Abstract
Glass samples with general formula NaCo1−x (VO) x PO4 (x = 0.1, 0.3, 0.5 and 0.7) are synthesized via a simple melt quenching method followed by high-energy ball milling for 30 h to form the homogeneous nanoscaled glass powders. DTA traces of all the glass and glass–ceramic samples indicated exothermic processes confirming selective crystallization induced in the glass network. The formation of major crystalline phase [sodium cobalt pyrophosphate (Na2CoP2O7)] with an ordered layered structure was monitored by X-ray diffraction and the same was justified by SEM images. Structural illustration of major crystalline Na2CoP2O7 phase offered more intra-layer Co–Co distance (7.12 Å) than inter-layer Co–Co distance (5.37 Å) which facilitates two-dimensional Na-ion diffusion pathways to achieve the fast intercalation and de-intercalation phenomenon along the a and c directions. The ionic conductivity was monitored by Impedance analysis and achieved to be highest (6.41 × 10−7 S cm−1) for the glass–ceramic cathode x = 0.3, NaCo1−x (VO) x PO4. The initial discharge capacity for the highest conducting NaCo0.7(VO)0.3PO4 cathode is obtained as 93 mA h g−1 in 0.1 C and had 65% capacity retention even at high rate 10 C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lithium-ion batteries have been considered as the standard energy source for portable electronic devices and as stationary storage devices for electric vehicles, smart electric grids and solar and wind power sectors, because of their high gravimetric energy density, long life time and low self-discharge rate [1,2,3]. The advantage of bulk type battery to the thin film micro battery is that in case of bulk type battery any desired cell capacity will be achieved by the addition of sufficient amount of electrode active materials to the cell. When the bulk type battery is used particularly for large-scale energy storage applications, many batteries are required and large size will cause safety issues. Minimum battery capacity required for the large-scale energy storage applications is 10 kWh; therefore large amount of lithium is required which is expensive. In order to develop cost effective higher capacity cells, ambient temperature sodium ion batteries would appear to be an excellent choice [4, 5]. Though Na-ion batteries work on the same basic principle as Li-ion batteries, experimental investigations revealed that the structure functions as in Li intercalation compounds may not necessarily be true in the case of Na based compounds [6]. The reason is that during the process of fast charging, sodium ions could not diffuse rapidly into the active centres to fill them up and the interfacial resistance would be increased as sodium ions are 70% heavier than lithium. To facilitate the development of new sodium batteries that operate at ambient temperatures, the challenge is to investigate and characterize suitable cathodic, anodic materials and optimization with highly ion conducting electrolyte which are key issues for Na-ion battery success.
The performance of battery system largely determined by cathode capacity, however, very limited research work has been done specially on the cathode materials. Hence, it is worth to gather knowledge on the Na-ion battery cathode materials, with the aim of providing wide view of cathode materials. In this regard, we have investigated a series of cathode materials promoting Na-ion transport with improved electronic and ionic conductivities along with their chemical, electrochemical, thermal and mechanical properties via this paper.
Among the various types of crystalline cathode materials, phosphate polyanion-based cathode materials (NaMPO4 where M = Fe, Mn, Ni, Co) with olivine structure are of special interest as with highest theoretical specific capacity and optimal redox potential [7,8,9]. The existence of cobalt ions in the polyanion compounds enhances the d-space between the layers, structure stability and theoretical capacity, which leads to exhibit higher electrode potential and electronic conductivity over Fe- and Mn-based olivine compounds [10,11,12]. In addition, V2O5 contributes a lot to boost up the insertion/de-insertion properties and rate performance of cathode composites in view of the fact that it possesses rich electrochemical window towards lithium or sodium. However, each crystalline material has met some obstacles in achieving good cycle stability and conductivity that has caused it to not become commercialized [8, 13, 14].
