Abstract
The thermal stability up to 800 °C of a nanocrystalline (NC) Ni (mean grain size ~25 nm) with ~4 wt% Al dispersed in the form of ~160-nm-sized particles, which was fabricated by co-electrodeposition from a nickel sulfate bath, has been investigated using differential scanning calorimetry (DSC), transmission electron microscopy (TEM) and X-ray diffraction (XRD). The results showed that microstructural evolution of the composite is temperature dependent, i.e., normal grain growth of the NC Ni, ~0.6 wt% Al solution into the Ni matrix and direct reaction between Al and Ni to form Ni3Al precipitates occurred at ~290, ~325 and ~575 °C, respectively. The distribution of Al in Ni matrix with temperature is fully discussed.
Graphical Abstract
A DSC scanning up to 800 °C of a nanocrystalline Ni matrix dispersing ~4 wt% Al particles (mean size: 160 nm) shows the particles did not significantly affect the normal grain growth of NC Ni and they have been converted into Ni3Al and Al solute atoms.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ni, Ni-based alloy and composite electrodeposits (EDs), normally with ultrafine- or nano-grained structures, are widely used in many established and emerging engineering applications, such as recording heads and soft magnetic disk components, microelectromechanical systems (MEMs) components fabricated by the LIGA process (Lithographic, Galvanoformung, Abformung), parts acquired for repairing nuclear steam generator tubes—Electrosleeve™ and protective coatings for corrosion, erosion and wear [1–7]. Most of these applications involve high ambient temperature and/or pressure at some stage. For example, the Ni MEM components, with high-precision micrometer, or smaller, features, are subject to annealing as an essential part of a diffusion-bonding assembly method [2]. The tubes repaired by Electrosleeve™ are required to operate reliably for long times in high-pressure steam at temperatures up to 650 °C [3, 8]. Also EDs are used to protect substrates against high-temperature corrosion [4–7]. In these circumstances, microstructural changes, such as grain growth, recrystallization, solid solution and reaction, can occur and result in degradation of the associated structure-dependent properties of the EDs. Therefore, in most of these applications, thermal stability is a key concern.
Recently, the thermal stability of pure nanocrystalline (NC) Ni EDs has been extensively investigated, and was reviewed by Hibbard and coworkers in 2002 [9]. The results showed that the abnormal grain growth occurred at a temperature lower than 100 °C, and subsequently rapid normal grain growth occurred at approximately 290 °C [9, 10]. Additions of alloying elements, even in trace amounts, have been shown to significantly improve the thermal stability of Ni matrix EDs [9]. This was suggested to be the result of a solute drag effect. For example, the grain growth temperature was observed to be increased from 260 to 360 °C with the addition of 1.2 wt% P [11]. The effect of alloying with Mn and W was reported to be considerable [12, 13]. Furthermore, if there were some in situ precipitation formed during annealing, the thermal stability of the Ni matrix would be increased because of the Zener drag effect [11–13]. However, there are few publications about the thermal stability of NC Ni matrix composites with an ex situ addition of particles, especially for a Ni matrix plus metal nanoparticles. These may differently behave from in situ precipitates because of some different properties, e.g., the type of particle/matrix interfaces, the particle distribution, concentration and size.
Recently, Susan and coworkers [14, 15] reported oxidation-resistant alumina-forming Ni–Al composites by co-electrodeposition of Ni matrix and Al microparticles. Using a similar composite electrodeposition technique, we developed a new type of nanocomposites that consisted of an NC Ni matrix and dispersed Al and/or Cr nanoparticles [4–7]. The nanocomposites had enhanced ability to undergo selective oxidation of Cr or Al to form a protective scale of chromia or alumina in a very short initial and transient oxidation stage, compared with materials with the same composition that were either micro-grained alloys or composites having an NC Ni matrix with dispersions of microparticles. Peng [16] attributed the result to two nano-effects of oxidation, i.e., the nanosize effect of particles by which Al2O3/Cr2O3 nucleated on the ubiquitously distributed particles at/adjacent to the surface of the nanocomposites at the onset of oxidation and a nanosize effect of the Ni-matrix grains by which a rapid linkage of the formed Al2O3/Cr2O3 nuclei through their lateral growth can be achieved by short-circuit diffusion of Al/Cr atoms through the numerous grain boundaries in the Ni matrix. However, the novel nanocomposites are in a non-equilibrium state and their physical and chemical evolution with temperature and time undoubtedly leads to a variation in their structure and composition, which is expected to in turn to affect the selective oxidation process, particularly the long-term oxidation behavior of the nanocomposites. Accordingly, to study the thermal stability of these nanocomposites, it is essential not only to understand fully the early and stable stage oxidation process but also to tailor new nano- or ultrafine-grained materials with high thermal stability and oxidation resistance in the future. For example, Ni grain growth and Al particle dissolution as well as reaction between Ni and Al are expected to occur for the Ni–Al nanocomposite at high temperatures; however, its structural thermal stability has not been reported. In this work, the thermal stability up to 800 °C of a Ni–Al composite, which was prepared using Al particles in a size close to the nano range, has been investigated.
