Abstract
(1 − x)Nd(Co1/2Ti1/2)O3(NCT)–xCa0.8Sr0.2TiO3(CST) composite ceramics were prepared by the conventional solid-state reaction process. From the X-ray diffraction analysis, it indicates that the Nd(Co1/2Ti1/2)O3 phase coexists with Ca0.8Sr0.2TiO3 phase, and it is easy for the cobalt volatilization to form second-phase Nd2Ti2O7 as x = 0.5 and 0.7. As the content of CST increases from 0.1 to 0.9, the dielectric constant of the (1 − x)Nd(Co1/2Ti1/2)O3–xCa0.8Sr0.2TiO3 composite ceramics increases from 29.3 to 114.7, the Q × f value decreases from 60,000 to 8,500 GHz, and the τ f value varies from −33.8 to 271 ppm/oC. A near zero τ f could be achieved at 0.5NCT–0.5CST ceramics with ε r = 44.5 and Q × f = 20,000 (GHz) sintered at 1,340 °C for 4 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the explosive growth of wireless and mobile communication technology, the performance microwave dielectric ceramics have been widely used for microwave device applications, such as resonators, filters, antennas, and oscillators. Commercial microwave dielectrics with a high dielectric constant (ε r) from 30 to 45, a high-quality factor (Q × f) more than 10,000 (GHz) and a near-zero temperature coefficient of the resonant frequency (τ f ) are currently fabricated for the applications. A high dielectric constant can reduce the size of resonators because the wavelength in dielectric is inversely proportional to \( \sqrt {\varepsilon_{\text{r}} } \) of the wavelength in vacuum. The quality factor (Q × f) is required to be high to enhance the selectivity of the resonators. A near-zero temperature coefficient of the resonant frequency (τ f ) is demanded for achieving the stability required of dielectric resonators for practical use.
Recently, Nd(Co1/2Ti1/2)O3 ceramics possessing good dielectric properties have been reported. Nd(Co1/2Ti1/2)O3 has a dielectric constant (ε r) of 27 and a extremely high-quality factor (Q × f) of 140,000 (GHz); however, its rather high negative temperature coefficient of resonant frequency (τ f ) of −46 ppm/oC precludes its practically usage [1]. In experience, the typical way involves mixing two or more compositions, τ f having coefficients with opposite sign to improve the temperature characteristic of microwave ceramics [2, 3]. Strontium–calcium titanate, Sr x Ca(1 − x )TiO3 (\( 0\le {\text{x}} \le 0. 8 \)) has been investigated with considerable interest for tunable microwave application. With partial replacement of Ca by Sr, the Ca0.8Sr0.2TiO3 ceramics possess excellent dielectric characteristics. Ca0.8Sr0.2TiO3 ceramics have ε r = 181, Q × f = 8,300 GHz (at 1.36 GHz), and τ f = 991 ppm/oC as reported by Wise et al. [4]. In the similar cases, employing Sm, La, and Nd substitute for Ca site to form Ca0.8Sm0.4/3TiO3, Ca0.6La0.8/3TiO3, and Ca0.6Nd0.8/3TiO3 ceramics. Yoon et al. [5] investigated the Ca1 − x Sm2x/3TiO3 solid-solution system and they found that the Ca0.8Sm0.4/3TiO3 ceramic possesses a high dielectric constant value of 119.3, a maximum Q × f value of 12,000 (GHz), and a positive τ f of 400 ppm/oC. Ca0.6La0.8/3TiO3 was reported to have a ε r of 109, a Q × f of 17,600 GHz, and a τ f of 213 ppm/oC [6]. In addition, Ca0.6Nd0.8/3TiO3 ceramic has a ε r of around 98, a Q × f value higher than 8,600 GHz, and a τ f of 247 ppm/oC [7]. Comparing these similar ceramic composites, though the Q × f value of Ca0.8Sr0.2TiO3 ceramic is slightly low, the ε r and τ f values are more outstanding than that of the others. Hence, the Ca0.8Sr0.2TiO3 ceramic has been introduced to the Nd(Co1/2Ti1/2)O3 composite to improve the microwave dielectric properties for meeting commercial requirements. In this present study, the (1 − x)Nd(Co1/2Ti1/2)O3–xCa0.8Sr0.2TiO3 composites have been synthesized and reported to demonstrate the significant change in microwave dielectric properties by the introduction of Ca0.8Sr0.2TiO3.
