Abstract
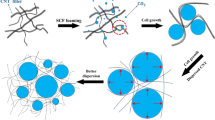
The high-density polyethylene (HDPE) nanocomposites were prepared by using graphite nanosheets (GNS) and expanded graphite (EG), followed by foaming with subcritical CO2 used as an environmentally benign and nonflammable foaming agent. The partially exfoliated GNS and EG endow the prepared microcellular nanocomposite foams with high electrical conductivity, improved mechanical properties, as well as density reduction up to ca. 20 %. Interestingly, insulator-to-semiconductor transition of microcellular nanocomposite foams shifts to lower nanofiller content compared to that of bulk nanocomposites. Whether the nanofiller is GNS or EG, its incorporation leads to uniformly small cells, resulting in a remarkable enhancement in ductility without sacrificing toughness. It has demonstrated that foaming of HDPE nanocomposites with EG or GNS provides tough and lightweight microcellular foams, exhibiting the potential for use in conductive high-performance lightweight nanocomposite systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymer foams are extensively used in a wide range of applications from packaging to tissue engineering. The growing trend of their applications has led researchers around the world to use such materials tailor-made for specific demands in diverse applications [1]. They benefit from several inherent advantages such as material saving, light weight, high specific strength, low thermal conductivity, as well as good acoustic properties. Considering extensive practical applications of polymer foams in electronic devices, the fabrication and development of electrically conductive foams with low cost and high performance have drawn a great deal of attention [2].
Incorporation of conducting nanofillers such as metal powders, carbon black, carbon nanotubes (CNTs), graphene, etc. into polymer foams is a straightforward route for preparing electrically conducting composite foams [3–6]. The electrical conductivity of nanocomposite foams is significantly affected by intrinsic conductivity, aspect ratio, and content of the nanofillers [4]. It has been shown that the high aspect ratio and good electrical conductivity of graphene sheets and CNTs not only improve their electrical performance, but also increase their mechanical strength as well as thermal stability [7]. Recently, by the incorporation of CNTs, nanocomposite foams based on polyurethane [8], polycaprolactone [9], and polystyrene [10] were prepared, which showed excellent electrical conductivity. In addition, Zhang et al. [4] prepared tough polymethylmethacrylate/graphene foams and observed good electrical conductivity along with mechanical properties. Furthermore, Ling et al. [11] produced lightweight microcellular polyetherimide/graphene with significantly increased electrical and low thermal conductivities. Although highly efficient nanocomposite foams have been prepared based on CNTs and graphene, high cost, high tendency for aggregation, and impurities from the additives (as reactant) strictly limit their usage. As applicable alternative candidates for the aforementioned nanofillers, expanded graphite (EG) and graphite nanaosheets (GNSs) due in particular to their low cost offer more advantages. For example, the addition of EG to poly(methyl methacrylate) [12], polyurethane [13], and polyester [14] improves electrical and mechanical properties.
In the light of above discussion, the present study reports the use of environmental-friendly CO2 foaming process to fabricate microcellular high-density polyethylene (HDPE) nanocomposites by using EG and GNS as the conductive nanofillers. Crystallinity and microstructure of the HDPE nanocomposites were evaluated. Then, electrical conductivity and mechanical properties of microcellular HDPE nanocomposite foams were investigated and compared to those of bulk nanocomposites.
Experimental section
Materials
High-density polyethylene (HDPE, 5030, Tabriz Petrochemical CO) with a density of 0.95 g/cm3 (ASTM 1505), melting temperature of 130 °C (ASTM D 2117), and a melt index of 2 g/10 min (ASTM D 1238) was used. The natural flake graphite (Beijing Invention Biology Engineering & New Material CO) with density 2.26 g/cm3 and an aggregate size of 300 μm was used. Sulfuric acid and nitric acid were supplied by Merck CO (Germany) and used as received.
Preparation of expanded graphite (EG)
10 g natural flake graphite was dried at 80 °C in a vacuum oven for 24 h. Then, it was mixed and saturated with acids consisting of concentrated sulfuric acid (200 cc) and concentrated nitric acid (50 cc) in a volume ratio of 4:1, for 24 h to form the graphite-intercalated compound (GIC). Nitric acid and sulfuric acid serve as an oxidizer and intercalant, respectively. The mixture was carefully washed and filtrated with deionized water until the pH level of the solution reached 6. After being dried at 80 °C in a vacuum oven for 24 h, the GIC was rapidly expanded at 1050 °C for 15 s in a muffle furnace to form EG [15, 16].
