Abstract
The microstructure studies of the reaction product region (RPR) obtained due to the interaction between the liquid aluminium and polycrystalline zinc oxide substrate at 1273 K has been studied. The RPR extended over the oxide substrate and showed a typical C4 (co-continuous-ceramic-composites) structure composed of two interpenetrating phases. The scanning electron microscopy studies revealed that the large crystals of alumina were surrounded by an Al(Zn) metallic phase. Moreover, the transmission electron microscopy investigation showed the presence of a thin (~250 nm) layer next to the ZnO. The chemical analysis accompanied by the selected area electron diffraction patterns indicated in both cases the same stoichiometric aluminium oxide but of different crystallographic structure, i.e., large crystals had α-Al2O3 structure while the layer was identified as metastable δ-Al2O3. The results were compared to those reported for interaction between liquid aluminium and monocrystalline ZnO.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Metal–ceramic Al–Al2O3 composites are a group of advanced engineering materials, which can be applied in the aviation and ground transport industry. Among methods available to fabricate such composites, one method utilizes in situ synthesis using the redox reactions between molten aluminium and binary or complex oxides such as SiO2, NiO, CoO, MgAl2O4, mullite, or kaolinite [1, 2]. As a result of these reactions, the ceramic and metallic networks interpenetrate each other and lead to the formation of a new material named co-continuous-ceramic-composite (C4). This extraordinary microstructure is responsible for the properties of the produced composites such as a high modulus, high strength, resistance to the thermal shocks and stability of the dimensions. Such a unique combination of advantages is practically impossible or much more expensive to obtain by conventional liquid-phase composites manufacturing methods. However, the mechanism of the formation of C4 structure is still not clear, particularly the role of processing parameters and type of initial oxide on the structure type and morphology of the reactively formed alumina phase [1–4].
The literature data provide information on the microstructure characterization of Al–Al2O3 composites reactively formed from various Al/MeO systems. Those most representative results are summarized in Table 1, showing that typical product of such reactions is α-alumina. However, some systems still need careful characterization and for others the existence of different types of Al2O3 should be confirmed.
The effect of temperature on the composite microstructure and the size of reactively formed alumina in the Al/SiO2 system at 973–1573 K was reported in [3, 5]. At low temperature of 973–1073 K, both Breslin et al. [3] and Yoshikawa et al. [5] evidenced the formation of the θ-Al2O3 only. At higher temperature, Breslin et al. [3] reported the formation of α-Al2O3 while Yoshikawa et al. [5] identified θ, γ and α-Al2O3 phases and commented that α-Al2O3 was not necessarily produced directly in the reaction, but due to the transformation from the θ-Al2O3. At 1073 K, the very fine θ-Al2O3 grains of several tens to hundreds of nanometers were observed, while at 1173 K the microstructure possessed complex morphology—fine grains of θ-Al2O3 phase and large α-Al2O3 (several micrometers in size) crystals coexisted. Yoshikawa et al. concluded that such a change in the microstructure can not be due to the thermal activation process but because of the differences in inherent size of the α-Al2O3 as the large grains formed separated regions. At the temperatures above 1273 K, α-Al2O3 became dominant and at 1373 K, the coalescence of the grains took place [5].
By applying high resolution transmission electron microscopy (HRTEM), Gao et al. [6] examined the microstructure of the reaction product region (RPR) formed after the interaction of aluminium and polycrystalline mulite at 1373 K. The authors distinguished two reaction zones in which the α-Al2O3 was identified as a main reactively formed oxide. Additionally, in the interface region between them Gao et al. found the presence of aluminium oxide of cubic structure with a lattice parameter of about 0.716 nm. The authors could not conclusively identify it as α-Al2O3 or any known metastable phase. These results are at variance with the investigations made later by Morgiel et al. [7] on the Al/mullite couples produced at 1273 K where only α-Al2O3 phase was identified.
