Abstract
Nanostructured palladium particles (nanorods, icosahedra, cubes) were synthesized in aqueous solution using a seeding-mediated approach with a structure-directing agent. These nanostructured Pd particles were then impregnated onto hydrogenotitanate nanotubes using two different impregnation procedures. The as-prepared catalysts were then tested in the selective hydrogenation of cinnamaldehyde at 323 K under 10 bars of H2. The selectivity is influenced by the morphology of the Pd nanostructured particles with a higher selectivity into saturated alcohols when the proportion of (111) Pd sites increases.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The selective hydrogenation of α,β-unsaturated aldehydes like cinnamaldehyde is of great importance for industrial applications. Indeed, the selective hydrogenation of C=O groups leading to the formation of unsaturated alcohols like cinnamyl alcohol is highly desirable since these compounds are important intermediates in the perfume industry. Similarly, the preferential hydrogenation of C=C bonds to unsaturated aldehydes such as hydrocinnamaldehyde have found applications in the drug industry [1].
This selective hydrogenation is still, however, a challenging task [2–5]. In this respect, the synthesis of new nanostructured materials opens new opportunities for creating more efficient and selective catalysts. Indeed, control of the morphology at the nanometer scale has attracted much attention recently due to the particular optical, electrical, and mechanical properties achievable through the formation of these new “tools” [6, 7].
Selective hydrogenation reactions are strongly influenced by strong metal–support interactions modifying the electronic properties of metallic sites. In this respect, TiO2 is well known to exhibit such properties. Panpranot et al. [8] have indeed shown that the generation of Ti3+ sites in TiO2 support can modify the electronic properties of Pd sites in close contact to each other resulting in a higher yield of ethylene during the selective hydrogenation of acetylene. Selectivity of hydrogenation reactions can also be enhanced through a control of the nanomorphology of the support. In this respect, titanate nanotubular materials, prepared by hydrothermal treatment of TiO2 particles in strong alkaline solutions [9–25], present high surface areas coupled with the possibility of controlling their nanomorphology making them potential candidates as catalytic supports. However, only one study by Sikhwivhilu et al. [26] showed that Pd particles supported on titanate nanotubes exhibit the highest activity and cyclohexanone selectivity among Pd/TiO2-related catalysts during the phenol hydrogenation.
The control of the morphology could also be achieved for metallic nanoparticles leading to well-defined morphologies (nanorods, icosahedra, cubes) with preferential exposition of either (100) or (111) planes. This shape-controlled formation of well-defined Pd nanoparticles is generally achieved through the selective interaction of ions, ligands, polymers, or surfactants with specific crystallographic facets leading to a preferential growth along a particular direction. About palladium, two different strategies were envisaged: (1) a polyol process in which a palladium salt is reduced by ethyleneglycol in the presence of a polymer, generally poly(vinylpyrrolidone (PVP) [27], or (2) in aqueous media through the reduction of the Pd salt by ascorbic acid [28] or citric acid [29] in the presence of cetyltrimethylammonium bromide (CTAB) [28] or PVP [29]. Better control over nucleation and growth is obtained by adding Pd seeds (isotropic 3–6 nm Pd particles) to the growth solution. Seed structure [30] and growth kinetics [31] are two important parameters controlling the final morphology of the nanocrystals. Controlling the proportion of the different crystallographic planes exposed to the reactants could greatly modify the selectivity for structure-sensitive reactions like the selective hydrogenation of α,β-unsaturated aldehydes. As demonstrated by Delbecq et al. [32, 33], the formation of the most dense (111) planes limits the C=C coordination of unsaturated aldehydes thus favoring the selectivity into alcohols.
In this work, titanate nanotubes (HNT) were elaborated by alkaline hydrothermal treatment of TiO2 (P25). The supports were then impregnated with nanostructured Pd particles presenting well-defined shapes (cubes, rods, icosahedra) and obtained through a seed-mediated approach in the presence of a structure-directing agent, cetyltrimethylammonium bromide (CTAB). The as-prepared catalysts were then tested in the selective hydrogenation of cinnamaldehyde.
Experimental
Support preparation
A commercial TiO2 powder (P 25, Degussa AG), with a surface area of 50 m2/g, was used as precursor. About 0.5 g of TiO2 was mixed with 15 mL of 11.25 mol/L NaOH aqueous solution, followed by hydrothermal treatment at 403 K in a teflon-lined autoclave for 20 h [34]. The treated powders were then washed with 1 L of ultrapure water and neutralized with a 0.1 mol/L HCl aqueous solution until pH = 7, and subsequently filtered and dried at 353 K overnight.
