samples is summarised in Table 2. As shown in the table, the nitrogen percentage decreased with the rising temperature, which is 1.80, 1.71, 1.43 and 1.35% for CK-4-600, CK-4-700, CK-4-800 and CK-4-900, respectively. However, the N-6 always accounts for a certain percentage of the sample, which can improve electron-donating properties and increase electron density [38]. Moreover, N-5 content of carbon material increased with the activation temperature increase. Previous reports indicated that N-5 benefits in the graphitisation degree increase and electrical conductivity of porous carbon [33, 39]. Generally, the combination of these N functional groups substantially improves the electrochemical properties of porous carbon materials.
Electrochemical performance
The electrochemical performances of CK-4-y as electrode materials were evaluated by using a three-electrode system in 6 M KOH solution. Figure 5a illustrates the CV curves of CK-4-y samples at a scan rate of 20 mVs−1. All samples show quasi-rectangular shapes, suggesting an electrochemical double-layer capacitance behaviour. Obviously, the CV curve of CK-4-900 displays the greatest rectangular shape among these CK-4-y samples owing to the largest specific surface area (2289 m2 g−1), a certain N content and special porous architecture [40]. To further research the favourable property of CK-4-900, its CV curves were obtained at various scan rates from 5 to 100 mV s−1, as shown in Fig. 5b. Apparently, the shapes of CV curves keep similar with the scan rate increasing, indicating good capacitive performance.
Electrochemical performance of the samples in 6 M KOH electrolyte: a cyclic voltammetry (CV) curves at 20 mV·s−1; b CV curves of CK-4-900 at different scan rate; c galvanostatic charge–discharge (GCD) curves at current density of 1 A g−1; d GCD curves of CK-4-900 at different current densities; e Nyquist plots; f The cycling performance of the CK-4-900 as electrode in three-electrode system at the current density of 2 A g−1 (the inset shows the GCD curves for the 1st and 10000th cycles)
GCD curves of CK-4-y samples at a current density of 1 A g−1 present a quasi-triangular shape, and their discharge voltages almost change linearly with time increase, as presented in Fig. 5c, indicating a typical double-layer capacitance characteristic. However, a little deviation from lines is observed at lower potential due to the existence of nitrogen functional groups, which provided a little pseudocapacitance [41]. Meanwhile, their specific capacitances were calculated by Eq. (1), and the value of C-600 only is 203 F g−1. After the activation of KOH, the specific capacitances of CK-4-600, CK-4-700, CK-4-800 and CK-4-900 reach 245, 277, 295 and 312 F g−1, respectively. In previous reports, porous carbon with a large number of microporous and mesoporous structures reveals the excellent electrochemical performance [42,43,44]. The CK-4-900 sample possesses ultrahigh surface area, and large volumes of micropores and mesopores make it show the greatest specific capacitance among those samples. Furthermore, nitrogen strengthens the wettability of the interface between electrolyte and electrode and improves the conductivity of electrode. Figure 5d shows the GCD curves of CK-4-900 at current densities of 0.5, 1, 2, 5, 8 and 10 A g−1, which correspond to specific capacitances of 338, 312, 284, 258, 224 and 198 F g−1. These values are relatively higher than some capacitive properties of porous carbon in previous reports (Table S2). Additionally, these curves keep a quasi-triangular shape even at 10 A g−1, which is consistent with previous CV curves.