Another system of interest, Al/TiO2, was examined in the temperature range of 1073–1373 K in [8]. It was demonstrated that molten Al reduces TiO2 to form fine alumina particles and Ti, which subsequently was dissolved in the Al drop. However, the reaction was remarkable only at high temperature of 1373 K and evidenced by the formation of well-distinguished RPR of C4 structure due to reactive metal penetration. Information about the type of alumina formed was not accessed. Avraham and Kaplan [9] performed the wetting experiments for Al/rutile system in the 973–1273 K temperature range. At 973 K, they did not detect any oxygen-rich layer at the drop/substrate interface. High resolution scanning electron microscopy analysis of the couples obtained at 1073 and 1173 K revealed the presence of very thin oxygen-rich interface layer of 0.16 and 0.3 μm in thickness, respectively. At 1273 K, a continuous (0.5 μm) Al2O3 layer was found both at the interface and within the cracks in its neighbourhood. It was described as α-Al2O3, however any experimental evidence was presented. Avraham et al. [10] studied the phase formation in ceramic matrix composites produced due to the pressure infiltration of Al–Si alloy and α-Al2O3–TiO2 preformes. It was found that the reduction of TiO2 (during further thermal annealing) resulted in the formation of intermediate phases TiO2 and γ-Al2O3. It was also postulated that γ-Al2O3 was formed after the reduction of TiO2 and transforms instantly to α-Al2O3.
With the aid of TEM characterization, the formation of α-Al2O3 phase was found by Sobczak et al. in the Al/B13O2 couple produced at 1173 and 1273 K [11]. Depending on processing temperature, the α-Al2O3 phase coexisted with two boron compounds, i.e. either the needles of AlB2 in low-temperature couples or both the AlB2 needles and coarse AlB12 crystals in high-temperature couples. What is interesting is that among the faceted α-Al2O3 grains Sobczak et al. detected fine precipitates identified as metastable spinel-like aluminium oxide of cubic structure similar to the γ-Al2O3 phase reported by Gao et al. [6] in Al/mullite couple. The possibility to form in situ the aluminium oxide of cubic structure in aluminium matrix is of practical importance since it increases significantly the wear resistance of such metal–ceramic composites, as reported by Sobczak et al. [11]. However, the formation of cubic aluminium oxide have been never confirmed in any other Al/MeO systems, except Al/mullite [6] and Al/B13O2 [11].
In the case of Al/NiO system, contradictory reports exist in the literature. Whereas Loehman et al. [12] were unsuccessful in infiltrating polycrystalline NiO by liquid Al at 1173–1473 K. On the other hand, Fahrenholtz et al. [13] reported infiltration using much higher temperature. They produced the NiAl–Al2O3 and Ni3Al–Al2O3 composites from the mixtures of Al and NiO or NiAl2O4 powders by reactive metal penetration at 1673 K for 2 h in ultra high purity argon gettered with titanium at temperatures above 973 K. On the contrary, a strong interaction in the temperature range of 973–1273 K was reported by Sobczak et al. for Al/NiO couples produced using single crystal NiO substrate [14]. Scanning electron microscopy analysis evidenced a thick RPR composed of very fine alumina particles surrounded by Al–Ni metallic matrix (having the same composition as inside the drop). After the solidification, both inside the drop and in the RPR, the formation of either Al3Ni (at 973–1073 K) or Al3Ni and Al3Ni2 phases took place. Nevertheless, the type of formed alumina was not discussed.
Studies concerning the interaction between liquid aluminium and cobalt oxide single crystal at 973 and 1273 K were described in [2]. This system showed significant reactivity accompanied with the formation of very fine α-Al2O3 particles enclosed within Al9Co2 or Al13Co4 phase. Similar to the Al/NiO couples, the process of RPR formation consisted of two stages: alumina formation and later reaction of produced Co metal with Al resulting in the formation of intermetallic compounds inside the metallic channels. More recent detailed examination of interfacial structure and chemistry in Al/CoOSC couples by HRTEM coupled with careful preparation of HRTEM specimens [15] clearly identified additional features such as the presence of a thin layer of pure Co at the CoO-side interface.
An interesting example of the redox reactions for the in situ synthesis of composites is the reaction between liquid aluminium and yttrium oxide, which was recently studied by two groups [16–19]. The RPR possessed C4 type microstructure [18, 19]; however, instead of alumina ternary oxides YAG (Al5Y3O12) or YAP (AlYO3) were observed after the interaction at 1273 K. They were interpenetrated by either Al3Y or Al2Y phases [19].