Preparation of graphite nanosheets (GNS)
1 g EG was immersed in a 70 % of aqueous alcohol solution (100 cc) in an ultrasonic bath (Power: 240 W, Hover & Boecker Co), operating at 100 W for 12 h. The dispersion was then filtered and dried at 80 °C to produce GNS [15, 16].
Preparation of HDPE/EG or GNS nanocomposites
The nanocomposites were prepared by intensive mixing of precise amounts of the EG and GNS additives with HDPE in the molten state (T = 180 °C) using a Brabender Plasticorder equipment with volume mixing chamber of 50 mL, at 70 rpm of shear rate, for a total time of 15 min. The equilibrium torque was recorded automatically by the machine. The abbreviation which will be used for the prepared samples are as HDPE/Xw: where X represents the type of used nanofiller (either GNS or EG), while w denotes the weight fraction of loaded nanofiller. It should be added that the mass fractions of EG and GNS in the nanocomposites were in the range of 0–20 wt%.
Preparation of HDPE nanocomposite microcellular foams
All microcellular HDPE nanocomposite foams were prepared by a batch foaming process with the aid of subcritical CO2. The bulk samples were saturated with CO2 at given conditions (125–130 °C, 3.5–5 MPa, and 24 h) in a pressure vessel [17]. The saturation condition used here depends on the thickness of bulk samples. For samples thinner than 2 mm, the conditions were 130 °C, 5.0 MPa, and 24 h, while for tensile specimens with a thickness of 4 mm, the conditions of 125 °C, 3.5 MPa, and 24 h were used to achieve adequate saturation. After the complete saturation, the pressure was rapidly released, and the samples were quickly immerged in a preheated hot water (70 °C) for 30 min. Then, the morphology of the foam was fixed in an ice/water mixture.
Characterizations and measurements
Wide-angle X-ray diffraction (WAXD) was performed on HDPE nanocomposites to evaluate the degrees of dispersion of EG and GNS in the host polymer. WAXD was carried out on an Equinox (model: 3000, INEL Co) diffractometer with Cu Kα radiation (k = 1.541 A°) with a scanning speed of 0.5°/min and operating at 40 kV and 20 mA. The layer-to-layer distance (d-spacing) can be calculated according to Bragg’s law: \( d = n\lambda /2\sin \theta \), where n is an integer determined by the given order, and λ is the wavelength.
The volume conductivity of the moderately conductive samples (>1 × 10−6 S/m) was measured using a standard four-probe method on a Physical Property Measurement System (Quantum Design, US). The samples with low conductivities (<1 × 10−6 S/m) were measured using a three-terminal fixture on an EST121 ultrahigh resistance and micro current meter (Beijing EST Science & Technology CO. Ltd.), according to ASTM D257. Circular plates with 7 cm in diameter were fabricated for conductivity measurements. The sample surfaces were coated with silver paste to reduce contact resistance between the sample and the electrodes.
The microstructures of the nanocomposites and foams were observed using a Hitachi S-4800 scanning electron microscopy (SEM). The samples were freeze-fractured after immersion in liquid nitrogen for 20 min, and the fractured surfaces were coated with a thin layer of gold before microscopic observation.
Thermal analysis was carried out on a modulated differential scanning calorimeter (DSC; Model Toledo Mettler). Before recording, the samples was heated quickly to 160 °C and maintained for 10 min to remove the thermal history. The sample was cooled to room temperature at a cooling rate of 10 °C/min to obtain the cooling curve. The sample was then heated to 160 °C at a heating rate of 10 °C/min to study the melting behavior of HDPE. The entire thermal scan was conducted under inert N2 with a gas flow rate of 50 mL/min.
Mechanical properties of the samples were tested with an Instron 5567 testing machine at a crosshead speed of 2.8 mm/min. The samples were cut in a type IV format according to ASTM D-638 with the thickness of 4 mm. At least three samples were tested to obtain average values.