Experimental procedure
Samples of (1 − x)Nd(Co1/2Ti1/2)O3−xCa0.8Sr0.2TiO3 were synthesized by conventional solid-state method. The starting materials were high-purity oxide powders (>99.9%): Nd2O3, CoO, TiO2, CaCO3, and SrCO3 which were separately prepared according to the desired stoichiometry of Nd(Co1/2Ti1/2)O3 and Ca0.8Sr0.2TiO3. The powders were ground in distilled water for 12 h in a ball mill with agate balls and then dried at 80 °C in an oven overnight. After drying, the Nd(Co1/2Ti1/2)O3 and Ca0.8Sr0.2TiO3 powder was forced through a 200-mesh sieve, and calcined at 1,250 °C for 2 h and 1,100 °C for 4 h, respectively. After calcinations, the calcined powders were mixed according to the molar fraction and then re-milled for 12 h. The fine powder with 3 wt% of a 10% solution of PVA as a binder was pressed into pellets with dimensions of 11 mm in diameter and 5 mm in thickness under a pressure of 2,000 kg/cm2. These pellets were sintered at temperatures of 1,310–1,460 °C for 4 h in air. The heating and the cooling rates were both set at 10 °C/min. On the other hand, the X-ray diffraction (XRD, Siemens D5000) data of powder and bulk samples were collected using Cu-Kα radiation and a graphite monochromator in the 2θ range of 20–60o. The microstructural observations and analysis of the sintered surface were performed using a scanning electron microscopy (SEM, Philips \( {\text{X}}\tilde{L} 40{\text{FEG}} \), Eindhoven. the Netherlands) and an energy dispersive X-ray spectrometer (EDS). The density of the sintered specimens, as a function of sintering temperature, was measured by the liquid Archimedes method using distilled water as the liquid.
The dielectric constants (ε r) and Q × f values at microwave frequencies were measured using the Hakki–Coleman dielectric resonator method, as modified and improved by Courtney [8, 9]. The dielectric resonator was positioned between two brass plates to form a cavity-like structure. The test cavity was placed over a thermostat and the temperature range used was 25–80 °C, with a heating rate of 1 °C/min for heating and the residence time was 10 min at each time. The τ f (ppm/°C) was calculated by noting the change in the resonant frequency using the formula,
where f 1 is the resonant frequency at T 1 and f 2 is the resonant frequency at T 2.
Results and discussions
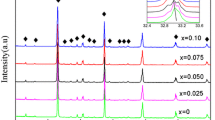
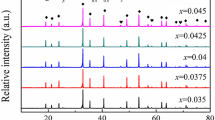
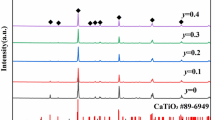
Figure 1a shows the X-ray diffraction patterns of (1 − x)Nd(Co1/2Ti1/2)O3–xCa0.8Sr0.2TiO3 mixed phases and Fig. 1b shows the XRD patterns of 0.5Nd(Co1/2Ti1/2)O3–0.5Ca0.8Sr0.2TiO3 composites sintered at different temperatures. All composites formed mixed phase ceramics with their diffraction peaks being indexed according to Nd(Co1/2Ti1/2)O3 phase (abbreviated as NCT) and Ca0.8Sr0.2TiO3 phase (abbreviated as CST). The XRD patterns suggest that Nd(Co1/2Ti1/2)O3 will not form a solid solution with Ca0.8Sr0.2TiO3. From Fig. 1a, it can be found that the intensity of the reflections of Ca0.8Sr0.2TiO3 increases greatly as the content of x increases. Compared with pure NCT phase, the reflection peaks shift systematically toward higher angle with increasing CST content, indicating a cell volume contraction of (1 − x)NCT–xCST. In addition to the primary phase, there is clear evidence of additional phases for sample with x = 0.5 and 0.7, as shown in Fig. 1a. The minor peaks are observed at about 2θ = 27.71, 28.63, and 43.6o. The peaks occur at similar 2θ values as those for Nd2Ti2O7 in NCT ceramics [1], and are inferred to be Nd2Ti2O7 secondary phase by analogy in this case. Tseng et al. [1] showed a secondary phase Nd2Ti2O7 had been formed in pure NCT ceramics after high sintering and this phase was formed due to cobalt volatilization. From Bain et al. [10] reported, it can also be observed that the Nd2Ti2O7 secondary phase can be formed easily from Nd2O3–TiO2 materials. These results are in good accord with our study result. However, the Nd2Ti2O7 secondary phase does not appear for sample with x = 0.9. The reason for no clear Nd2Ti2O7 peaks in the x = 0.9 might be that it is difficult to observe in XRD because of the extremely less amount of Nd2Ti2O7 and a tendency toward CST component. In addition, a phenomenon can be observed in Fig. 1b; the intensities of diffraction peaks related to the Nd2Ti2O7 phase increase gradually with increasing sintering temperature.