In the study by Sobczak et al. [20], strong reactivity with Al was reported for zinc oxide. The reactively formed oxide was suggested to be aluminium oxide without detailed identification of its type and structure. In the reviews [1, 2], Sobczak has examined the Al/ZnO system because, compared to numerous reactive Al/MeO systems, it is characterized by the formation of RPR composed of very large Al2O3 crystals while the RPR growth takes place despite a lack of wettability in the Al/ZnO system. High contact angles (θ = 111° on single crystalline substrate and θ = 140° on polycrystalline substrate) were recorded even at high temperature of 1273 K at which the reaction product (Al2O3) is wettable by molten Al (θ < 90°). Moreover, the in situ removal of oxide film from Al drop by capillary purification procedure was found to not improve the wetting properties as it is commonly observed for a wide group of Al/ceramic systems. Sobczak [2] suggested two reasons for such unusual behaviour: secondary roughening of initially smooth ZnO single crystal surface and secondary drop surface oxidation. The first conclusion came from the observation of fine alumina precipitates detected at the substrate surface around the Al drop. Their formation was explained by evaporative–reactive deposition caused from enlarged transport of Al vapour under UHV and its reaction with ZnO. The second conclusions came from the presence of fine Al2O3 precipitates at the drop surface, particularly localized close to the triple line. Their formation was explained by enhanced transfer of oxygen from the ZnO substrate to the drop surface caused by copious evaporation of Zn formed at the Al/ZnO interface due to the reduction of ZnO by Al.
Although previous studies [1, 2, 20] have demonstrated that Al/ZnO is a good candidate for in situ synthesis of Al–Al2O3 composites of C4 structure, the type of reactively formed Al2O3 as well as the detailed structure of reaction products region are still not well recognized since these investigations were limited to optical and scanning electron microscopy characterizations.
The aim of this study was to identify, by applying advanced techniques for structural characterization, the type and morphology of aluminium oxide formed due to the interaction between liquid aluminium and polycrystalline ZnO. The results obtained were compared to recent data reported for ZnO(0001) single crystal [21].
Experimental
The materials used were pure Al (99.9999%) and ZnO polycrystalline substrate (ZnOPC) produced by hot-pressing synthesis and polished to 150 nm finish. For comparison, the results of structural characterization of Al/ZnO interfaces recently obtained in [21] with ZnO single crystal substrate (ZnOSC) produced by crystal growth technology (hydrothermal method) with 0001 orientation and 2 nm roughness are also discussed.
The Al/ZnOPC couple was prepared in the sessile drop wettability test under dynamic vacuum produced by continuously working turbomolecular pump [20]. A special procedure, known as capillary purification or dispended drop method, described in [8], was applied. This procedure gives at least two important advantages, compared to classical sessile drop method, since it makes possible to avoid phenomena that may mask a real interaction in the Al/ZnO system, i.e., (1) non-contact heating of a couple of materials to avoid the effect of the interaction between reactive materials during heating to the to test temperature, (2) in situ removal of primary oxide film from the aluminium specimen directly in UHV chamber by squeezing the droplet through a graphite capillary and its deposition on the ZnO substrates at required temperature. Although the primary oxide film on aluminium has only 2-4 nm thickness it prevents the formation of a true drop/substrate contact and affects interfacial phenomena in the Al/ceramic systems [22].
For the Al/ZnOPC couple examined in this study, the droplet was produced at 1073 K in order to avoid unwanted interaction of liquid Al with graphite capillary used as a dispenser. Next, immediately after drop deposition, the Al/ZnOPC couple was rapidly heated (~14 K/s) to the test temperature of 1273 K, held isothermally at this temperature for 30 min, followed by slow cooling at a rate of ~12 K/s.
The reference Al/ZnOSC couple recently examined in study [21] was produced under dynamic vacuum using contact step heating to the same temperature of 1273 K starting from 973 K for 40 min, followed by the holding of the couple at 1073 K for 10 min, at 1273 K for 55 min and cooling at the same rate as the Al/ZnOPC couple. It should be noted that it was impossible to reproduce such heating conditions in the Al/ZnOPC test because of strong evaporation, compared to the Al/ZnOSC test.