Result and discussion
Thermal properties
Figure 1a, b displays the DSC cooling scans of the EG- and GNS-containing HDPE nanocomposites, respectively. As can be seen, the crystallization temperature increases to higher values as nanofiller content increases. Also, the degree of crystallization of HDPE is influenced by the presence of EG and GNS nanofillers. The crystallinity of HDPE (X0) can be calculated based on the following equation (Eq. 1) [18]:
where d, d c, and d a are the densities of the HDPE, completely crystalline HDPE (0.985 g/cm3), and completely amorphous HDPE (0.824 g/cm3), respectively. The density (d) of HDPE used in these experiments was 0.958 g/cm3. Therefore, according to the Eq. 1, the calculated crystallinity of HDPE is about 86 %. On the other hand, the crystallinity of HDPE in the nanocomposites can be calculated based on Eq. 2 [18]:
where ∆H 0, and ∆H respectively are the crystallization enthalpies per gram of pure HDPE, and HDPE in nanocomposites in the cooling run which are obtained from DSC scans.
Figure 2 depicts the obtained values of crystallization temperature, and the degree of crystallinity versus nanofiller contents. As can be seen, crystallization temperature of HDPE/GNS nanocomposites is higher than that of HDPE/EG ones except for nanocomposites containing 7 wt% of EG or GNS. This is due to the fact that GNS has higher surface-to-volume ratio compared to EG, resulting in more nucleating sites to be formed. These results are rather consistent with the studies reported on other types of nanofillers such as glass and carbon fibers [19, 20].
As can be seen in Fig. 2, the degree of crystallinity first increases and then decreases by the incorporation of GNS and EG. Before discussing this behavior, it should be mentioned that, in general, there are two competing factors affecting the degree of crystallinity. On the one hand, when a suitable nanofiller, with a good state of dispersion, is added into a host polymer, nucleation of crystallization is facilitated which then leads to higher degree of crystallinity. On the other hand, as reported by several researchers, the addition of nanofillers is associated with the reduced chain mobility [21–24]. The latter case becomes more pronounced in particular when a strong interaction exists between nanofillers and the host polymer. Under such a situation, the crystallizable chains are no longer able to be folded and ordered. As a consequence, the crystallinity percentage will decrease. In the light of the fact that the initial increase of crystallinity is followed by a decrease, one can infer that, at low nanofiller content, nucleation effects of GNS and EG prevail over the reduced chain mobility effect, while at higher nanofiller contents, the converse is true. It should also be mentioned that in good agreement with the aforementioned studies, melting point did not change significantly by the addition of GNS and EG, such that all the samples had melting point at around 132 °C [25, 26].
X-ray scattering
Figure 3 shows the XRD diffraction patterns of EG, GNS, and the HDPE nanocomposites containing 3 wt% of EG and GNS. The diffracted intensity is represented as a function of the scattering angle 2θ. The characteristic diffraction peaks of semicrystalline HDPE are widely reported to be at 21.6° and 24° which correspond to the (110) and (200) reflections of the orthorhombic phase of polyethylene, respectively [27]. Diffractograms of nanocomposites, however, exhibit an additional reflection at 26.2° which is attributed to the (002) reflection from the graphitic layer structure with d-spacing of ~0.337 nm [5, 28]. As to the XRD patterns of the nanocomposites, the characteristic peak of graphitic layers slightly shifted to the lower 2θ values, which corresponds to a relatively larger d-spacing, indicating that EG and GNS could not exfoliate or separate completely. In addition, compared with XRD spectra of pure EG and GNG, the spectra of nanocomposites exhibited the characteristic peak of the graphitic layer structure with a much lower intensity. It should also be mentioned that the lower diffraction intensity of GNS-containing nanocomposites compared to that of EG ones implies that the level of dispersion in the case of the former is better.
The change in total crystallinity fraction by the addition of nanofillers can also be calculated by normalizing the area under the characteristic diffraction peaks of HDPE. The fraction of the crystalline phase can be estimated using a curve-fitting program from the ratio of the area below the crystalline peaks to the total area of the diffractogram [27]. The obtained results from XRD patterns were in good accordance with those from DSC results (not shown).