Experimental
Al particles were added to a sulfate bath containing 150 g l−1 NiSO4·6H2O, 15 g l−1 H3BO3, 15 g l−1 NH4Cl, 0.1 g l−1 C12H25NaSO4 and 1 g l−1 saccharin for electrodeposition of the Ni–Al composite using a pulse power. The pulse reversal plating parameters are listed in Table 1. Pure copper (>99.5 %) plate with dimensions 20 × 20 × 0.5 mm and nickel (99.9 %) plate with dimensions 15 × 10 × 2 mm were used as substrates. The Ni–Al composites were ~70-μm thick and contained ~4 wt% Al (determined by energy dispersive spectroscopy after abrading ~5 μm from the surface). The material deposited on the copper plate was peeled off for transmission electron microscopy (TEM) and differential scanning calorimetry (DSC) tests, and that on the Ni plate was used for X-ray diffraction (XRD) characterization. The calorimetric investigations were performed in a NETZSCH STA 449C instrument using high-purity argon (99.999 %) as purge gas. The measurements were conducted from room temperature to 800 °C. After cooling to room temperature at a rate of 20 °C min−1, a second DSC run provided the baseline for data analysis.
Results and discussion
The Al particles used were in the range of 60–400 nm and with a mean size of ~160 nm. The TEM results, given in Fig. 1a, indicated that the particles were dispersed in an NC Ni matrix with a mean grain size of ~25 nm. No microcracks or micropores could be observed in Ni/Al interfaces. The dark-field image presented in Fig. 1b indicates some agglomeration of the finer particles.
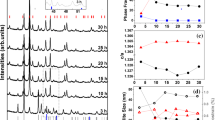
Figure 2 shows the DSC curve at a heating rate of 10 °C min−1. It exhibited two exothermic peaks. A shallow one (P 1 ) was in the temperature range 254~339 °C, with the peak at 290.1 °C and heat release of ~8.1 J g−1 (calculated by integrating the peak area). P 1 is mainly ascribed to normal grain growth of the NC Ni matrix as extensively reported elsewhere [9, 10, 17]. The peak temperature and amount of heat released are very close to the corresponding values (~290 °C and ~7.1 J g−1) of pure Ni EDs with a similar grain size (20 nm) measured by Wang et al. [17] using DSC at the same heating rate (10 °C min−1). Furthermore, according to the Kissinger equation [18]:
where B, T p and R are the heating rate, the peak temperature and the gas constant, respectively, the activation energy (E a) for grain growth can be calculated to be 121.3 kJ mol−1 from the slope of ln(B/T 2) versus 1/(RT) given in Fig. 3. This value is also similar to the E a (131.5 kJ mol−1) for the pure NC Ni EDs [17]. The results suggest that the presence of the finer 4 wt% Al particles has an insignificant effect on the normal grain growth of NC Ni. The other peak (P 2 ) was sharp and occurred in the temperature range 394~713 °C, with the peak at 575.4 °C and a heat release of ~260 J g−1. Such a sharp peak in this temperature range was not found in the non-isothermal DSC scanning of an NC Ni ED [17].