Normally, the amorphous/glass phase is expected to offer good capacity, desirable voltage and conductivity than crystalline counterparts of similar compositions. In order to gain the stable cycle life, conductivity and high rate capability, disordered mixed polyanion glass network is one which has the advantage of achieving high theoretical capacities than promising crystalline polyanion network. The disordered covalently bonded polyanion structure in the glass network controls the ability of structure to rearrange and also improves not only physical properties but also electrochemical behaviour. The specific characteristics such as higher electronic and ionic conductivity, compositional tuning of redox potential, higher specific energy and higher phase stability, make these polyanion glasses as the most potential cathode materials [15, 16]. The polyanions in the glass network must be with correct valency to achieve theoretical capacity which makes these materials more flexible and create wide channels for the Na ion to migrate within the structural framework; hence any desired voltage can be easily achieved. It is also speculated that glass ceramic exhibits higher reversibility and cycle capacity over its precursor glass material [17]. Multiple advantages and highly desired properties of these materials come along with few drawbacks which have to be addressed before successful realization of this family of materials. Some of the disadvantages include poor electronic and ionic conductivity, less relative density, lack of chemical stability and toxicity. Hence, efforts will be aimed in this paper to design and characterize the new mixed polyanion NaCo1−x (VO) x PO4 (x = 0.1, 0.3, 0.5, 0.7) glass and glass–ceramic system to realize Na-ion battery competent to Li-ion battery technology.
Experimental
Stoichiometric ratios of Na2CO3, CoC2O4, (NH4)2PO4 and V2O5 were mixed thoroughly and ground in an agate mortar for 30 min to form homogeneous mixture. Then, the mixture is melted in alumina crucible at 1200 °C for 1 h in an electric furnace and the resulting melts were quenched on the brass plate and rolled by copper roller yield to form a glass. Further, high-energy ball milling method was used for 30 h to form the homogeneous nanoscaled glass powders. The glass transition temperature (T g), crystallization temperature (T c) and the crystalline phase transitions of crystallized glass powders were determined using differential thermal analysis (SII Exstrar 6300) at a heating rate of 10 K/min. Glass–ceramics of precursor glass samples was prepared by heat treating the precursor glasses in 5% H2–95% Ar atmosphere in a tubular electric furnace at their corresponding crystallization temperatures (T c) for 5 h. The crystallization phase details were studied by X-ray diffraction (XRD) method using PANanalytical Diffractometer B.V fitted with Cu target (both K(α1+α2) wavelengths) and Ni filter at 40 kV and 30 mA (2 h range). The crystallite sizes were examined with SEM pictures (Zeiss Gemini 1530 operated at 1 kV). All glass–ceramic compounds were coated with 7 wt% reduced graphene oxide in a planetary ball mill with 1:10 ball ratio for 30 min at 300 rpm.
Working electrode preparation
The electrochemical performances of as-synthesized nanoscaled glass–ceramic powder combinations were monitored by Swagelok-type half-cell configuration with sodium metal foil as counter electrode and Whatman® glass microfiber filter paper (Grade GF/F) was used as separator. The working glass–ceramic cathode was fabricated from a mixture of active material, polyvinylidene fluoride and conductive carbon black in a weight ratio of 85:5:10. The active material was loaded on the working electrodes ~3 mg. N-Methylpyrrolidone was used to make slurry mixture. The slurry coated on thin aluminium foil and dried at 90 °C for 10 h in vacuum oven after homogenization. 1 M NaClO4 in the mixture of ethylene carbonate and diethyl carbonate (1% V/V) was used as electrolyte [18]. Cyclic voltammograms were carried out using a battery test unit model 1470 coupled with a FRA model 1255 from Solartron, Inc. (Corrware and ZPlot software, Scribner Associates), and a multichannel battery test unit from Maccor, Inc., model 2000 at a scan rate of 0.1 mV s−1 with a potential window ranging between 0.001–4 V.