Directly before each test, the ZnO substrate and freshly cut and mechanically cleaned Al specimen were ultrasonically cleaned in acetone and placed in a vacuum chamber. Following the wettability test, the solidified couples were cross-sectioned perpendicular to the substrate surface, ground and polished with a diamond suspension as the final step. Special attention was paid to obtain accurate parallel surfaces of the specimens prepared for the chemical analysis examination. Their microstructure was examined by optical microscopy using conventional and polarized light. Next, the samples were investigated with Philips XL 30 and FEI E-SEM XL30 Scanning Electron Microscopes (SEM) equipped with Link ISIS, EDAX energy dispersive X-ray (EDX) spectrometers.
Focused ion beam (FIB) technique accompanied with the transmission electron microscopy (TEM) was applied in order to characterize the material at the submicron scale. The selected areas of RPR were cut using Quanta 3D in order to prepare thin foils for the TEM observations. The TEM investigations were performed using TECNAI G2 FEG super TWIN (200 kV) microscope equipped with High Angle Angular Dark Field (HAADF) detector and integrated EDAX energy dispersive spectroscopy system. Selected area diffraction patterns were obtained using TEM Philips CM 20 Twin microscope.
Results
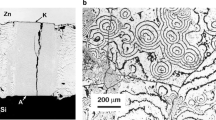
Structural examination of the Al/ZnOPC specimens was focused on the detailed characterization of the RPR of C4 structure formed inside the ZnO substrate under the drop (Fig. 1a, b, see arrow). The RPR had ~30 μm thickness and it was composed of the crystals from 1 to 10 μm in size. The EDS analysis confirmed that these crystals consisted of the Al2O3 phase, while Al with dissolved Zn was present in the surrounding channels.
The same phase composition was found recently in the Al/ZnOSC couple [21]. However, compared to the Al/ZnOPC, the RPR layer was almost twice as thick in Al/ZnOSC while the shape of the alumina crystals could be described as more rounded and much bigger (Fig. 1c, d).
The TEM combined with FIB preparation method was applied for the detailed observation under high magnification the microstructure of the region reactively formed in the ZnO substrate under the Al drop (reactive product region—RPR). Figure 2a, b illustrates the most representative bright-field images of the RPR/ZnOPC interface in comparison to those of RPR/ZnOSC in Fig. 2c, d. The interface was marked with white dotted line.
It can be noticed that in both cases, the large faceted crystals are surrounded by the metal matrix. Next to the ZnOPC substrate, a layer of about 250 nm in thickness was present (Fig. 2b). A closer inspection showed that it had a complex morphology: fine-grained one in the area closer to the substrate and columnar at the RPR side. The growth of the layer also took place in the case of single crystal ZnO substrate (Fig. 2d). However, the layer was thinner than that at RPR/ZnOPC interface and in its microstructure, the columnar grains were not clearly distinguished.
The chemical analysis of both large crystals and the thin interfacial layer showed the same chemical composition corresponding to the Al2O3 compound while their selected area electron diffraction patterns were different, as illustrated in Fig. 3a and b, respectively. The first was hexagonal α-Al2O3 phase characterized by lattice parameters of 4.76 and 12.99 nm, and the other was tetragonal δ-Al2O3 phase of 5.6 and 23.37 nm lattice parameters. The same phase type was found in the RPR/ZnOSC sample [21].
Figure 4 shows the maps of distribution of aluminium, zinc, and oxygen in the RPR/ZnOPC couple that were performed in order to find the composition of the areas between the columns of the layer, as marked by arrows in Fig. 4a.
They were concluded to be the voids as the Al, Zn and O maps revealed that inside the long channels none of the elements (Al, Zn and O) were present.