EIS measurements were conducted to verify the resistance of ions and charges transfer. All Nyquist plots (Fig. 5e) contain three different sections, which are semicircle curve at high frequency, 45° slant line at intermediate frequency and vertical line at low frequency. The semicircle exhibits inherent ohmic resistance from charge-transferring in electrolyte, current collector, porous carbon and even separator; the 45° slant line indicates ion diffusion resistance in the porous structure of CK-4-y samples; the vertical line presents favourable electrical double-layer capacitive performance [45,46,47]. Apparently, the Nyquist plot of CK-4-900 possesses the most vertical line, the shortest 45° slant line and the smallest diameter size semicircle among these plots, suggesting the most favourable capacitive property and the lowest resistance. Figure 5f illustrates the cycling performance of the CK-4-900 as electrode in the three-electrode system at 2 A g−1. No serious attenuation of specific capacitance was observed after 10000 cycles. According to the calculation, the activated material electrode still maintains at approximately 91.6% specific capacitance. Therefore, the CK-4-900 exhibits excellent cycling stability, which can be the promising electrode materials of EDLCs.
To clarify the actual application of as-prepared porous carbon materials, an all-solid-state symmetrical supercapacitor, which was based on two-electrode system principle with CK-4-900 as electrodes and H2SO4/PVA gel as solid-state electrolyte, was fabricated to test the electrochemical property (Fig. 6a). As shown in Fig. 6b, CV curves of the two-electrode system were measured at several scan rates from 5 to 100 mV s−1 in a voltage window of 0–1.6 V. The CV curves present nearly rectangular shapes even at 100 mV s−1, indicating a prominent capacitive behaviour and an excellent rate capability. Meanwhile, the nearly triangular-shaped GCD curves are displayed at different current densities in Fig. 6c. They maintain similar shapes without obvious deformation, which is consistent with the result of CV and further confirms the prominent performance of as-obtained porous carbon materials. According to Eq. (2), the specific capacitance was 80.4, 72.1, 65.7, 47.9 and 38.3 F g−1 at 0.5, 1, 2, 5 and 10 A g−1, respectively. Likewise, the cycling performance of the solid-state supercapacitor was measured at 2 A g−1 (Fig. 6d). No serious capacitance loss was observed, and the specific capacitance retention was 96.2% after 3000 cycles. Furthermore, the inset in Fig. 6d illustrates the comparison of Ragone plots before and after 3000 cycles corresponding to the result of charge–discharge measurement. The energy density (E) and the power density (P) of this all-solid-state symmetrical supercapacitor were calculated by Eqs. (3) and (4), with the current increasing (from 0.5 to 10 A g−1); E is 21.9, 19.6, 17.9, 13.0 and 10.4 W h kg−1; the corresponding P is 700.8, 1397.2, 2801.7, 6974.6 and 12910.3 W kg−1, respectively. Apparently, the E value maintains 47.49% at 10 A g−1. After 3000 cycles, E is approximately 21.06 W h kg−1 at 0.5 A g−1. Therefore, the CK-4-900 sample was evidenced to have favourable electrochemical capacity.
Electrochemical performance of the CK-4-900 as electrodes in all-solid-state supercapacitor: a assembled capacitor schematic; b CV curves at scan rate from 5 to 100 mV s−1; c GCD curves at different current densities; d cycling stability at current density of 2 A g−1, the inset are Ragone plots before and after 3000 cycles
Conclusions
A facile method was described to synthesise nitrogen-containing porous carbons with numerous micropores for electrodes of EDLCs. The as-prepared carbon materials possess high BET specific surface area (up to 2289 m2 g−1) and a rational nitrogen content. As a result, the porous carbon materials exhibit excellent electrochemical performance of specific capacitance reaching 338 F g−1 at a current density of 0.5 A g−1 and cycling stability maintaining 91.6% after 10000 cycles. Meanwhile, the assembled all-solid-state symmetrical supercapacitors present favourable energy densities of 21.9 to 10.4 W h kg−1 at power densities from 700.8 to 12910.3 W kg−1. Considering the above conclusions, this kind of material has potential for future application.