Results and discussion
DTA Results
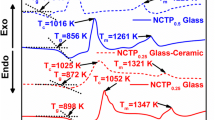
DTA traces of all the NaCo1−x (VO) x PO4 (x = 0.1, 0.3, 0.5 and 0.7) precursor glass and glass–ceramic samples, exhibit an endothermic effect due to glass transition temperature T g, in the temperature range 660–720 K (Fig. 1). Exothermic peaks (T c1 and T c2) are observed in the temperature range 820–870 K which is ascribed due to the precipitation of crystals in the amorphous network. Further, the endothermic effect due to the melting effect (T m) in the temperature range (1120–1180 K) is also observed in all the traces (Fig. 1). The glass transition temperature (T g) decreases up to x = 0.3 (NaCo0.7(VO)0.3PO4) glass cathode sample, however, beyond this concentration, the value of T g increases (Table 1). In order to check the details of crystalline phases in the glass–ceramics, the precursor glass samples as-synthesized were further subjected to heat treatment at their corresponding crystallization temperatures (T c2) determined by DTA patterns (Fig. 1) for about 5 h in a tubular furnace with 5% H2–95% Ar gas flow. The clear exothermic effect and endothermic effect of glass–ceramic samples revealed that the glass matrix is induced with selective crystallization, leading to the precipitation of crystalline phases along with the residual amorphous phase (Fig. 1) and subsequent melting through the endothermic peak. The details of the different crystalline phases of all the glass–ceramics are discussed later in the XRD studies. The glass thermal stability parameter ΔT (ΔT = T c2 − T g) and glass-forming ability parameter K gl (K gl = \( \frac{{T_{\text{c2}} \; - \;T_{\text{g}} }}{{T_{\text{m}} \; - \;T_{\text{c2}} }} \)) are calculated for all the mixed polyanion glass samples and glass–ceramic samples (Table 1) [19]. The K gl values of all the glass samples are greater than the values of glass–ceramic samples, which is ascribed due to the presence of nanocrystalline phases in the mixed polyanion glass network, leading to the modification in the structure of glass network during the transfer of glass into glass–ceramic network (Table 3). The lowest value of K gl for the NaCo0.7(VO)0.3PO4 glass–ceramic cathode predicts its lowest degree of de-polymerization. Interestingly, the same composition recorded the highest thermal stability of the glass against crystallization (ΔT) after the heat treatment. Hence, it has been confirmed again that the thermal stability against devitrification of mixed polyanion glass–ceramic samples is larger than that of the corresponding precursor glass samples via this article [19,20,21]. Though the K gl of NaCo0.7(VO)0.3PO4 is low compared to other samples under investigation, it has become our choice of interest because of two reasons: (a) low glass transition temperature (658 K) that would exhibit the good electrical properties, (b) optimum degree of crystallization by the precipitation of nanocrystalline phases which would yield correlation between electrical conductivity and electrochemical performance.
X-ray diffraction studies
Powder XRD patterns for all the glasses and glass–ceramic samples of NaCo1−x (VO) x PO4 network are shown in Fig. 2. The absence of peaks in the NaCo0.9(VO)0.1PO4 glass confirms its amorphous nature (inset of Fig. 2). XRD pattern of all the glasses was similar in nature. A full pattern matching Rietveld refinement was also performed using X’pert High score Plus and yields the actual compound up to 90% in addition to impurity profiles such as NaVO3 (7%), maricite NaCoPO4 (1.5%) and NaCoVO4 (1.4%) [22, 23]. Though these impurity phases are thermally stable, they could not influence the overall electrochemical performance of mixed polyanion glass–ceramic cathodes. The broad peaks in all the mixed glass–ceramic cathodes are well indexed corresponding to the major crystalline phase Na2CoP2O7 (sodium cobalt pyrophosphate) with an ordered layered structure indexed to the orthorhombic (P21cn) space group framework which is the most stable structure among all the polymorphs along with retained amorphous nature even after the heat treatment [24, 25]. In addition, some weak peaks are also identified corresponding to the phase NaVO3 (sodium metavanadate). The slight variation in the peak intensity is ascribed due to the influence of polyanion content of these glass–ceramic samples. The refined lattice parameters (a, b, c and cell volume) for orthorhombic phase crystals (P21cn) are calculated and also compared with experimental crystal structures (Table 2). The size of crystallites of all the mixed polyanion glass and glass–ceramic samples was estimated using Williamson–Hall (W–H) equation by taking into account the lattice broadening and strain broadening at full width of half maximum (FWHM) of XRD peaks (Fig. 3) [26].
where β, θ, C, D, ε and λ are the FWHM, Bragg angle, correction factor (C \( \approx 1), \) size of crystallites (nm), lattice strain and the wavelength (0.1540562 nm) of X-ray, respectively. The average crystallite size varies from 118 to 206 nm as a function of ‘x’ (Fig. 3).
Structural illustration
Crystal structure of orthorhombic Na2CoP2O7 (sodium cobalt pyrophosphate; Space group: P21cn) phase was drawn along a, b, c axis using the VESTA software [27] precipitated in NaCo0.7(VO)0.3PO4 glass–ceramic cathode as depicted in Fig. 4. Orthorhombic (Na2CoP2O7) phase is formed by the layer structure with the stacking of {Co(P2O7)−2} slabs separated by sodium ions. Wherein, tetrahedral units of CoO4 and PO4 are interconnected alternatively by the layers that exist along [100] direction (Fig. 4c). It can be clearly seen that oxygen atoms are shared between CoO4 tetrahedral units with four neighbouring P2O7 units. Furthermore, it offers more intra-layer Co–Co distance (7.12 Å) than inter-layer Co–Co distance (5.37 Å), which facilitates two-dimensional Na-ion diffusion pathways to achieve the fast intercalation and de-intercalation phenomena along the a and c directions. Inspired by the significant structural features of orthorhombic phase (Na2CoP2O7) precipitated during the transfer of glass into glass–ceramics at our present investigation, further efforts have been aimed in this paper to monitor the electrochemical performance of all the mixed polyanion glass–ceramic cathode samples and also to target best composition with high electrochemical performance as a potential candidate.
