Abstract
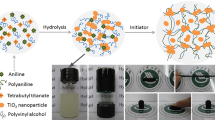
In the present work, by using the in situ polymerization of aniline in acidic aqueous solution of PVA and freezing–thawing method, the reinforced conducting polymer hydrogel of polyaniline/polyvinyl alcohol (PANI/PVA) was prepared and directly used as the self-supported electrode for supercapacitor. The chemical structure of PANI/PVA hydrogel was characterized by UV–Vis, and the three-dimensional (3D) interconnected hierarchical nanoporous structure was observed by scanning electron microscopy. The measurement of compressive strength demonstrated the high mechanical strength of the hydrogel. The electrochemical properties of PANI/PVA hydrogel electrodes were evaluated by using cyclic voltammetry, electrochemical impedance spectroscopy and galvanostatic charge/discharge, which indicated their good responsiveness and rate capability, low resistance, high specific capacitance (240 F/g at current density of 1 A/g) and excellent cycling stability. The easily fabricated PANI/PVA hydrogel combined hierarchical nanoporous microstructure, self-supported feature and favorable capacitive behavior and provided a new strategy for constructing high-performance electrode for supercapacitors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Supercapacitors, a type of novel device of electrochemical energy storage, have attached more and more attentions in recent 10 years [1–4]. Due to the high power density, long recycle life and fast charging and discharging, the supercapacitors have achieved wide range of applications in electromobile, portable electronic products and uninterrupted power supply [4, 5]. In the system of supercapacitors, the electrode materials are the most important factor for the performance of the device, and it is well known that the electrode materials with nanostructure are considerably necessary for the high charge/discharge capacities due to fast kinetics, efficient contact with electrolyte ions and abundant electroactive sites for energy storage [5]. Moreover, at present, the bulk electrode for supercapacitor is commonly prepared by mixing the active materials, bonders and conductive additives and compressing the mixture under high pressure [6, 7], which not only would bring contact resistance, but also is considered to be a relatively complicated process. It is reported that developing self-supported electrodes without bonders and additives is an effective way to increase the energy density of supercapacitor [8, 9]. Due to the 3D interconnected hierarchical nanostructure, large specific surface and fast electron/ion transportation, conducting hydrogels are the ideal materials for self-supported electrode of supercapacitors. The graphene-based conducting hydrogel is a candidate of self-supported electrode for the 3D nanoporous structure [10–12]. Unfortunately, the graphene-based supercapacitors are known as electric double-layer capacitors, whose specific capacitance is generally considered lower the pseudocapacitors, e.g., conducting polymers. Thus, the self-supported electrodes of conducting polymer hydrogels are greatly developed due to the high theoretical specific capacitance, low cost and facile fabrication.
Conducting polymer hydrogels are used to be fabricated by two-step method [13–15], including the preparation of non-conductive hydrogels and subsequent compositing with conducting polymer. This process not only leads to a complicated preparation and high cost, but also is difficult to achieve high loading of conducting polymer and uniform composite structure. Considering these shortcomings of two-step method, the one-pot process to fabricate conducting polymer hydrogels has been developed. The reported conducting polymer hydrogels could be composed of a single component, such as the hydrogels of polyaniline and polypyrrole [16, 17], or a complex system, such as the composite hydrogels of polyaniline/phytic acid [18, 19], polypyrrole/phytic acid [20], polyaniline/poly(styrene sulfonate) [21] and polypyrrole/Prussian blue [22]. They all showed favorable electrochemical behaviors. However, most of the above conducting hydrogels suffer from considerably poor mechanical strength that slight agitation can lead to the collapse of the hydrogels. Our group has fabricated a reinforced hydrogel of polyaniline/sodium alginate (PANI/SA) resulting from the entanglement of the polyaniline and sodium alginate molecular chains [23], but the mechanical strength of the hydrogel will also decrease after 1 week due to the inherent character of degradation of sodium alginate in the water. Lu et al. [24] prepared an elastic polypyrrole hydrogel; nevertheless, it would not turn to be elastic state unless it has been placed at room temperature for more than 30 days. An elastic polypyrrole nanotube aerogel was also fabricated, but the template and cross-linker have to be adopted [25]. Thus, it is still a challenge to develop more novel reinforced conducting polymer hydrogels with facile synthetic process for self-supported electrode materials.