To understand fully the relationships between the exothermic peaks and the microstructural changes, samples were heated in a vacuum system to the temperatures near the end of P 1 (325 °C) and P 2 (625 °C) (as arrowed with the dashed line in Fig. 2) using a similar heating rate to that of the DSC measurement and then air cooling to room temperature for XRD characterization. Figure 4 shows the XRD patterns of as-deposited and as-annealed samples. In contrast to the as-deposited sample, the width of the Ni peaks decreased after heating to 325 °C, which was mainly caused by grain growth of the NC Ni matrix. Additionally, the position of the Ni peaks was shifted toward lower angles (as listed in Table 2). This was because the larger Al atoms (0.143 nm in radius) were incorporated into the Ni lattice (0.124 nm) according to the Bragg equation [19]. Provided that no lattice distortion occurred in the as-deposited state, the lattice constants of Ni and Al were 0.35238 and 0.40494 nm, respectively. The solid solubility of Al (c) was calculated to be ~0.4 wt% by the Vegard’s law (Eq. 2) [20],
where a, a 1 and a 2 are the lattice constants of solid solution, solvent (Ni) and solute (Al), respectively. Intriguingly, the solid solubility of Al in the Ni matrix was much larger than the value, <0.2 wt%, reported by Susan et al. for Ni–7 wt% Al microcomposites composed of a micrometer-sized Ni matrix and Al micro-particles, even though their temperature was 415 °C and their annealing time was much longer [21]. This different particle solubility results from the difference in the size of Ni matrix grains and Al particles. However, we recently found (Zheng et al. unpublished) that although a similar grain growth DSC peak was acquired for an Ni–11.7 wt% Al composite that had a similar Ni matrix nanostructure to that in this study but with dispersions of Al microparticles (mean particle size ~1.4 μm), no significant XRD line position displacement was seen, even after heating to a much higher temperature of 556 °C. The result demonstrates that the particle size rather than the Ni matrix grain size is the dominant factor for the solution kinetics of Al into the Ni matrix, i.e., finer particles lead to higher solubility. One reason for the result is related to the fact that the thermal stability of the particle decreases with its size, because of an increase of the surface energy. Another reason may be that the Ni matrix in this study during its structural evolution with temperature contained a higher density of defects (such as twins and stacking faults), which increased the solid solubility of Al. Furthermore, the Ni matrix with a more uniform particle dispersion that was achieved by replacing the conventionally used micro-particles with the nearly nano-sized particles [16] had a more homogeneous distribution of Al solid solute atoms. This may be an additional reason for the higher Al solubility.
When the temperature was increased to 625 °C, a significant change in the XRD patterns occurred compared with those at 325 °C (Fig. 4). Ni3Al peaks emerged, the Al peaks disappeared and the positions of diffraction peaks (200) and (220) of Ni decreased further (see Table 2). This result suggests that the Al solubility was continuously increased from 0.4 to 0.6 wt% and P 2 was mainly caused by solid phase reaction between Al and Ni to form Ni3Al. Susan et al. [21] found that before the final Ni3Al phase occurred after heating a Ni–7 wt% Al composites (>1.5-μm-sized particles) to 1,000 °C, an intermediate NiAl phase was formed. The formation of this phase is understandable, because the activation energy of the reaction between Ni and Al to form NiAl is lower than that to form Ni3Al. The difference in the activation energies is consistent with the fact that the formation temperature of Ni3Al, 575.4 °C, obtained by DSC in this study is much higher than that of NiAl, 532 °C, obtained by DTA by Susan et al. [21]. However, NiAl was not formed in this study, possibly because the rapid dissolution of the finer Al particles into the Ni matrix led to the local amount of Al available for the reaction between Ni and Al to be lower than the critical value for the formation of NiAl but was sufficient for the formation of Ni3Al. The activation energy for the reaction between Ni and Al to form Ni3Al was calculated from Eq. 1 to be 233 kJ mol−1. This value is similar to that of 249 kJ mol−1 for Ni3Al phase formation reported by Watanabe et al. [22], but much higher than that for the normal NC Ni grain growth (Fig. 3).