SEM Observations
Figure 5a, b shows SEM micrographs of NaCo0.7(VO)0.3PO4 glass sample before and after heat treatment, respectively. The presence of randomly oriented crystallites in the glass–ceramic surface leads to the formation and the distribution of well-interconnected grains of major phase Na2CoP2O7 (sodium cobalt pyrophosphate) in addition to another secondary phase NaVO3 (sodium metavanadate) with some residual glassy phase which is in good agreement with XRD pattern (Fig. 5b). Hence, the precipitation of crystalline phase structures in the amorphous network during the heat treatment prevents the aggregation of nanocrystallites which paves the way for the improved conducting channels and also for electronic conductivity [28].
Transport properties
As the electrical conductivity plays a vital role in estimating the electrochemical performance, to evaluate the Na+ ion conductivity of all the mixed polyanion glass–ceramic cathodes under investigation, pellets are pressed at 380 MPa with 8 mm diameter for Impedance analysis of samples. The Nyquist plots in Fig. 6 depict low internal resistance for the best mixed polyanion glass–ceramic cathode sample NaCo0.7(VO)0.3PO4 among all the samples under investigation which justifies the better performance of this composition. It is reported that depressed semicircle in the high frequency side stands for the Na+-ion migration across the surface [29, 30]. The inclined line towards the low frequency side presents the Warburg diffusion line. The ionic conductivity is achieved to be 6.41 × 10−7 S cm−1 (Table 3), highest for the sample (NaCo0.7(VO)0.3PO4). This accounts for the well formation of interfacial regions between crystalline and residual glassy matrix, where the large concentration of pairs of CoO6 octahedrons is responsible for hopping of electrons as mentioned in the structural illustration [29, 30]. Furthermore, ordered layer structure polymorph will form in grains and these grains are expected to provide very large open channels for the Na+ ion migration along b-axis which leads to the boosting of ionic conductivity.
Electrochemical analyses
The galvanostatic charge and discharge profiles of each cycle from the first to tenth for the highest conducting glass–ceramics cathode (NaCo0.7(VO)0.3PO4) at fixed current density of 0.1 C rate, as depicted in Fig. 7a and from first to fiftieth cycle for the same sample is more obvious, as confirmed in Fig. 7b. From the Fig. 7a, b it is to be noted that the voltage decreases consistently with increase in sodium insertion into the material. The charge and discharge profile super-curves from first to fiftieth time confirm that this mixed glass–ceramic cathode composition follows the homogeneous phase sodiation mechanism which would be applicable to all the other samples under investigation [31]. The initial discharge capacity for the highest conducting sample NaCo0.7(VO)0.3PO4 is obtained as 93 mA h g−1 in 0.1 C and ~91% of its discharge capacity retention (84 mA h g−1) is shown in Fig. 8 even after 50 cycles which is a remarkable feature for NaCo0.7(VO)0.3PO4 cathode composition (Fig. 8). The cycle performance of NaCo0.7(VO)0.3PO4 cathode is shown in Fig. 8 and discharge capacity is reported as ~65% (60 mA h g−1) even at a high rate of 10 C for the initial discharge. Table 3 summarizes the theoretical and experimental reversible discharge capacities of all the mixed polyanion glass–ceramic samples of NaCo1−x (VO) x PO4 network. Thus, the results have clearly proved that the higher concentration of CoO6 octahedrons enlarges the d-spacing of the sodium diffusion layers of crystalline phase Na2CoP2O7 (sodium cobalt pyrophosphate) upon the substitution of cobalt for vanadium sites which hinders the migration barrier and improves the structure stability due to shrinkage of Co–O and O–O bond lengths which leads to boost up the rate capability and cycle stability as explained in the structure illustration part.