In this paper, the conducting polymer hydrogel of polyaniline/polyvinyl alcohol (PANI/PVA) with high mechanical strength and typical hierarchical nanoporous structure was facilely prepared by using in situ polymerization of aniline in an acidic aqueous solution of PVA and the freezing–thawing method. The as-prepared PANI/PVA hydrogel can be used as self-supported electrodes for supercapacitors and shows favorable capacitive behaviors. It provides a new way to construct ideal electrode materials for supercapacitors and actuators.
Experimental section
Materials
Polyvinyl alcohol (PVA, analytically pure, 1788) was purchased from Aladdin Industrial Inc. Aniline (An, chemically pure) was purchased from Sinopharm Chemical Reagent Co. and was distilled before use. Ammonium persulphate (APS, analytically pure) was purchased from Shanghai Chemical Co. and used as received. All other chemicals and solvents were of analytical grade and used as received.
Preparation of PANI/PVA conducting polymer hydrogels
In a water bath, 6 g of PVA was added to 100 mL of deionized water under heating and stirring for 2 h to obtain a fully dissolved solution. After the solution was cooled to room temperature, 10 mL concentrated hydrochloric acid (36–38 wt%) and a known amount of An were added. Then, in an ice-water bath, the APS with the same molar weight of An was added to the above solution under fast stirring. Subsequently, the mixture was subjected to three cycles of freeze–thawing, in which the mixture was frozen for 12 h at −20 °C, and then thawed for 12 h at room temperature as one cycle. The hydrogel formed by the above freeze–thawing method was immersed into a large amount of deionized water for 3 days to remove inorganic impurities and oligoaniline, and the deionized water was changed every 12 h. Finally, the PANI/PVA conducting polymer with high mechanical strength was obtained. As a reference, the pure PVA hydrogel was also prepared by the same procedure without adding HCL, An and APS.
Characterization
UV–Vis spectroscopic measurements were taken with a UV–Vis spectrophotometer (TU-1810, Beijing Pushi General Co.) using deionized water as the solvent. The morphologies of the conducting polymer hydrogels were observed by a field emission scanning electron microscope (FE-SEM, Sirion, FEI) at an acceleration voltage of 5 kV. The compressive strength of hydrogels was measured by using an electromechanical universal testing machine (CMT-4104, SANS) at room temperature. The cylindrical samples (φ15 × 10 mm) were placed between two plates, and the compression speed was 2 mm/min.
Electrochemical properties
The as-prepared PANI/PVA hydrogels were cut into slices (1.0 × 1.0 cm) and pressed onto a stainless steel mesh. Then, the hydrogel slices were immersed in H2SO4 aqueous solution (1 mol/L) for 24 h and used as working electrodes. The electrochemical tests were performed through the three-electrode system in H2SO4 aqueous solution (1 mol/L). The Pt wire and saturated calomel electrode were used as the counter electrode and reference electrode, respectively. The cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and galvanostatic charge/discharge (GCD) were performed on an electrochemical workstation (CHI, 660D). The CV curves were collected in the potential window of −0.2–0.9 V, and four scan rates at 10, 50, 100 and 200 mV/s were used. The frequency of EIS was in the range of 100 kHz–0.01 Hz, and perturbation AC voltage was 10 mV. The GCD measurements were taken with varying voltages ranging from 0 to 0.8 V and at the current densities of 1, 2, 3 and 5 A/g. All of the above electrochemical tests were carried out at room temperature.
Results and discussion
In the present work, An was firstly dissolved in the acidic aqueous solution of PVA. After the oxidant APS was added, the freezing–thawing method, as described in experimental section, was immediately used to allow the mixture to form hydrogel. In this freezing–thawing process, the microcrystalline domains, viz., physical crossing points, were generated and the three-dimensional network, viz., hydrogel, was further formed. Meanwhile, the PANI was obtained through the in situ polymerized of An and doped by hydrochloric acid. Thus, the PANI/PVA conducting hydrogels were prepared. Figure 1 presents the UV–Vis spectra of PVA and PANI/PVA hydrogel using deionized water as solvent. The PVA does not show any obvious absorption band in the range of 200–1000 nm. In the UV–Vis spectrum of PANI/PVA hydrogel, three obvious absorption bands at 336, 430 and 805 nm can be seen. Thus, these bands must be attributed to the PANI in situ polymerized in the hydrogel. The band at 336 nm is assigned to the π–π* transition of the benzene ring, and the bands at 430 and 805 nm are ascribed to the polaron band π–π* transition and the π to the localized polaron band of doped PANI, respectively [23, 26]. The results of the UV–Vis spectra confirm that the PANI is formed by using in situ polymerization and exists in the hydrogel as conductive emeraldine salt.