Discussion
In this study, the advanced SEM and TEM analysis of the Al/ZnO couples confirm the observations reported previously in [1, 2, 20] on the following redox reaction taking place between molten aluminium and zinc oxide:
The results show that at 1273 K, the formation of the RPR of typical C4 composite structure takes place within the ZnO substrate, and for both singe crystal and polycrystalline substrates it is composed of Al(Zn) and alumina interpenetrating networks. Phase analysis using selected electron diffraction patterns revealed that independently of the substrate type (ZnOPC and ZnOSC), the reactively formed alumina is α-Al2O3. However, in the Al/ZnO couples, the α-Al2O3 crystals are of greater size than those reported by Sobczak [1, 2] for other reactive Al/oxide systems (NiO, CoO, ZrO2, TiO2, SiO2, B13O2, mullite, kaoline, fly ash) over a comparable temperature range of 973–1273 K as well as those noted by Yoshikawa et al. [5] and Breslin et al. [3] for the Al/SiO2 couples at high temperatures.
According to a critical analysis of factors affecting the RPR formation of C4 structure in reactive Al/MeO systems by Sobczak [23], the large size of reactively formed alumina in the Al/ZnO system can be explained by unusually high volumetric mismatch between initial oxide (molar volume VZnO = 14.28 cm3/mol) and reactively formed alumina (\( V^{{{\text{Al}}_{ 2} {\text{O}}_{ 3} }} = 2 5. 6 2 \) cm3/mol). The formation of alumina by the redox reaction (2Al + 3ZnO = Al2O3 + 3Zn) results in the transformation of three moles of ZnO to one mole of Al2O3. This process, accompanied by ~40% reduction in volume, causes differential strains, breakage of freshly formed Al2O3 interfacial layer as well as the formation of wide discontinuities in the layer, all contributing to the growth of larger Al2O3 crystals.
Furthermore, the effect of the ZnO type on the thickness and morphology of the RPR was noted, i.e., it is almost twice as thin and its metallic Al(Zn) channels are much narrower in the case of polycrystalline substrate, compared to the single crystal couples examined in [20, 21]. The same relationship between the microstructure refinement and the type of substrate was observed by Rapp et al. [24] in the Fe/NiO reaction couple interacted at 1273 K. For both polycrystalline and single crystal NiO, the PRR was composed of the two interpenetrating phases (metallic Ni–Fe alloy and oxide FeO). However, for polycrystalline NiO, the microstructure was finer. The authors explained the difference in the morphology of this layer by dissimilar reaction mechanisms at the NiO/RPR interface. For NiO sintered-powder compacts, the fabrication method led to void formation. Such NiO compacts have pores that during reaction with Fe affect the growth and morphology of RPR. In addition, pores size depends on the grade of initial powder. Therefore, the interaction of Fe with sintered-powder compacts made from finer mesh NiO indeed resulted in a finer product RPR microstructure formed at the NiO/product interface.
Following explanation by Rapp et al. [24], thinner and finer microstructure of the RPR formed in the Al/ZnOPC samples contrary to Al/ZnOSC ones can be explained via an analogy. The use of sintered-powder compacts as end-members is not an unambiguous procedure if the experiment is performed like a diffusion couple experiment. In such a case, the density, segregation of impurities, etc. influence the morphology and growth rate of the product. Even, at the initial stage, a “barrier” for nucleation can take place. The crystallographic orientation can also govern the rate of the RPR growth. Moreover, dissimilar heating procedures used for the preparation of Al/ZnOPC and Al/ZnOSC samples may affect the growth of reactively formed alumina and thus more work is needed in order to understand how to control the morphology of in situ Al–Al2O3 composites.
Additionally, in this study, contrary to all available literature data on reactive Al/MeO systems, the detailed TEM + SEM examinations of Al/ZnOPC specimens revealed the formation of an unusual layer at the RPR/ZnOPC interface that is composed of δ-Al2O3 columnar crystals growing in the direction perpendicular to the surface of ZnO grains. Its dual microstructure can be compared to the chill and columnar zones in the ingot cross section. This suggests its possible formation during the cooling when at lower temperature, the δ-Al2O3 phase might be formed instead of α-Al2O3 and the solid-state diffusion is responsible for creation of dissimilar morphology of the reactively formed interfacial layer. For comparison, in the Al/ZnOSC specimens, this effect is less pronounced and the δ-Al2O3 layer consists of smaller crystals of 200 nm in size (Fig. 2d).