Conclusions
The clear exothermic effect of NaCo1−x (VO) x PO4 glass–ceramic cathodes confirmed that the glass matrix is induced with selective crystallization after the heat treatment at its crystallization temperature. The broad peaks in all the mixed glass–ceramic cathodes are well indexed corresponding to the major crystalline phase Na2CoP2O7 (sodium cobalt pyrophosphate) with an ordered layered structure indexed to the orthorhombic (P21cn) space group framework which is the most stable structure among all the polymorphs. The size of crystallites of all the mixed is polyanion glass–ceramic cathodes vary from 118 to 206 nm which are in good agreement with SEM images. The highest ionic conductivity (6.41 × 10−7 S cm−1) is achieved for NaCo0.7(VO)0.3PO4 cathode which accounts for the well formation of interfacial regions between crystalline and glassy matrix, where the large concentration of pairs of CoO6 octahedrons is responsible for hopping of electrons as mentioned in the structural illustration. The Warburg diffusion line in the Nyquist plots indicates the ionic nature of sodium pyrophosphate conducting phase. The highest initial discharge capacity is achieved to be 93 mA h g−1 in 0.1 C and 91.4% of its discharge capacity retention (84.5 mA h g−1) even after 50 cycles for the highest conducting sample NaCo0.7(VO)0.3PO4, which is a remarkable feature to deliver the operational safety although the net capacity is moderate. Nevertheless, the results achieved for these glass–ceramic compositions are very encouraging and further efforts will be aimed on various glass–ceramic cathode combinations that could encompass the improved capacity for use in large-scale energy storage devices.
References
Nitta N, Wu F, Lee JT, Yushin G (2015) Li-ion battery materials: present and future. Mater Today 18(5):252–264
Takada K (2013) Progress and prospective of solid-state lithium batteries. Acta Mater 61(3):759–770
Tarascon JM (2010) Key challenges in future Li-battery research. Philos Trans R Soc A 368(1923):3227–3241
Palomares V, Serras P, Villaluenga I, Hueso KB, Carretero-González J, Rojo T (2012) Na-ion batteries, recent advances and present challenges to become low cost energy storage systems. Energy Environ Sci 5(3):5884–5901
Pan H, Hu YS, Chen L (2013) Room-temperature stationary sodium-ion batteries for large-scale electric energy storage. Energy Environ Sci 6(8):2338–2360
Ma X, Chen H, Ceder G (2011) Electrochemical properties of monoclinic NaMnO2. J Electrochem Soc 158(12):A1307–A1312
Johnson C (2016). Synthesis and evaluation of NaMPO4 (M = Fe, Mn, Co) framework polyanion cathodes for sodium-ion batteries (SIB). In: 18th international meeting on lithium batteries, Ecs, 19–24 June
Zaghib K, Trottier J, Hovington P, Brochu F, Guerfi A, Mauger A, Julien CM (2011) Characterization of Na-based phosphate as electrode materials for electrochemical cells. J Power Sources 196(22):9612–9617
Zhu Y, Xu Y, Liu Y, Luo C, Wang C (2013) Comparison of electrochemical performances of olivine NaFePO4 in sodium-ion batteries and olivine LiFePO4 in lithium-ion batteries. Nanoscale 5(2):780–787
Li ZY, Zhang J, Gao R, Zhang H, Hu Z, Liu X (2016) Unveiling the role of Co in improving the high-rate capability and cycling performance of layered Na0.7Mn0. 7Ni0.3-xCoxO2 cathode materials for sodium ion batteries. ACS Appl Mater Interfaces. doi:10.1021/acsami.6b04073
Amine K, Yasuda H, Yamachi M (2000) Olivine LiCoPO4 as 4.8 V electrode material for lithium batteries. Electrochem Solid State Lett 3(4):178–179
Okada S, Sawa SI, Uebou Y, Egashira M, Yamaki JI, Tabuchi M, Kageyama H (2003) Charge-discharge mechanism of LiCoPO4 cathode for rechargeable lithium batteries. Electrochemistry 71(12):1136–1138
Xu J, Lee DH, Clément RJ, Yu X, Leskes M, Pell AJ, Meng YS (2014) Identifying the critical role of Li substitution in P2–Nax[LiyNizMn1–y–z] O2 (0 < x, y, z < 1) intercalation cathode materials for high-energy Na-ion batteries. Chem Mater 26(2):1260–1269