Figure 2 shows the FE-SEM images of PVA and PANI/PVA hydrogel. It can be found that the pure PVA hydrogel exhibits a typical nanoporous morphology with pore size from several micrometers to nanometers (Fig. 2a). In the case of PANI/PVA hydrogel, the microstructure not only maintains the nanoporous morphology formed by PVA chains, but also contains nanoaggregates formed by PANI chains, which exhibit random morphologies including nanorods, nanosheets and nanoparticles 50–300 nm in size (Fig. 2b). Because the aniline monomer was added to the acidic solution of PVA to obtain a fully dissolved mixture and underwent in situ polymerization, accompanied with freezing–thawing gelation of PVA, the PANI nanoaggregates were uniformly dispersed within the hydrogel and intercrossed with the PVA nanonetworks to form a hierarchical nanoporous microstructure, which can provide large specific surface and ion/electron transmission channel for electrochemical reactions within the PANI/PVA hydrogel.
The aqueous solution of PVA can form hydrogel through the freezing–thawing process, in which the hydrogen bond and microcrystalline domains between PVA chains are generated. The formed PVA hydrogel owns the ability to resist the external force and maintain the gel state with good deformability [27–29]. Thus, it is expected that the conducting hydrogel of PANI/PVA fabricated by this freezing–thawing method would possess high mechanical strength, which is very important for the application of the hydrogels. Herein, the excellent mechanical strength of the as-prepared PANI/PVA hydrogel can be directly seen from that it can withstand a large external force (Fig. 3a). It is also noted that the mechanical strength of hydrogels can be enhanced with the increase in freezing–thawing cycles. In order to achieve high mechanical strength and appropriate experimental period, all hydrogels are prepared through three freezing–thawing cycles. Figure 3b shows the diagrams of compressive stress/strain of the as-prepared hydrogels. Owing the limited freezing–thawing cycles, the compressive strength of pure PVA hydrogel is relatively poor, lower than 0.2 MPa. By contrast, the mechanical strength of PANI/PVA hydrogel has greatly improved, and the larger amount of An used, the higher mechanical strength of hydrogels. For instance, the compressive strengths of the PANI/PVA hydrogels prepared with 0.4 and 0.8 mol/L of An in the original PVA solution are 0.62 and 3.38 MPa, 3.4 and 18.7 times higher than that of pure PVA hydrogel, respectively. Comparing with this PANI/PVA hydrogel, the compressive strengths of reported conducting hydrogels are indeed relatively low, such as triple-network hydrogel (1.8 MPa) [30], PANI/SA hydrogel (41 kPa) [23] and the other brittle hydrogels [16, 18, 20, 21]. Therefore, the results suggest that the PANI nanoaggregates in situ polymerized within the hydrogel networks, as shown in the SEM images in Fig. 2, not only endow the hydrogel with electrochemical properties, but also lead to an increase in the hydrogel strength [15]. The synergy between the microcrystalline of PVA and the reinforcement of PANI nanoaggregates endows the PANI/PVA hydrogel favorable mechanical strength (Fig. 3c).
Considering the inherent faradic pseudocapacitance of PANI and the reinforced 3D interconnected structure, the PANI/PVA hydrogel is used as self-supported electrode for supercapacitors. For the electrode materials, the hierarchical nanoporous structure of PANI/PVA hydrogel can provide large specific surface for the electrochemical reactions and can be greatly beneficial to the ion exchange and electron transmission between active materials and electrolyte. In addition, because the supercapacitor electrodes could undergo expansion and shrinkage in the processes of charging and discharging, the excellent mechanical strength of PANI/PVA hydrogel is also distinctly important for the recycle life of electrode materials. Thus, the self-supported electrodes were prepared through directly pressing the PANI/PVA hydrogels onto a stainless steel mesh, and the electrochemical performance was evaluated by means of cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and galvanostatic charge/discharge (GCD).