Nanostructured palladium preparation
The preparation of nanostructured Pd particles was made in two steps. The first step consists in the preparation of a seed solution following the method developed by Nikoobakht et al. [35] and Berhault et al. [36]. An aqueous solution containing 25 mL of Na2PdCl4 (5.10−4 M) and 50 mL of CTAB (0.3 M) was prepared. Next, 6 mL of ice-cold 0.1 M freshly prepared NaBH4 solution was injected into the solution all at once while stirring vigorously. The color of the solution turned dark immediately indicating the formation of small Pd nanoparticles or “seeds”. If ex situ prepared (see below), the second step is the preparation of the growth solution obtained by mixing 50 mL of CTAB (0.24 M) with 50 mL of Na2PdCl4 (3.10−3 M) and 2.8 mL of freshly prepared ascorbic acid (0.08 M). Finally, 120 μL of silver seed solution was added. The growth solution was left for 48 h before TEM analysis.
Catalyst preparation
The HNT supported Pd nanoparticles were obtained using two different impregnation procedures: (1) an ex-situ method in which the nanostructured Pd particles were first obtained by injecting seeds into the growth solution before impregnating it onto the support, or (2) an in situ method in which Pd seeds were first impregnated onto the support before contacting with the growth solution. This latter method maximizes the interference of the support during the growth step leading to different final nanomorphologies of the Pd nanocrystals compared to the ex situ impregnation in which the support did not influence at all the growth process. These slightly modified impregnation procedures provide a simple way for modifying the relative proportions of crystallographic facets [36]. In both cases, after maturation 24 h at 303 K, the solid was washed with ethanol before being dried at 303 K overnight. This washing procedure was essential to remove efficiently the CTAB precursor around the Pd particles [28]. Indeed, by blocking selectively (100) facets [37], CTAB can strongly modify the final selectivity avoiding any relationship between selectivity modifications and the catalyst nanomorphology. The Pd contents are 2.1 and 1.1 wt% for the Pd/HNT ex situ and Pd/HNT in situ catalysts, respectively.
For comparison purposes, an isotropic Pd/HNT catalyst was also prepared as follows: (1) incipient wetness impregnation of 350 mg of HNT with 1 mL of a 6.6 mM solution of Pd(NO3)2 prepared in an ammoniacal buffered solution at pH higher than the PZC (point of zero charge) of titanate (pH = 9), and (2) drying overnight at 353 K. The Pd content was 0.2 wt%.
Characterization techniques
X-ray diffraction (XRD) patterns of the prepared support were obtained on a PANanalytical XPERT MPD Pro diffractometer using Co Kα radiation (λ = 1.789 Ǻ). The N2 adsorption–desorption isotherm at 77 K was performed using a Micromeritics ASAP 2000 instrument to determine the Brunauer–Emmett–Teller (BET) surface area and to estimate the pore size distribution using the Barrett–Joyner–Halenda (BJH) method. Degassing pre-treatment was performed at 423 K 16 h at 10−6 atm. Transmission electron microscopy (TEM) studies were performed in a JEOL 2010 instrument operating at 200 kV.
Catalytic test
Cinnamaldehyde hydrogenation was performed in liquid phase using a laboratory scale stainless-steel batch reactor working under static conditions. The batch was first filled with 150 mL of isopropanol. About 50 mg of the catalyst was then put into contact with 400 mg of cinnamaldehyde at room temperature under 10 bars of H2. The autoclave was then purged three times with N2 before being heated at 323 K. When the final temperature was reached, the autoclave was stirred at 500 rpm. The course of the reaction was followed by analyzing samples by gas chromatography (VARIAN CP 3380—plot alumina column PONA, L = 50 m, split injector; FID detection) at regular time intervals considering the beginning of the stirring as the initial reaction time. Due to the low surface-to-volume ratios of the Pd particles, turnover frequency (TOF) values were only calculated based on dispersion values determined by considering the percentages of the different nanoobjects and their respective proportions of (100) and (111) facets.
Results and discussion
Characterisation of the HNT support
The HNT support was extensively characterized in a previous study [34, 38]. Therefore, herein only a short summary of its characterization will be presented. XRD study of the HNT support showed that titanium oxide P25 was transformed into a H2Ti2O5-like phase during the hydrothermal treatment under concentrated NaOH solution (Fig. 1). Sodium was partly replaced by hydrogen during the HCl treatment leading to the following HxNa2−xTi2O5·H2O (x ~ 1.61) stoichiometry. This support also presents a high-surface area and a mesoporous organization characterized by a type IV isotherm and a well-developed hysteresis loop [34, 38]. Textural properties for this HNT support are summarized in Table 1.