Chen CY, Matsumoto K, Nohira T, Hagiwara R (2014) Na2MnSiO4 as a positive electrode material for sodium secondary batteries using an ionic liquid electrolyte. Electrochem Commun 45:63–66
Kercher AK, Ramey JO, Carroll KJ, Kiggans JO, Dudney NJ, Meisner RA, Veith GM (2014) Mixed polyanion glass cathodes: iron phosphate vanadate glasses. J Electrochem Soc 161(14):A2210–A2215
Kercher AK, Kolopus JA, Carroll KJ, Unocic RR, Kirklin S, Wolverton C, Dudney NJ (2016) Mixed polyanion glass cathodes: glass-state conversion reactions. J Electrochem Soc 163(2):A131–A137
Aoyagi T, Fujieda T, Mitsuishi K, Kawaji J, Toyama T, Kono K, Naito T (2014) V2O5-P2O5-Fe2O3-Li2O glass-ceramics as high-capacity cathode for lithium-ion batteries. Mater Res Soc Symp Proc 1643:13–1643. doi:10.1557/opl.2014.246
Barpanda P, Ye T, Nishimura SI, Chung SC, Yamada Y, Okubo M, Yamada A (2012) Sodium iron pyrophosphate: a novel 3.0 V iron-based cathode for sodium-ion batteries. J Electrochem Commun 24:116–119
Murugan GS, Varma KBR (2002) Lithium borate–strontium bismuth tantalate glass nanocomposite: a novel material for nonlinear optic and ferroelectric applications. J Mater Chem 12(5):1426–1436
Delaizir G, Seznec V, Rozier P, Surcin C, Salles P, Dolle M (2013) Electrochemical performances of vitreous materials in the system Li2O–V2O5–P2O5 as electrode for lithium batteries. Solid State Ionics 237:22–27
Hassaan MY, Salem SM, Moustafa MG (2014) Study of nanostructure and ionic conductivity of Li1.3Nb0.3V1.7(PO4)3 glass ceramics used as cathode material for solid batteries. J Non-Cryst Solids 391:6–11
Rietveld H (1969) A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr 2(2):65–71
Rietveld HM (1967) Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr 22(1):151–152
Erragh F, Boukhari A, Elouadi B, Holt EM (1991) Crystal structures of two allotropic forms of Na2CoP2O7. J Cryst Spectrosc 21(3):321–326
Sanz F, Parada C, Rojo JM, Ruiz-Valero C, Saez-Puche R (1999) Studies on tetragonal Na2CoP2O7, a novel ionic conductor. J Solid State Chem 145(2):604–611
Williamson GK, Hall WH (1953) X-ray line broadening from filed aluminium and wolfram. Acta Metall Mater 1(1):22–31
Momma K, Izumi F (2011) VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J Appl Crystallogr 44(6):1272–1276
Honma T, Sato A, Ito N, Togashi T, Shinozaki K, Komatsu T (2014) Crystallization behavior of sodium iron phosphate glass Na2-xFe1+0.5xP2O7 for sodium ion batteries. J Non-Cryst Solids 404:26–31
Chowdari BVR, Rao GS, Lee GYH (2000) XPS and ionic conductivity studies on Li2O–Al2O3–(TiO2 or GeO2)–P2O5 glass–ceramics. Solid State Ionics 136:1067–1075
Zhang Q, Wen Z, Liu Y, Song S, Wu X (2009) Na+ ion conductors of glass–ceramics in the system Na1+xAlxGe2−xP3O12 (0.3 ≤ x ≤ 1.0). J Alloys Compd 479(1):494–499
Afyon S, Krumeich F, Mensing C, Borgschulte A, Nesper R (2014) New high capacity cathode materials for rechargeable Li-ion batteries: vanadate-borate glasses. Sci Rep 4:7113. doi:10.1038/srep07
Acknowledgements
This work has been funded by NAVAL RESEARCH BOARD, DRDO, Govt. of INDIA, Grant No: NRB-311/MAT/13-14. The authors thank Dr. S.K. Martha, Department of Chemistry, IIT Hyderabad for his kind help in acquiring CV studies and discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
R. Balaji Rao has received Grants from NAVAL RESEARCH BOARD, DRDO, Govt. of INDIA, Grant No: NRB-311/MAT/13-14.
Rights and permissions
About this article
Cite this article
Suman, G., Rao, C.S., Ojha, P.K. et al. Mixed polyanion NaCo1−x (VO) x PO4 glass–ceramic cathode: role of ‘Co’ on structural behaviour and electrochemical performance. J Mater Sci 52, 5038–5047 (2017). https://doi.org/10.1007/s10853-016-0741-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0741-7