Structure and morphology
Figure 4 shows the SEM micrographs of natural graphite flakes, EG, and GNS. The differences in microstructures between the two different fillers can be clearly noted. The EG has a worm-like morphology obtained by rapid heating of GIC [29]. As can be seen in Fig. 4b, the ensuing EG sheets are also entangled with each other, resulting in big pores inside them. Figure 4b also reveals the loose structures containing multipores of EG. The prepared EG has the average pore diameter and thickness range of about 2 μm and 100–200 nm, respectively. On the other hand, the integrated and entangled morphologies of EG are no longer seen in GNS micrograph, which is due to the breakdown of those structures by the ultasonication (Fig. 4c). Such a high surface area of GNS could strongly facilitate the processing and formation of an efficient polymer/GNS nanocomposite [26, 29].
Figure 5a–d shows SEM micrographs of the fractured surfaces of HDPE nanocomposites containing 3 wt% of GNS and EG. As can be seen, there is no obvious sign of agglomeration of EG and GNS, and nanofillers with layered structure are homogenously dispersed in HDPE matrix, further verifying the XRD results. Detailed observation of the fracture surface of HDPE/EG and HDPE/GNS nanocomposites reveals a difference in the polymer/nanofiller interfacial interaction. Although the multilayer EG nanofillers protrude cleanly from the fracture surface, suggesting a weak interfacial adhesion, the protruding GNS nanofillers are thickly coated with adsorbed polymer, indicating strong polymer/GNS interactions [30, 31]. Such regions are illustrated by circles in Fig. 5 as adhered HDPE to the pulled-out nanofillers.
Figure 6 shows SEM micrographs taken from cross sections of the microcellular HDPE/EG and HDPE/GNS nanocomposite foams with different EG or GNS contents. As it can be seen, as the amount of GNS or EG content increases, the size of pores sharply decreases, and the distribution of cell size becomes more homogenous [32–34]. These early spherical cells exhibit a cell size distribution ranging from 1 to 10 μm in the both cases, categorizing them as microcellular foams. The formation of microcellular structure is attributed to the use of subcritical CO2 as the foaming agent [35]. Moreover, heterogeneous nucleation induced by the incorporation of GNS and EG is responsible for the sharp reduction in the average size of cells and more uniform size distribution. These morphological results are in good agreement with previous studies on polymer nanocomposite foams produced using supercritical CO2 [32, 34, 36]. It has been widely reported that nanofillers serve not only as heterogeneous nucleating agents, but also as inhibitors for the coalescence of cells during growth step. It should also be mentioned that in the presence of nanofillers, the activation energy barrier is decreased. Density reduction (DR) results further confirm the reduction in activation energy of nucleation (Table 1). The density reduction values for both HDPE nanocomposites containing EG and GNS continuously increased as the amount of nanofiller increased.
In addition, thanks to the presence of –COOH, –OH, and –O– groups on the surfaces of GNS and EG, the overall polarity of the nanocomposites is boosted as the amount of added nanofillers increases, thus enhancing CO2 solubility in the samples [1, 32, 37, 38]. It should be noted that one of the biggest challenges involved in CO2 foaming of highly nonpolar polymers such as HDPE that could be met by nanofillers’ inclusion, is that of low solubility of CO2. Li et al. [35] indicated that one of the other crucial factors for an efficient CO2 foaming process is low diffusivity of the CO2. It has been well understood that nanofillers with platelet-like geometry such as clay mineral, EG, and GNS can efficiently reduce gas diffusivity by providing tortuous pathway to the gas molecules, consequently resulting in a more effective CO2 foaming process. So, in light of the aforementioned discussion, EG and GNS, due to their high surface area and polar functional groups, contribute to the improved foaming process.
Considering all that was discussed, the importance of dispersion of nanofillers in the polymer matrix is highlighted since the better the state of dispersion is, the more the surface functional groups will be available for CO2 dissolution, and also the farther the path will be created for CO2 diffusion.