The calculated value of the formation enthalpy of Ni3Al is −467.4 kJ mol−1 per mol of Al [23]. The value obtained in this study was ~260 J g−1. From the difference between these two values, the amount of Al consumed by the reaction has been calculated to be ~1.5 wt%. This value plus the solid solubility (~0.6 wt%) of Al in the Ni matrix is ~2.1 wt%, which is much smaller than the as-codeposited particle content (~4 wt% Al). This result suggests that during the DSC scanning, the rest of the Al may segregate to the Ni GBs driven by the lattice misfit strain because of the relatively larger size of the Al atom to Ni. Although significant Ni grain growth occurred during the DSC scanning, the Ni still remained ultrafine-grained ([6] Zheng et al. unpublished), which makes possible the accommodation of such a large amount of Al at the numerous GBs.
Conclusions
The DSC scanning up to 800 °C of the electrodeposited Ni–4 wt% Al composite shows that the dispersion of the ~160-nm-sized Al particles had no significant effect on the normal grain growth of the ~25-nm-sized Ni (which occurred at 290.1 °C), but changed the composite composition by its reaction with Ni to form Ni3Al at 575.4 °C. Part of the total as-deposited 4 wt% Al particles formed a ~0.6 wt% solid solution in the Ni and part formed novel Ni3Al precipitates (~1.5 wt% Al). The rest might exist as segregated atoms at the Ni GBs.
Research highlights
-
1.
Thermal stability of an NC Ni dispersing ultrafine Al particles was investigated.
-
2.
No obvious effect of ~4 wt% Al dispersoids on NC Ni grain growth at 290.1 °C.
-
3.
Reaction of Al particles with Ni to form Ni3Al at 575.4 °C.
-
4.
Al particles have been converted into solute atoms and Ni3Al in the DSC scanning.
-
5.
Segregation of part Al at the Ni GB is suggested in the DSC scanning up to 800 °C.
References
Clark D, Wood D, Erb U (1997) Nanostruct Mater 9:755
Thomas E, David A, Joseph R, Todd R, Steven D (2002) Metall Mater Trans A 33:539
Palumbo G, Gonzalez F, Brennenstuhl AM, Erb U, Shmayda W, Lichtenberger PC (1997) Nanostruct Mater 9:737
Zhang Y, Peng X, Wang F (2004) Mater Lett 58:1134
Zhou Y, Peng X, Wang F (1050) Scripta Mater 50(2004):1429
Yang X, Peng X, Wang F (2007) Scripta Mater 56:891
Yang X, Peng X, Wang F (2009) J Electrochem Soc 156:C167
Viswanathan R, Bakker WJ (2001) J Mater Eng Perform 10:81
Hibbard G, Erb U, Aust KT, Klement U, Palumbo G (2002) Mater Sci Forum 386–388:387
Klement U, Erb U, El-Sherik AM, Aust KT (1995) Mater Sci Eng A 203:177
Mehta SC, Smith DA, Erb U (1995) Mater Sci Eng A 204:227
Talin AA, Marquis EA, Goods SH, Kelly JJ, Miller MK (2006) Acta Mater 54:1935
Choi P, Al-Kassab T, Gärtner F, Kreye H, Kirchheim R (2003) Mater Sci Eng A 353:74
Susan DF, Marder AR (2002) Oxid Met 57:131
Susan DF, Marder AR (1000) Oxid Met 57(2002):159
Peng X (2010) Nanoscale 2:262
Wang N, Wang ZR, Aust KT, Erb U (1997) Acta Mater 45:1655
Kissinger HE (1957) Anal Chem 29:1702
Bragg WH (1913) Nature 91:477
Vegard L (1921) Z Phys 5:17
Susan DF, Misiolek WZ, Marder AR (2001) Metall Mater Trans A 32:379
Watanabe M, Horita Z, Sano T, Nemoto M (1994) Acta Metall Mater 42:3389
Liang YJ, Che YC (1993) Handbook of inorganic thermodynamic data (in Chinese), 1st edn. Northeastern University Press, Shenyang
Acknowledgements
The work is supported by National Basic Research Program (No. 2010CB934604) of China, Ministry of Science and Technology China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zheng, L., Peng, X. & Wang, F. Thermal stability up to 800 °C of a Ni–4 wt% Al nanocomposite. J Mater Sci 47, 7759–7763 (2012). https://doi.org/10.1007/s10853-012-6501-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6501-4