References
Guo H, Gao Q (2009) Boron and nitrogen co-doped porous carbon and its enhanced properties as supercapacitor. J Power Sources 186(2):551–556
Long C, Zhuang J, Xiao Y, Zheng M, Hu H, Dong H (2016) Nitrogen-doped porous carbon with an ultrahigh specific surface area for superior performance supercapacitors. J Power Sources 310:145–153
Han LN, Wei X, Zhu QC, Xu SM, Wang KX, Chen JS (2016) Nitrogen-doped carbon nets with micro/mesoporous structures as electrodes for high-performance supercapacitors. J Mater Chem A 4(42):16698–16705
Jia D, Yu X, Tan H, Li X, Han F, Li L (2017) Hierarchical porous carbon with ordered straight micro-channels templated by continuous filament glass fiber arrays for high performance supercapacitors. J Mater Chem A 5(4):1516–1525
Liu S, Zhao Q, Tong M, Zhu X, Wang G, Cai W (2016) Ultrafine nickel–cobalt alloy nanoparticles incorporated into three-dimensional porous graphitic carbon as an electrode material for supercapacitors. J Mater Chem A 4(43):17080–17086
Li W, Liu J, Zhao D (2016) Mesoporous materials for energy conversion and storage devices. Nat Rev Mater 1(6):16023. https://doi.org/10.1038/natrevmats.2016.23
Wu Z, Li L, Yan JM, Zhang XB (2017) Materials design and system construction for conventional and new-concept supercapacitors. Adv Sci 4(6):1600382. https://doi.org/10.1002/advs.201600382
Wei Q, Xiong F, Tan S, Huang L, Lan EH, Dunn B (2017) Porous one-dimensional nanomaterials: design, fabrication and applications in electrochemical energy storage. Adv Mater 29(20):1602300
Qie L, Chen W, Xu H, Xiong X, Jiang Y, Zou F (2013) Synthesis of functionalized 3D hierarchical porous carbon for high-performance supercapacitors. Energy Environ Sci 6(8):2497–2504
Wang Q, Yan J, Fan Z (2016) Carbon materials for high volumetric performance supercapacitors: design, progress, challenges and opportunities. Energy Environ Sci 9(3):729–762
Dutta S, Bhaumik A, Wu KCW (2014) Hierarchically porous carbon derived from polymers and biomass: effect of interconnected pores on energy applications. Energy Environ Sci 7(11):3574–3592
Tang D, Hu S, Dai F, Yi R, Gordin ML, Chen S (2016) Self-templated synthesis of mesoporous carbon from carbon tetrachloride precursor for supercapacitor electrodes. ACS Appl Mater Interfaces 8(11):6779–6783
Wei H, Chen H, Fu N, Chen J, Lan G, Qian W (2017) Excellent electrochemical properties and large CO2 capture of nitrogen-doped activated porous carbon synthesised from waste longan shells. Electrochim Acta 231:403–411
Qi D, Liu Y, Liu Z, Zhang L, Chen X (2017) Design of architectures and materials in in-plane micro-supercapacitors: current status and future challenges. Adv Mater 29(5):1602802. https//doi.org/10.1002/adma.201602802
Chmiola J, Yushin G, Gogotsi Y, Portet C, Simon P, Taberna PL (2006) Anomalous increase in carbon capacitance at pore sizes less than 1 nanometer. Science 313(5794):1760–1763
Galhena DT, Bayer BC, Hofmann S, Amaratunga GA (2016) Understanding capacitance variation in sub-nanometer pores by in situ tuning of interlayer constrictions. ACS Nano 10(1):747–754
Lu WJ, Huang SZ, Miao L, Liu MX, Zhu DZ, Li LC (2017) Synthesis of MnO2/N-doped ultramicroporous carbon nanospheres for high-performance supercapacitor electrodes. Chin Chem Lett 28(6):1324–1329
Miao L, Duan H, Liu M, Lu W, Zhu D, Chen T (2017) Poly(ionic liquid)-derived, N, S-codoped ultramicroporous carbon nanoparticles for supercapacitors. Chem Eng J 317:651–659