Electrical conductivity
Figure 7 shows a comparison of electrical conductivities between EG- and GNS-filled HDPE nanocomposites and foams. Three distinct regions could be identified on the conductivity curves in Fig. 7a. At low nanofiller contents less than 2.5 vol% for EG and 3.2 vol% for GNS, the conductivity is negligible (10−13 S/cm) resembling that of an insulator. At slightly higher nanofiller concentration, the nanocomposites experience a distinctive transition from low conductivity to a rapid increase in conductivity. When a percolation threshold is achieved, a steady-state region occurs whereat the conductivity for EG-containing composites is 10−4 S/cm and that for GNS composites 10−3 S/cm, which are consistent with those of a semiconductor. Homogenously dispersed GNS or EG layers in the matrix can form an interconnected nanofiller network throughout the nanocomposites. This continuous conducting network is responsible for the improved electrical conductivity [39, 40]. It was reported that the conductivity increased because of direct tunneling between adjacent conductive nanofiller clusters [41, 42]. According to the classical percolation theory [43, 44], in the close vicinity of the percolation threshold (V c), which corresponds to the onset of the transition from an insulator to a semiconductor, the relationship of electrical conductivity (σ) of materials with nanofiller volume fraction (V) should follow the scaling law regardless of the nanofiller’s shape and distribution [43]:
where α is known to be the critical exponent for percolation transition of electrical conductivity. In the percolation model, the exponents are 2 and 1.3 for three-dimensional and two-dimensional randomly distributed objects, respectively [43]. The experimental data obtained from Fig. 7 for HDPE/GNS and HDPE/EG are fitted using Eq. 3. Clearly, α can be estimated from the slope of the log linearity. Therefore, the amounts of α for HDPE/GNS and HDPE/EG nanocomposites are 7.07 and 7.33, respectively. The compliance of the experimental data with Eq. 3 presents an indirect proof that the conductivity transition of HDPE nanocomposites containing EG and GNS is governed by the percolation concept. The critical exponent for HDPE/GNS is lower. This is attributed to the partial orientation of the GNS layers in the nanocomposites. For the HDPE/EG composites, V c ~ 4.6 vol %, but it reduces to V c ~ 2.5 vol %, when EG is replaced by GNS. In other words, the GNS are more effective in enhancing the electrical conductivity of HDPE nanocomposites than EG. Clearly, the GNS system exhibits a markedly lower percolation threshold. Balberg et al. reported the percolation threshold in a three-dimensional stick system [45, 46]. It was shown that, the percolation threshold depends on the form factor of the sticks. The percolation threshold of stick systems could be determined based on the excluded volume, which is defined as the volume around an object in which the center of another similar object is not allowed if overlapping of the two objects is to be avoided, of the sticks for nonspherical nanofillers. For an infinitely large system, the relationship of the critical total nanofiller volume fraction (V c) with nanofiller volume (V) and excluded volume (V ex) is expressed as follows [46–48]:
where K is a constant. Since K is known to be a constant in the order of 1, a small V/V ex ratio means a low V c value. In the case of HDPE/GNS, we could consider the nanofiller as randomly oriented sheets with a thickness of T and diameter d,
and,
when d/T ≫ 1; the percolation criterion can be given as follows [47]:
where f is the form factor, f = d/T; and K/π is a constant estimated to be about 1. In Eq. 7, it is assumed that all nanofillers are of the same size. So, Eq. 7 should be replaced by
where [d]3/[d]2 term, defined as the normalized nanofiller diameter, is usually larger than d in Eq. 7 [46]. From equations 7 and 8, it could be readily shown that, the larger the nanofiller form factor, which means d = T, the lower the critical volume fraction, V c, for conductive nanofiller in the composites. If one substitutes V c ~ 2.5 and ~4.6 vol % for HDPE/GNS and HDPE/EG nanocomposites, respectively, into Eq. 7, the nanofiller form factor could be calculated to be 940 and 320 for GNS and EG nanofillers, respectively.