It is widely accepted that the δ-Al2O3 is the intermediate phase in the transformation from γ-Al2O3 to θ-Al2O3; therefore, its formation in the Al/ZnO couples under conditions of our study is not clear. Furthermore, the δ-Al2O3 columnar crystals seem to be not surrounded by metallic network since the presence of numerous voids between them was clearly distinguished (Fig. 4). Taking into account the fact that the same layer recorded in Al/ZnOSC specimens was free of any discontinuities [21] more work is needed in order to understand the mechanism of the formation and growth of the δ-Al2O3 layer at the RPR/ZnO interface.
The formation of different alumina types due to redox reaction took place also in other metal/oxide systems. Yoshikawa et al. [5] examined the Al/SiO2 couples by X-ray analysis and concluded that the α-Al2O3 is the main component in the couples produced at T > 1273 K while in couples obtained at 1073 K < T < 1273 K, different types of alumina were present: fine-grained θ and γ, which were subsequently and isothermally transformed into α-Al2O3 and coarse-grained α-alumina formed directly at higher temperatures [5]. Shen et al. [25] identified different Al2O3 phases in the Al/MgO couples, showing that the type of reactively formed alumina is not dependent on the production temperature but on the crystallographic orientation of the MgO single crystal: α for (100), κ, κ′ and δ for (110) and (111). This last study gives a new look at the complicity of redox reactions in the metal/oxide systems accompanied by diverse mechanisms of the formation of C4 structure. Therefore, further high resolution structural investigations of Al/ZnO couples produces with ZnO single crystals of different crystallographic orientations should be done for understanding the formation mechanism of such microstructure in Al/ZnO system, particularly the factors affecting the type and morphology of reactively formed alumina.
Moreover, the redox reactions may lead also to the formation of ternary oxides. Rapp et al. [24] investigated the interaction between the Fe and NiO and they found relatively thin layer next to the NiO substrate, which was composed of NiFe2O4 and Ni phases. Nevertheless, the authors consider its effect on the growth rate of the main RPR as negligible because of its relatively small thickness. The formation of ternary oxides was also reported for the Al/MeO systems. For Al/Y2O3, Barzilai et al. [16, 17] and Wojewoda-Budka et al. [19] evidenced the formation of YAG and YAP instead of Al2O3. For Al/MgO, Fujii and Nakae [26], Shen et al. [25] and Nowak et al. [27] noted Al2MgO4 together with Al2O3. Despite no clear evidence of any ternary oxides in the Al/ZnO specimens of our study the formation of AlZn2O4 phase in the Al/ZnO system may not be excluded. Thus, additional high resolution structural characterization should be performed on the specimens obtained under different contact time and temperature.
Summary
The interaction between liquid aluminium and polycrystalline zinc oxide substrate at 1273 K under vacuum is accompanied by redox reaction resulting in the formation of the RPR of about 30 μm in thickness that is composed of the alumina precipitates interpenetrated by Al(Zn) metallic channels. The RPR structure is typical to the C4 type reported previously in other reactive Al/Metal oxide systems. However, the Al/ZnO is found to be unusual not only in the sense of creation of very coarse Al2O3 particles inside the RPR, but also because of the formation of additional thin layer at the RPR/ZnOPC interface well distinguished under high TEM magnifications.
Moreover, two types of alumina have been indentified within the RPR in Al/ZnOPC, i.e., hexagonal α-Al2O3 crystals, as a main constituent and δ-Al2O3 columnar crystals, as a component of the interfacial layer between RPR and ZnOPC. In this study, the metallic network was not identified around the δ-Al2O3 columnar crystals.
Taking into account some important observations of the differences in the RPR structures formed with ZnOPC and ZnOSC substrates, further careful and systematic structural examination of the Al/ZnO couples must be performed using polycrystalline and single crystal zinc oxides. The influence of the processing temperature, temperature profile, and crystallographic orientation of ZnO on the morphology and type of reactively formed alumina must be determined. This will allow to understand the mechanisms of the formation and growth of both δ-Al2O3 interfacial layer as well as to identify the key factors affecting the morphology and phase composition of RPR of C4 structure formed between liquid aluminium and polycrystalline ZnO.