Figure 4 shows the CV curves of PANI/PVA hydrogel electrode with varying scan rate at 10, 50, 100 and 200 mV/s in aqueous solution of H2SO4 (1 mol/L). It can be seen that two pairs of redox peaks appeared in the scan range of −0.2–0.9 V. Herein, the two pairs of redox peaks observed are assigned to the transition between the leucoemeraldine base state of PANI to an emeraldine state and a further transition to a pernigraniline state [31, 32], which suggest the pseudocapacitance feature of PANI/PVA hydrogels. Moreover, in the CV curves, the obvious increase of peak currents with the increase of scan rates indicates the good responsiveness and rate capability [27, 33]. These favorable capacitive behaviors could be attributed to hierarchical nanoporous structure of PANI/PVA hydrogel electrode which facilitates the ion exchange and electron transmission [34]. Meanwhile, because the hydrogel electrodes are made up of 3D interconnected networks and prepared without binders and conductive fillers, the contact resistance is also avoided.
Figure 5 shows the EIS spectra of PANI/PVA hydrogel electrodes prepared with 0.4 and 0.8 mol/L of An in the original PVA solution. The semicircles can be seen in the both EIS spectra at high-frequency region, and the bigger diameter of the semicircle corresponds to the higher charge-transfer resistance of the electrode [35, 36]. For instance, when the PANI/PVA hydrogel was prepared with increasing the An amount from 0.4 to 0.8 mol/L, a distinct decrease of the diameter of the semicircle indicates that the resistance of the electrode declined significantly. Generally, the EIS spectra in the low-frequency region show a straight line, whose slope can indicate the capacitive behavior of the electrode materials. The more vertical the straight line, the more closely the supercapacitor behaves as an ideal capacitor [6, 37]. Thus, from the EIS spectra in the low-frequency region, it indicates that the hydrogel prepared with 0.8 mol/L of An possesses better supercapacitor behavior than the hydrogel prepared with 0.4 mol/L of An. It originated from the high content of PANI in situ polymerized in the hydrogel system. On the one hand, the capacitance of the hydrogel electrode is derived from the pseudocapacitance of PANI, the higher content of PANI, the better supercapacitor behavior of hydrogel electrode. On the other hand, the higher content of PANI contributes to lower resistance as indicated by the diameter of semicircle in high-frequency region, which can be beneficial to the charge transport in electrode to enhance supercapacitor behavior. In this work, 0.8 mol/L was the largest amount of An, because in the case of too large amount of An, the in situ polymerization is so fast that the synthetic processes of hydrogels are out of control.
Figure 6a, b is the GCD curves of PANI/PVA hydrogel electrodes at current densities of 1, 2, 3 and 5 A/g. According to the discharging time, the specific capacitance of electrode can be calculated through the following formula [38]:
where C is the specific capacitance of the hydrogel electrode (F/g), I/m is the current density (A/g), t is the discharging time(s), and ∆V is 0.8 V. The values of the specific capacitance for the hydrogel electrodes at different current densities are shown in Fig. 6c. At the situation of high current density, the relatively slow speed of the diffusion of electrolyte ions leads to an uncompleted doping and dedoping of PANI of PANI/PVA hydrogel electrode. That is, only part of active materials in the electrode undergo the processes of charging and discharging, resulting in a decrease of utilization rate of the electrode materials [39, 40]. Thus, the values of the specific capacitance for the hydrogel electrodes declined with promoting the current density (Fig. 6c). For the hydrogel electrode prepared with 0.4 mol/L of An, the unsatisfactory capacitance was obtained. At current densities of 1, 2, 3 and 5 A/g, the specific capacitances were as small as 105, 85, 55 and 37 F/g, respectively. When the amount of An used was increased to 0.8 mol/L, an obvious increase of specific capacitance can be seen. For instance, the specific capacitance with a current density at 1 A/g significantly increased to 240 F/g, which is 2.3 times higher than the former. It is due to the low resistance of the hydrogel electrode prepared with larger amount of An (Fig. 5), contributing to the electron/ion transportation and electrochemical redox reaction. At the situation of 1 A/g of current density and 0.8 mol/L of An, the specific capacitance (240 F/g) of PANI/PVA hydrogel electrode is much higher than that of most carbon materials whose capacitances are commonly below 200 F/g [5], which can be attributed to the hierarchical nanoporous and self-supported structure, and pseudocapacitance feature. Although the capacitance of PANI/PVA hydrogel is equivalent to that of reported composite conducting hydrogel, such as PANI/PSS and PANI/SA hydrogels [21, 23], even lower than that of pure polyaniline and polypyrrole hydrogels [16, 20], it still has great potential in the fields of flexible supercapacitor, actuators and soft tissue engineering scaffolds due to the facile preparation and excellent mechanical strength.