It is seen in Fig. 7b, c that the electrical conductivity increases with the increasing EG and GNS contents for both the bulk and microcellular foamed nanocomposites. Interestingly, the insulator-to-semiconductor transition of the microcellular nanocomposite foams shifts to lower EG and GNS contents compared to that of the bulk nanocomposites [4]. The low absolute values and trends at EG and GNS concentrations above the critical value led us to assume a similar approach as the one used for the bulks, i.e., tunnel conduction. In general, obtaining the lower percolation threshold value in polymer foams stems from two effects: one is that cell formation followed by in situ-generated strong extensional flow pushes nanosheets together, and considerably facilitates the orientation of nanofillers in cell wall [48]. In other words, CO2 foaming process excluding volume related to cell formation pushed the nanofillers together, causing them to be closer in the microcellular foamed nanocomposites, and in turn reducing the critical distance for tunnel conduction. The other effect of the foaming process is the volume expansion, which tends to increase the distance of adjacent nanofillers [11]. Therefore, the respective values of critical EG and GNS concentrations for conduction in this case were reduced by foaming process (bulk: 4.6 vol% EG, 2.5 vol% GNS; Microcellular foams: 3.2 vol% EG, 1.8 vol% GNS) [49].
Mechanical behavior
The mechanical properties of bulk HDPE nanocomposites and their microcellular foams are shown in Fig. 8a–c. As observed, the tensile strength as well as elastic modulus increases with the increasing nanofiller contents in both bulk and foam forms. However, the obtained values, whether it is tensile strength or elastic modulus, are higher for bulk nanocomposites compared to those of foamed ones. The other noticeable fact is that the incorporation of GNS is much effective than that of EG in improving both tensile strength and elastic modulus due in particular to its high surface area and better dispersion in the HDPE matrix. The obtained results are in a good accordance with other polymer composites containing various nanofillers [50–53].
Regarding the Fig. 8c, as can be seen, the elongation at break, in both foam and bulk nanocomposites, first improves and then deteriorates which is due to nanofiller aggregations. In addition, similar to what was observed in tensile strength and elastic modulus, GNS compared to EG again exhibits higher efficiency in enhancing ductility. More importantly, the microcellular HDPE nanocomposite foams with 10 wt% GNS give an elongation at break of 281.6 % compared to 194 % for its bulk counterpart. The reinforcement of cell walls/junctions by the GNS is responsible for these improvements [4]. Moreover, uniformly small cells of the microcellular HDPE nanocomposite foams, acting in a similar way as the cavitated rubber particles in rubber-toughened plastics, are resistant to rupture and favorable for simultaneous enhancement of both ductility and toughness [54, 55].
Conclusion
HDPE nanocomposites containing GNS and EG were prepared by simple blending and then foamed by using environmentally benign subcritical CO2 foaming technique. The crystallization process of HDPE in the nanocomposites was accelerated, and the crystallinity increased compared with neat HDPE. However, at higher amounts of EG or GNS, crystallinity decreased which was attributed to the reduced chain mobility. The GNS/HDPE nanocomposites exhibited better conductivity and mechanical properties than the EG/HDPE nanocomposites due to the high form factor of the sticks. The insulator-to-semiconductor transition of the microcellular HDPE nanocomposite foams shifts to lower EG and GNS contents compared to that of bulk nanocomposites. Interestingly, the presence of microcellular cells produced by CO2 foaming makes GNS- and EG-containing nanocomposites more ductile.
References
Jacobs LJ, Kemmere MF, Keurentjes JT (2008) Sustainable polymer foaming using high pressure carbon dioxide: a review on fundamentals, processes and applications. Green Chem 10(7):731–738
Fugetsu B et al (2008) Electrical conductivity and electromagnetic interference shielding efficiency of carbon nanotube/cellulose composite paper. Carbon 46(9):1256–1258