References
Sobczak N (2005) Solid State Phenom 101–102:221
Sobczak N (2006) In: Gupta N, Hunt WH (eds) Solidification processing of metal matrix composites. TMS Publications, OH, USA, pp 133–146
Breslin MC, Ringnala J, Seeger J, Marasco AL, Daehn GS, Fraser HL (1994) Cer Eng Sci Proc 15(4):104
Liu W, Koster U (1996) Scripta Mater 35(1):35
Yoshikawa N, Kikuchi A, Taniguchi S (2002) J Am Cer Soc 85(7):1827
Gao Y, Jia J, Loechman RE, Ewsuk KG (1995) J Mater Res 10(5):1216
Morgiel J, Sobczak N, Pomorska M (2009) In: Sobczak J (ed) Innovations in foundry, Part III (in Polish). Foundry Research Institute, Krakow, Poland, pp 101–108
Sobczak N, Stobierski L, Radziwill W, Ksiazek M, Warmuzek M (2004) Surf Interface Anal 36:1067
Avraham S, Kaplan WD (2005) J Mater Sci 40:1093. doi:10.1007/s10853-005-6922-4
Avraham S, Beyer P, Janssen R, Claussen N, Kaplan WD (2006) J Eur Ceram Soc 26:2719
Sobczak N, Morgiel J, Kharlamow A, Ksiazek M, Radziwill W, Baliga S (1998) Inzynieria Materialowa 4(105):754
Loehman RE, Ewsuk KG, Tomsia AP (1996) J Am Ceram Soc 79(1):27
Fahrenholtz WG, Ewsuk KV, Loehman RE, Tomsia AP (1996) Met Mater Trans 27A:2100
Sobczak N, Oblakowski J, Nowak R, Kudyba A, Radziwill W (2005) J Mater Sci 40:2313. doi:10.1007/s10853-005-1951-6
Morgiel J, Major L, Wojewoda-Budka J, Grzonka J, Pomorska M, Sobczak N (2007) In: Sobczak J (ed) Innovations in foundry, Part II (in Polish). Foundry Research Institute, Krakow, Poland, pp 239–246
Barzilai S, Aizenshtein M, Froumin N, Frage N (2006) Mater Sci Eng A 420:291
Barzilai S, Aizenshtein M, Shapiro-Tsoref E, Froumin N, Frage N (2007) Int J Adhesion Adhesive 27:358
Sobczak N, Nowak R, Radziwill W, Stobierski L (2008) In: Sobczak J (ed) Innovation in foundry, Part II (in Polish). Foundry Research Institute, Krakow
Wojewoda-Budka J, Sobczak N, Morgiel J (2010) J Microsc 237(3):253. doi:10.1111/j.1365-2818.2009.03237.x
Sobczak N, Kudyba A, Nowak R, Radziwill W, Oblakowski J (2005) Ceramika/Ceramics (Polish Ceramic Bull) 80:661
Wojewoda-Budka J, Sobczak N, Morgiel J, Nowak R (2010) Arch Metall, to be published
Sobczak N, Asthana R, Radziwill W, Nowak R, Kudyba A (2007) Arch Metall Mater 52(1):55
Sobczak N (2007) In: J. Sobczak (ed) Innovations in foundry (in Polish), Part II. Foundry Research Institute, Krakow, Poland, pp 187–198
Rapp RA, Ezis A, Yurek GJ (1973) Met Trans 4:1283
Shen P, Fujii H, Matsumoto T, Nogi K (2004) Acta Mater 52:887
Fujii H, Nakae H (1996) Acta Mater 44(9):3567
Nowak R, Sobczak N, Kudyba A, Radziwill W, Korpala B (2009) In: Sobczak J (ed) Innovations in foundry, Part III (in Polish). Foundry Research Institute, Krakow, Poland
Acknowledgement
This work has been supported by the Ministry of Science and Higher Education of Poland within Project No. N N507 272836.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wojewoda-Budka, J., Sobczak, N., Morgiel, J. et al. Reactivity of molten aluminium with polycrystalline ZnO substrate. J Mater Sci 45, 4291–4298 (2010). https://doi.org/10.1007/s10853-010-4379-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-010-4379-6