Miao L, Zhu D, Zhao Y, Liu M, Duan H, Xiong W (2017) Design of carbon materials with ultramicro-, supermicro- and mesopores using solvent- and self-template strategy for supercapacitors. Microporous Mesoporous Mater 253:1–9
Wang C, Sun L, Zhou Y, Wan P, Zhang X, Qiu J (2013) P/N co-doped microporous carbons from H3PO4-doped polyaniline by in situ activation for supercapacitors. Carbon 59(7):537–546
Ramakrishnan P, Shanmugam S (2016) Nitrogen-doped porous multi-nano-channel nanocarbons for use in high-performance supercapacitor applications. ACS Sustain Chem Eng 4(4):2439–2448
Wei T, Wei X, Gao Y, Li H (2015) Large scale production of biomass-derived nitrogen-doped porous carbon materials for supercapacitors. Electrochim Acta 169:186–194
Zhang L, Su Z, Jiang F, Yang L, Qian J, Zhou Y (2014) Highly graphitized nitrogen-doped porous carbon nanopolyhedra derived from ZIF-8 nanocrystals as efficient electrocatalysts for oxygen reduction reactions. Nanoscale 6(12):6590–6602
Tan H, Wang X, Jia D, Hao P, Sang Y, Liu H (2017) Structure-dependent electrode properties of hollow carbon micro-fibers derived from Platanus fruit and willow catkins for high-performance supercapacitors. J Mater Chem A 5(6):2580–2591
Chen LF, Zhang XD, Liang HW, Kong M, Guan QF, Chen P (2012) Synthesis of nitrogen-doped porous carbon nanofibers as an efficient electrode material for supercapacitors. ACS Nano 6(8):7092–7102
Qie L, Chen WM, Wang ZH, Shao QG, Li X, Yuan LX (2012) Nitrogen-doped porous carbon nanofiber webs as anodes for lithium ion batteries with a superhigh capacity and rate capability. Adv Mater 24(15):2047–2050
Xu B, Duan H, Chu M, Cao G, Yang Y (2013) Facile synthesis of nitrogen-doped porous carbon for supercapacitors. J Mater Chem A 1(14):4565–4570
Safarpour M, Khataee A, Vatanpour V (2015) Effect of reduced graphene oxide/TiO2 nanocomposite with different molar ratios on the performance of PVDF ultrafiltration membranes. Sep Purif Technol 140:32–42
Wang L, Zhang G, Han B, Chang Y, Li H, Wang J (2017) A two-volt aqueous supercapacitor from porous dehalogenated carbon. J Mater Chem A 5(14):6734–6739
Ma L, Liu R, Niu H, Xing L, Liu L, Huang Y (2016) Flexible and freestanding supercapacitor electrodes based on nitrogen-doped carbon networks/graphene/bacterial cellulose with ultrahigh areal capacitance. ACS Appl Mater Interfaces 8(49):33608–33618
Guo N, Li M, Sun X, Wang F, Yang R (2017) Enzymatic hydrolysis lignin derived hierarchical porous carbon for supercapacitor in ionic liquid with high power and energy density. Green Chem 19(2017):2595–2602
Fan Y, Liu P-F, Huang Z-Y, Jiang T-W, Yao K-L, Han R (2015) Porous hollow carbon spheres for electrode material of supercapacitors and support material of dendritic Pt electrocatalyst. J Power Sources 280:30–38
Lu C, Wang D, Zhao J, Han S, Chen W (2017) A continuous carbon nitride polyhedron assembly for high-performance flexible supercapacitors. Adv Funct Mater 27(8):1606219. https//doi.org/10.1002/adfm.201606219
Sun K, Yu S, Hu Z, Li Z, Lei G, Xiao Q (2017) Oxygen-containing hierarchically porous carbon materials derived from wild jujube pit for high-performance supercapacitor. Electrochim Acta 231:417–428
Liu M, Ma X, Gan L, Xu Z, Zhu D, Chen L (2014) A facile synthesis of a novel mesoporous Ge@C sphere anode with stable and high capacity for lithium ion batteries. J Mater Chem A 2(40):17107–17114
Hueso JL, Espinós JP, Caballero A, Cotrino J, González-Elipe AR (2007) XPS investigation of the reaction of carbon with NO, O2, N2 and H2O plasmas. Carbon 45(1):89–96