Im JS, Kim JG, Lee Y-S (2009) Fluorination effects of carbon black additives for electrical properties and EMI shielding efficiency by improved dispersion and adhesion. Carbon 47(11):2640–2647
Zhang H-B et al (2011) Tough graphene–polymer microcellular foams for electromagnetic interference shielding. ACS Appl Mater Interfaces 3(3):918–924
Dehsari HS et al (2014) Efficient preparation of ultralarge graphene oxide using a PEDOT: PSS/GO composite layer as hole transport layer in polymer-based optoelectronic devices. RSC Adv 4(98):55067–55076
Xu K, Chen G, Qiu D (2013) Convenient construction of poly (3, 4-ethylenedioxythiophene)–graphene pie-like structure with enhanced thermoelectric performance. J Mater Chem A 1(40):12395–12399
Zhang Z et al (2015) Template‐Directed In Situ Polymerization Preparation of Nanocomposites of PEDOT: PSS‐Coated Multi‐Walled Carbon Nanotubes with Enhanced Thermoelectric Property. Chem 10(1):149–153
Xu XB et al (2007) Ultralight conductive carbon-nanotube–polymer composite. Small 3(3):408–411
Thomassin J-M et al (2008) Foams of polycaprolactone/MWNT nanocomposites for efficient EMI reduction. J Mater Chem 18(7):792–796
Yang Y et al (2005) Novel carbon nanotube-polystyrene foam composites for electromagnetic interference shielding. Nano Lett 5(11):2131–2134
Ling J et al (2013) Facile preparation of lightweight microcellular polyetherimide/graphene composite foams for electromagnetic interference shielding. ACS Appl Mater Interfaces 5(7):2677–2684
Ye L et al (2009) Synthesis and characterization of expandable graphite–poly (methyl methacrylate) composite particles and their application to flame retardation of rigid polyurethane foams. Polym Degrad Stab 94(6):971–979
Li Y et al (2014) Effect of expandable graphite particle size on the flame retardant, mechanical, and thermal properties of water–blown semi–rigid polyurethane foam. J Appl Polym Sci 131(3):39885
Sorrentino L et al (2012) Mechanical behavior of solid and foamed polyester/expanded graphite nanocomposites. J Cell Plast 48(4):355–368
Celzard A et al (2000) Electrical conductivity of anisotropic expanded graphite-based monoliths. J Phys D 33(23):3094
Zheng W, Wong S-C (2003) Electrical conductivity and dielectric properties of PMMA/expanded graphite composites. Compos Sci Technol 63(2):225–235
Zeng C et al (2003) Polymer–clay nanocomposite foams prepared using carbon dioxide. Adv Mater 15(20):1743–1747
Peacock A (2000) Handbook of Polyethylene: Structures, Properties, and Applications. Taylor & Francis, New York
Pisharath S, Wong SC (2003) Development of the morphology and crystalline state due to hybridization of reinforced toughened nylon containing a liquid-crystalline polymer. J Polym Sci Part B 41(6):549–559
Zhang C et al (2000) Morphology, crystallization and melting behaviors of isotactic polypropylene/high density polyethylene blend: effect of the addition of short carbon fiber. J Mater Sci 35(3):673–683. doi:10.1021/ma071025m
Hoang T et al (2014) Tensile, rheological properties, thermal stability, and morphology of ethylene vinyl acetate copolymer/silica nanocomposites using EVA-g-maleic anhydride. J Compos Mater 48(4):505–511
Wilson R et al (2012) Clay intercalation and its influence on the morphology and transport properties of EVA/clay nanocomposites. J Phys Chem C 116(37):20002–20014
Jolfaei AF et al (2015) Effect of organoclay and compatibilizers on microstructure, rheological and mechanical properties of dynamically vulcanized EPDM/PP elastomers. Polym Bull 72(5):1127–1144
Bidsorkhi HC et al (2015) Preparation and characterization of ethylene-vinyl acetate/halloysite nanotube nanocomposites. J Mater Sci 50(8):3237–3245. doi:10.1007/s10853-015-8891-6
Li YC, Chen GH (2007) HDPE/expanded graphite nanocomposites prepared via masterbatch process. Polym Eng Sci 47(6):882–888
Wang L, Chen G (2010) Dramatic improvement in mechanical properties of GNs-reinforced HDPE nanocomposites. J Appl Polym Sci 116(4):2029–2034
Young RJ, Lovell PA (2011) Introduction to Polymers, 3rd edn. Taylor & Francis, New York
Aboutalebi SH et al (2011) Spontaneous formation of liquid crystals in ultralarge graphene oxide dispersions. Adv Funct Mater 21(15):2978–2988