Figure 2 presents SEM photographs and EDS analysis of the (1 − x)NCT–xCST ceramics sintered at different temperatures for 4 h. The grain size increases significantly with increasing x, which is consistent with the XRD patterns exhibiting weak and wide peak for 0.9NCT–0.1CST specimens. From the micrograph, it is seen that there are three types of grains in the specimens. To clarify, the composition of the grains, the EDS analysis is performed for (1 − x)NCT–xCST ceramics. The EDS analysis shows that the large grain as A belongs to CST phase and small grains as B and D belong to NCT. For the sample with x = 0.5 and 0.7, noodle-shaped phase (C and E) exists, which is identified as Nd2Ti2O7 compound by EDS analysis. These results are in agreement with the XRD patterns presented in Fig. 1. Figure 2f, c, g, h, i shows the 0.5NCT–0.5CST specimens sintered from 1,310 to 1,430 °C for 4 h, respectively. It depicts the amount of Nd2Ti2O7 secondary-phase increase with increasing sintering temperature. The Nd2Ti2O7 secondary phase is not observed in Fig. 2f. Nevertheless, it can be found, the phase in XRD reflection pattern for the 0.5NCT–0.5CST specimens sintered at 1,310 °C. It might be that the grain size of Nd2Ti2O7 secondary phase is too small to be observed from the SEM photograph.
The microwave dielectric properties related with various amounts of CST content in (1 − x)NCT–xCST ceramics sintered at their optimal temperatures are shown in Fig. 3. As indicated with the increasing content of CST, the ε r and τ f values increase because CST possesses a high ε r (181) and a large positive τ f value (991 ppm/oC). The dielectric constant ε r changes from 29.3 to 114.7 while the τ f values range from negative value of −33.8 ppm/oC to positive value of 271 ppm/oC as CST ranged from 0.1 to 0.9. It can be seen that a near-zero τ f value is obtained as x = 0.5. The effect of Nd2Ti2O7 secondary phase in dielectric constant and τ f value can be observed as shown in Fig. 3. As x = 0.5 and 0.7, the trend of ε r is slightly low because the ε r of Nd2Ti2O7 (ε r = 33) is similar to NCT (ε r = 27). Owing to Nd2Ti2O7 secondary phase not existing at x = 0.9, the dielectric constant and τ f value increase substantially. On the contrary, the densities and Q × f values decrease with increasing CST. This behavior can be expected because the decrease in the densities and Q × f values is mainly related to the much lower densities and Q × f value of CST (density<3.78 g/cm3, Q × f ~ 8,300 GHz) than that of NCT (density ~6.6 g/cm3, Q × f = 140,000 GHz). In addition, the resonant frequency of dielectric ceramics in the mixed phase measured using a microwave cavity was plotted in Fig. 3 with respect to the content of CST. The resonant frequency of samples with the same dimensions decreases near-linearly from NCT to CST rich composites, which have larger dielectric constant.
Figure 4a and b shows the variation of density and dielectric constant of (1 − x)NCT–xCST ceramics as function of sintering temperature. The densities increase with increasing sintering temperature and reach maximum value, and then reduce with further increasing sintering temperature. The decrease in densities at higher sintering temperature is due to the rapid grain growth as observed in Fig. 2. For x ≥ 0.5, the ceramics achieve clearly the maximum densities at 1,340 °C while the others obtain the values sintered at 1,430 °C. The relationships between ε r values and sintering temperatures reveal the same trend with those between densities and sintering temperatures. However, the variation of ε r values is too small to be observed.