Sevilla M, Valle-Vigón P, Fuertes AB (2011) N-doped polypyrrole-based porous carbons for CO2 capture. Adv Funct Mater 21(14):2781–2787
Lin T, Chen IW, Liu F, Yang C, Bi H, Xu F (2015) Nitrogen-doped mesoporous carbon of extraordinary capacitance for electrochemical energy storage. Science 350(6267):1508–1513
Wan L, Wang J, Xie L, Sun Y, Li K (2014) Nitrogen-enriched hierarchically porous carbons prepared from polybenzoxazine for high-performance supercapacitors. ACS Appl Mater Interfaces 6(17):15583–15596
Guo N, Li M, Wang Y, Sun X, Wang F, Yang R (2016) Soybean root-derived hierarchical porous carbon as electrode material for high-performance supercapacitors in ionic liquids. ACS Appl Mater Interfaces 8(49):33626–33634
Zhou M, Pu F, Wang Z, Guan S (2014) Nitrogen-doped porous carbons through KOH activation with superior performance in supercapacitors. Carbon 68(3):185–194
Tian W, Zhang H, Sun H, Suvorova A, Saunders M, Tade M (2016) Heteroatom (N or N-S)-doping induced layered and honeycomb microstructures of porous carbons for CO2 capture and energy applications. Adv Funct Mater 26(47):8651–8661
Lei Q, Song H, Chen X, Li M, Li A, Tang B (2016) Effects of graphene oxide addition on the synthesis and supercapacitor performance of carbon aerogel particles. RSC Adv 6(47):40683–40690
Zhou C, Chen X, Liu H, Zhou J, Ma Z, Jia M (2017) Heteroatom-doped multilocular carbon nanospheres with high surface utilization and excellent rate capability as electrode material for supercapacitors. Electrochim Acta 236:53–60
Wang H, Xu Z, Kohandehghan A, Li Z, Cui K, Tan X (2013) Interconnected carbon nanosheets derived from hemp for ultrafast supercapacitors with high energy. ACS Nano 7(6):5131–5541
Sevilla M, Fuertes AB (2014) Direct synthesis of highly porous interconnected carbon nanosheets and their application as high-performance supercapacitors. ACS Nano 8(5):5069–5078
Tian W, Gao Q, Tan Y, Yang K, Zhu L, Yang C (2015) Bio-inspired beehive-like hierarchical nanoporous carbon derived from bamboo-based industrial by-product as a high performance supercapacitor electrode material. J Mater Chem A 3(10):5656–5664
Acknowledgements
This project was supported by the Shanghai Leading Academic Discipline Project (Project Number J51503), National Natural Science Foundation of China (Project Number 2097610551641208 and 21606151), Shanghai excellent technical leaders (Project Number 17XD1424900), Shanghai Association for Science and Technology Achievements Transformation Alliance Program(Project Number LM201559), Shanghai Municipal Education Commission boosting project (Project Number 15cxy39), Science and Technology Commission of Shanghai Municipality Project (Project Number 14520503200), Shanghai Municipal Education Commission (Plateau Discipline Construction Program), Shanghai Talent Development Funding (Project Number 201335), 2016 laboratory technique project—Chemical Engineering Simulation Training Center (Project Number 3921NH163004007).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors listed have declared that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, J., Wei, H., Fu, N. et al. Facile synthesis of nitrogen-containing porous carbon as electrode materials for superior-performance electrical double-layer capacitors. J Mater Sci 53, 2137–2148 (2018). https://doi.org/10.1007/s10853-017-1664-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-017-1664-7