She Y, Chen G, Wu D (2007) Fabrication of polyethylene/graphite nanocomposite from modified expanded graphite. Polym Int 56(5):679–685
Li J et al (2007) Br treated graphite nanoplatelets for improved electrical conductivity of polymer composites. Carbon 45(4):744–750
Gavgani JN, Adelnia H, Gudarzi MM (2014) Intumescent flame retardant polyurethane/reduced graphene oxide composites with improved mechanical, thermal, and barrier properties. J Mater Sci 49(1):243–254. doi:10.1007/s10853-015-8891-6
Lee LJ et al (2005) Polymer nanocomposite foams. Compos Sci Technol 65(15):2344–2363
Nam PH et al (2002) Foam processing and cellular structure of polypropylene/clay nanocomposites. Polym Eng Sci 42(9):1907–1918
Taki K et al (2004) Visual observation of CO2 foaming of polypropylene-clay nanocomposites. Polym Eng Sci 44(6):1004–1011
Li Y et al (2011) Numerical simulation of polypropylene foaming process assisted by carbon dioxide: bubble growth dynamics and stability. Chem Eng Sci 66(16):3656–3665
Hodlur R, Rabinal M (2014) Self assembled graphene layers on polyurethane foam as a highly pressure sensitive conducting composite. Compos Sci Technol 90:160–165
Realinho V et al (2011) Influence of nanoclay concentration on the CO2 diffusion and physical properties of PMMA montmorillonite microcellular foams. Ind Eng Chem Res 50(24):13819–13824
Tomasko DL et al (2009) Development of CO2 for polymer foam applications. J Supercrit Fluids 47(3):493–499
Zhao Y et al (2007) Preparation and properties of electrically conductive PPS/expanded graphite nanocomposites. Compos Sci Technol 67(11):2528–2534
Lu J et al (2006) The piezoresistive behaviors of polyethylene/foliated graphite nanocomposites. Eur Polymer J 42(5):1015–1021
Van der Putten D et al (1992) Evidence for superlocalization on a fractal network in conductive carbon-black–polymer composites. Phys Rev Lett 69(3):494
Calberg C et al (1999) Electrical and dielectric properties of carbon black filled co-continuous two-phase polymer blends. J Phys D Appl Phys 32(13):1517
Stauffer D, Aharony A (1991) Introduction to percolation theory. Taylor & Francis, New York
Grimmett G (1999) What is percolation?. Springer, Berlin
Balberg I, Binenbaum N, Wagner N (1984) Percolation thresholds in the three-dimensional sticks system. Phys Rev Lett 52(17):1465
Balberg I (1986) Excluded-volume explanation of Archie’s law. Phys Rev B 33(5):3618
Zheng W, Wong S-C, Sue H-J (2002) Transport behavior of PMMA/expanded graphite nanocomposites. Polymer 43(25):6767–6773
Zhai W et al (2012) The orientation of carbon nanotubes in poly (ethylene-co-octene) microcellular foaming and its suppression effect on cell coalescence. Polym Eng Sci 52(10):2078–2089
Ezquerra TA et al (2001) Alternating-current electrical properties of graphite, carbon-black and carbon-fiber polymeric composites. Compos Sci Technol 61(6):903–909
Hu Z, Chen G (2014) Aqueous dispersions of layered double hydroxide/polyacrylamide nanocomposites: preparation and rheology. J Mater Chem A 2(33):13593–13601
Hu Z, Chen G (2014) Novel nanocomposite hydrogels consisting of layered double hydroxide with ultrahigh tensibility and hierarchical porous structure at low inorganic content. Adv Mater 26(34):5950–5956
Gavgani JN et al (2014) Intumescent flame retardant polyurethane/starch composites: Thermal, mechanical, and rheological properties. J Appl Polym Sci 131(23):41158
Bidsorkhi HC et al (2014) Mechanical, thermal and flammability properties of ethylene-vinyl acetate (EVA)/sepiolite nanocomposites. Polym Testing 37:117–122
Dasari A, Yu Z-Z, Mai Y-W (2009) Electrically conductive and super-tough polyamide-based nanocomposites. Polymer 50(16):4112–4121
Collias DI, Baird DG, Borggreve RJ (1994) Impact toughening of polycarbonate by microcellular foaming. Polymer 35(18):3978–3983
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baseghi, S., Garmabi, H., Gavgani, J.N. et al. Lightweight high-density polyethylene/carbonaceous nanosheets microcellular foams with improved electrical conductivity and mechanical properties. J Mater Sci 50, 4994–5004 (2015). https://doi.org/10.1007/s10853-015-9048-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-015-9048-3