The cycling stability is an important performance for the electrode materials for supercapacitors. On account of the expansion and shrinkage in cycles of charge/discharge, the mechanical strength and faradic-capacitive performance of the polymeric electrode materials may degrade after multiple cycles [5, 18], leading to a bad cycling stability. Figure 7 presents the cycling stability of PANI/PVA hydrogel electrode prepared with 0.8 mol/L of An at the current density of 3 A/g. The retention ratio of 79 % after 1000 cycles, which matches with the pellet electrode prepared with PANI electroactive materials, acetylene black and polytetrafluoroethylene [21, 40], indicates the excellent cycling stability of the as-prepared PANI/PVA hydrogel electrode. It is attributed to the hierarchical nanoporous structure and high mechanical strength of the hydrogel (Figs. 2, 3), which can efficiently overcome the expansion and shrinkage in cycles of charge/discharge [22, 41].
According to the above evaluation of electrochemical performance of the self-supported PANI/PVA hydrogel, the mechanism of its favorable supercapacitor behavior can be illustrated in Fig. 8. On the one hand, the excellent mechanical strength (Fig. 3), which is ascribed to the synergy between the microcrystalline of PVA and the reinforcement of PANI nanoaggregates, endows PANI/PVA hydrogel with self-supported feature and facilitates the charge/discharge cycling stability of the hydrogel electrode (Fig. 7). On the other hand, the hierarchical nanoporous microstructure (Fig. 2) can provide large specific surface for the thorough electrochemical redox (faradic pseudocapacitance) and sufficient channel for the fast transportation of electron and electrolyte ions, which is considerably beneficial for the favorable electrochemical performance, including the high specific capacitance (Fig. 6) and good rate capability (Fig. 4). Moreover, this self-supported electrode is such a continuous conductive network without binders and additives that the charge-transfer resistance is very low (Fig. 5). Therefore, the PANI/PVA hydrogel possesses favorable electrochemical performance due to the excellent mechanical strength, hierarchical nanoporous microstructure and self-supported feature, which are crucial important for the high-performance supercapacitor electrode.
Conclusions
In this work, the conducting polymer hydrogel of PANI/PVA was prepared via in situ polymerization and freezing–thawing method. The easily fabricated hydrogel with high mechanical strength and typical 3D interconnected hierarchical nanoporous structure can be directly used as self-supported electrode for supercapacitors and shows favorable capacitive behaviors. The microstructure and chemical structure were analyzed by using SEM and UV–Vis, and the electrochemical study of cyclic voltammetry, electrochemical impedance spectroscopy and galvanostatic charge/discharge indicated the good responsiveness and rate capability, low resistance, high specific capacitance (240 F/g at a current density of 1 A/g) and excellent cycling stability of PANI/PVA hydrogel electrode. The self-supported PANI/PVA hydrogel with hierarchical nanoporous structure and favorable electrochemical performance shows great potential as ideal electrode materials for supercapacitors.