Figure 4c and d illustrates the Q × f and τ f values dependence of sintering temperature for (1 − x)NCT–xCST ceramics. For a given x value, the Q × f values initially increase to a maximum value and decrease thereafter. The correlations between Q × f values and sintering temperature nearly reveal the same trend as those between densities and sintering temperatures, as observed in Fig. 4a. It is believed that the densification of the ceramics plays an important role in controlling the dielectric loss. Furthermore, it has been noted that the microwave dielectric loss is mainly caused not only by the lattice vibrational modes, but also by the pore, density, and second phase [11]. The further increase in sintering temperature will result in the appearance of rapid grain growth and lead to the degradation of Q × f values. When x value reaches 0.5 and 0.7, cobalt volatilization results in Nd2O3–TiO2 materials to form Nd2Ti2O7 phase which indicates that Nd2Ti2O7 phase has a Q × f value of about 3,000 GHz. Hence, it can be seen that the Q × f values decrease substantially for x = 0.5 and 0.7. Figure 4d shows the τ f value of (1 − x)NCT–xCST ceramics sintered at different temperatures. The τ f value of the ceramics does not alter with the sintering temperature. As mentioned above, the τ f values of the ceramics for x = 0.5 and 0.7 are inhibited because of the existence of Nd2Ti2O7 phase. A near-zero τ f can be obtained for 0.5NCT–0.5CST ceramics at different sintering temperatures for 4 h. In summary, 0.5Nd(Co1/2Ti1/2)O3–0.5Ca0.8Sr0.2TiO3 ceramics sintered at 1,340 °C for 4 h have good dielectric properties; these properties are a dielectric constant (ε r) of 44.5, a quality factor (Q × f) of 20,000 GHz, and a τ f of −2 ppm/oC.
Conclusion
The microwave dielectric properties of (1 − x)Nd(Co1/2Ti1/2)O3–xCa0.8Sr0.2TiO3 ceramics have been studied. As expected, the dielectric constant and τ f increases with increasing the amount of CST, but the Q × f value decreases. The X-ray analysis and SEM reveal that the Nd2Ti2O7 secondary phase appears for x = 0.5 and 0.7. Nd2Ti2O7 secondary phase degrades the microwave dielectric properties. Under the same content of CST, the densities, ε r and Q × f values increase initially and then decrease with increasing sintering temperature; meanwhile, no significant change of the τ f is observed. The (1 − x)Nd(Co1/2Ti1/2)O3–xCa0.8Sr0.2TiO3 (x = 0.5) ceramics sintered at 1,340 °C for 4 h exhibit microwave dielectric properties with ε r of 44.5, Q × f value of 20,000 GHz, and τ f of −2 ppm/oC, respectively.
References
Tseng CF, Hsu CH, Huang CL (2007) J Am Ceram Soc 90:1619
Seabra MP, Avdeev M, Ferreira VM, Pullar RC, Alford McNN (2003) J Eur Ceram Soc 23:2403
Hsu CS, Huang CL, Tseng JF, You CC (2004) Ceram Int 30:2067
Wise PL, Reaney IM, Lee WE, Price TJ, Iddles DM, Cannell DS (2001) J Eur Ceram Soc 21:1723
Yoon KH, Kim WS, Kim ES (2003) Mater Sci Eng B99:112
Huang CL, Liu SS (2008) Mater Lett 62:3205
Wakino K (1989) Ferroelectrics 91:69
Hakki BW, Coleman PD (1960) IEEE Trans Microw Theory Tech 8:402
Courtney WE (1970) IEEE Trans Microw Theory Tech 18:476
Bain JJ, Song GX, Yan K (2008) Ceram Int 34:893
Saagala DA, Nambu S (1992) J Am Ceram Soc 75:2573
Acknowledgement
This work was sponsored by the National Science Council of the Republic of China under grant NSC 99-2221-E-239-018.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tseng, CF., Huang, CC. Microwave dielectric properties of (1 − x)Nd(Co1/2Ti1/2)O3–x(Ca0.8Sr0.2)TiO3 composite ceramics. J Mater Sci 47, 3982–3988 (2012). https://doi.org/10.1007/s10853-012-6251-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-012-6251-3