References
Miller JR, Simon P (2008) Electrochemical capacitors for energy management. Science 321:651–652
Simon P, Gogotsi Y (2008) Materials for electrochemical capacitors. Nat Mater 7:845–854
Armaroli N, Balzani V (2011) Towards an electricity-powered world. Energy Environ Sci 4:3193–3222
Ji J, Zhang X, Liu J, Peng L, Chen C, Huang Z, Li L, Yu X, Shang S (2015) Assembly of polypyrrole nanotube@ MnO2 composites with an improved electrochemical capacitance. Mater Sci Eng B 198:51–56
Wang GP, Zhang L, Zhang JJ (2012) A review of electrode materials for electrochemical supercapacitors. Chem Soc Rev 41:797–828
Huang H, Yao J, Chen H, Zeng X, Chen C, She X, Li L (2016) Facile preparation of halloysite/polyaniline nanocomposites via in situ polymerization and layer-by-layer assembly with good supercapacitor performance. J Mater Sci 51:4047–4054. doi:10.1007/s10853-016-9724-y
Li N, Tang S, Dai Y, Meng X (2014) The synthesis of graphene oxide nanostructures for supercapacitors: a simple route. J Mater Sci 49:2802–2809. doi:10.1007/s10853-013-7986-1
Wang J, Shen L, Nie P, Yun X, Xu Y, Dou H, Zhang X (2015) N-doped carbon foam based three-dimensional electrode architectures and asymmetric supercapacitors. J Mater Chem A 3:2853–2860
Zhang L, Shi GQ (2011) Preparation of highly conductive graphene hydrogels for fabricating supercapacitors with high rate capability. J Phys Chem C 115:17206–17212
Xu Y, Lin Z, Huang X, Wang Y, Huang Y, Duan X (2013) Functionalized graphene hydrogel-based high-performance supercapacitors. Adv Mater 25:5779–5784
Chen S, Duan J, Tang Y, Zhang Qiao S (2013) Hybrid hydrogels of porous graphene and nickel hydroxide as advanced supercapacitor materials. Chem Eur J 19:7118–7124
Peng Z, Lin J, Ye R, Samuel ELG, Tour JM (2015) Flexible and stackable laser-induced graphene supercapacitors. ACS Appl Mater Interfaces 7:3414–3419
Siddhanta SK, Gangopadhyay R (2005) Conducting polymer gel: formation of a novel semi-IPN from polyaniline and crosslinked poly(2-acrylamido-2-methyl propanesulphonicacid). Polymer 46:2993–3000
Tang Q, Wu J, Sun H, Lin J, Fan S, Hu D (2008) Polyaniline/polyacrylamide conducting composite hydrogel with a porous structure. Carbohydr Polym 74:215–219
Xia YY, Zhu HL (2011) Polyaniline nanofiber-reinforced conducting hydrogel with unique pH-sensitivity. Soft Matter 7:9388–9393
Guo H, He W, Lu Y, Zhang X (2015) Self-crosslinked polyaniline hydrogel electrodes for electrochemical energy storage. Carbon 92:133–141
Wei D, Lin X, Li L, Shang S, Yuen MC, Yan G, Yu X (2013) Controlled growth of polypyrrole hydrogels. Soft Matter 9:2832–2836
Pan L, Yu G, Zhai D, Lee HR, Zhao W, Liu N, Wang H, Tee BCK, Shi Y, Cui Y, Bao Z (2012) Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc Natl Acad Sci USA 109:9287–9292
Wang K, Zhang X, Li C, Zhang H, Sun X, Xu N, Ma Y (2014) Flexible solid-state supercapacitors based on a conducting polymer hydrogel with enhanced electrochemical performance. J Mater Chem A 2:19726–19732
Shi Y, Pan L, Liu B, Wang Y, Cui Y, Bao Z, Yu G (2014) Nanostructured conductive polypyrrole hydrogels as high-performance, flexible supercapacitor electrodes. J Mater Chem A 2:6086–6091
Dai T, Jia Y (2011) Supramolecular hydrogels of polyaniline-poly(styrene sulfonate) prepared in concentrated solutions. Polymer 52:2550–2558
Tuo X, Li B, Chen C, Huang Z, Huang H, Li L, Yu X (2016) Facile assembly of polypyrrole/Prussian blue aerogels for hydrogen peroxide reduction. Synth Met 213:73–77
Huang H, Zeng X, Li W, Wang H, Wang Q, Yang Y (2014) Reinforced conducting hydrogels prepared from the in situ polymerization of aniline in an aqueous solution of sodium alginate. J Mater Chem A 2:16516–16522
Lu Y, He W, Cao T, Guo H, Zhang Y, Li Q, Shao Z, Cui Y, Zhang X (2014) Elastic, conductive, polymeric hydrogels and sponges. Sci Rep UK 4:5792
Ying S, Zheng W, Li B, She X, Huang H, Li L, Huang Z, Huang Y, Liu Z, Yu X (2016) Facile fabrication of elastic conducting polypyrrole nanotube aerogels. Synth Met 218:50–55
Adhikari S, Banerji P (2009) Polyaniline composite by in situ polymerization on a swollen PVA gel. Synth Met 159:2519–2524
Abitbol T, Johnstone T, Quinn TM, Gray DG (2011) Reinforcement with cellulose nanocrystals of poly(vinyl alcohol) hydrogels prepared by cyclic freezing and thawing. Soft Matter 7:2373–2379
Jiang H, Zuo Y, Zhang L, Li J, Zhang A, Li Y, Yang X (2014) Property-based design: optimization and characterization of polyvinyl alcohol (PVA) hydrogel and PVA-matrix composite for artificial cornea. J Mater Sci 25:941–952. doi:10.1007/s10856-013-5121-0
Hatakeyema T, Uno J, Yamada C, Kishi A, Hatakeyama H (2005) Gel-Sol transition of poly(vinyl alcohol) hydrogels formed by freezing and thawing. Thermochim Acta 431:144–148
Dai T, Qing X, Lu Y, Xia Y (2009) Conducting hydrogels with enhanced mechanical strength. Polymer 50:5236–5241
Kotal M, Thakur AK, Bhowmick AK (2013) Polyaniline–carbon nanofiber composite by a chemical grafting approach and its supercapacitor application. ACS Appl Mater Interfaces 5:8374–8386
Bhadra S, Khastgir D, Singha NK, Lee JH (2009) Progress in preparation, processing and applications of polyaniline. Prog Polym Sci 34:783–810
Li M, Xue J (2014) Integrated synthesis of nitrogen-doped mesoporous carbon from melamine resins with superior performance in supercapacitors. J Phys Chem C 118:2507–2517
Ouyang W, Sun J, Memon J, Wang C, Geng J, Huang Y (2013) Scalable preparation of three-dimensional porous structures of reduced graphene oxide/cellulose composites and their application in supercapacitors. Carbon 62:501–509
Li W, Zeng X, Wang H, Wang Q, Yang Y (2015) Polyaniline-poly (styrene sulfonate) conducting hydrogels reinforced by supramolecular nanofibers and used as drug carriers with electric-driven release. Eur Polym J 66:513–519
Saha S, Schön EM, Cativiela C, Díaz Díaz D, Banerjee R (2013) Proton-conducting supramolecular metallogels from the lowest molecular weight assembler ligand: a quote for simplicity. Chem Eur J 19:9562–9568
Sarker AK, Hong JD (2012) Layer-by-layer self-assembled multilayer films composed of graphene/polyaniline bilayers: high-energy electrode materials for supercapacitors. Langmuir 28:12637–12646
Wang YG, Li HQ, Xia YY (2006) Ordered whiskerlike polyaniline grown on the surface of mesoporous carbon and its electrochemical capacitance performance. Adv Mater 18:2619–2623
She X, Zhang X, Liu J, Li L, Yu X, Huang Z, Shang S (2015) Microwave-assisted synthesis of Mn3O4 nanoparticles@ reduced graphene oxide nanocomposites for high performance supercapacitors. Mater Res Bull 70:945–950
Miao YE, Fan W, Chen D, Liu T (2013) High-performance supercapacitors based on hollow polyaniline nanofibers by electrospinning. ACS Appl Mater Interfaces 5:4423–4428
Xiong P, Huang H, Wang X (2014) Design and synthesis of ternary cobalt ferrite/graphene/polyaniline hierarchical nanocomposites for high-performance supercapacitors. J Power Sources 245:937–946
Acknowledgements
The work was supported by Outstanding Youth Scientific Innovation Team of Colleges and Universities in Hubei Province (T201406), National Natural Science Foundation of China (51403167, 51374155), Science and Technology Support Program of Hubei Province (2014BCB034) and Scientific Research Foundation of Wuhan Institute of Technology (K201508).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Huang, H., Yao, J., Li, L. et al. Reinforced polyaniline/polyvinyl alcohol conducting hydrogel from a freezing–thawing method as self-supported electrode for supercapacitors. J Mater Sci 51, 8728–8736 (2016). https://doi.org/10.1007/s10853-016-0137-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-016-0137-8