Complementary TEM studies also showed clearly that the HNT support presents a tubular morphology with nanotubes exhibiting an average diameter of about 8–12 nm.
Characterization of HNT-supported nanostructured palladium
The TEM studies showed that different nanostructured palladium particles with well-defined shapes (mainly icosahedra, rods, cubes, or triangular plate-like particles) were obtained after the ex situ impregnation of the well-defined Pd nanoparticles on the HNT support (Fig. 2a). Previous work has elucidated the morphology and the different proportions of facets exposed on the surface of the different Pd nanocrystals [31, 39]. While icosahedra and triangular-like nanoplates exposed mainly (111) facets, cubes preferentially expose (100) facets. Fivefold-symmetrical nanorods exhibit (100) lateral facets and (111) planes at their extremities. Finally, HAADF-STEM tomographic studies have unambiguously shown that bipyramids expose 50% of (100) and 50% of (111) facets [39]. These proportions evidently correspond to perfectly defined geometries, which are sometimes not observed for deformed particles. These particles present the same relative shape proportion and size than the one found in the growth solution before impregnation. Pd particles were then simply impregnated onto hydrogenotitanate nanotubes without modification of their shape during this step (Figs. 2, 3, and Table 2). This situation greatly differs from the one observed for the Pd particles obtained by the in situ impregnation. In this case, 90% of nanoparticles were multiply twinned icosahedra particles (MTPs) with a more or less deformed hexagonal aspect (Figs. 2b, 3b). This difference might result from the different procedure of preparation used for the in situ method. Indeed, in that case, small Pd isotropic particles (“seeds”) were impregnated first onto the HNT support leading to a strong interaction between the support and the seeds before any growth step. The further stabilization induced by the support may strongly modify the electronic properties of the seeds and/or the seed structure. In this latter case, it was recently demonstrated that the initial seed structure could strongly influence the final morphology [30]. Multiply twinned particles with (111) facets would evolve into icosahedral particles if stabilization of (100) facets is not possible. This suggests that the stabilization induced by the titanate support would strongly favour the restructuration of seeds in order to expose almost only (111) facets without any possible further stabilization of (100) facets during the growth step. Their deformed aspect is another proof that the titanate support strongly perturbs the growth of the icosahedral particles. This thermodynamic-controlled evolution of the morphology of Pd nanocrystals is, however, not related to any change of the experimental conditions for the growth solution since similar concentrations in surfactant, reducing agent, and Pd salt were used in both in situ and ex situ methods. This evolution is more related to the further stabilization of the seed structure induced by the support during its growth.
The size of the nanostructured Pd particles is also dependent on the method of preparation. While large Pd nanoparticles were obtained following the ex situ impregnation, much smaller particles were synthesized during the in situ procedure. For instance, the size of the icosahedra particles decreased from 67 to 22 nm when going from the ex situ to the in situ method. This situation differs from the one observed for Pd particles supported on α-Al2O3 [36]. In this previous case, the smallest Pd particles were obtained with the in situ preparation method. The difference of support interaction between α-Al2O3 and titanate could explain such a result. On α-Al2O3, the weak interaction of the support led to a loss of seeds, which were not impregnated on the support leading after contact with the growth solution to a lower intrinsic Pd seed/Pd growth ratio. This lower ratio favors the formation of bigger particles [40]. On titanate, the strong support interaction will minimize the loss of seeds during the impregnation procedure. Moreover, recent results have shown that the injection of Pd seeds into a growth solution led to a second heterogeneous nucleation forming small nuclei in solution, which are selectively aggregated to the bigger seeds during the growth step [41]. The fact that in the situ method of preparation, seeds are already impregnated would still lead to a second heterogeneous nucleation but the nuclei could interact strongly with the support before aggregating to the former seeds generating in fine new centers for growth by agglomeration (new “seeds”). This would lead finally to a higher intrinsic Pd seed/Pd growth ratio, which would favor the formation of smaller Pd particles.
Cinnamaldehyde hydrogenation catalytic activity
The different Pd/HNT catalysts were tested in the selective hydrogenation of cinnamaldehyde. The reaction scheme of the cinnamaldehyde hydrogenation proceeds via parallel and consecutive pathways that involve hydrogenation of C=O and C=C groups forming cinnamyl alcohol (CA), hydrocinnamaldehyde (HCALD), and 3-phenylpropanol (PP). On Pd catalytic systems, the reaction occurs preferentially through the initial hydrogenation of the C=C bond leading to hydrocinnamaldehyde, whereas selective C=O bond hydrogenation is quite limited [42]. The reaction scheme herein is formed of two parallel pathways, one corresponding to a preferential C=C bond hydrogenation of cinnamaldehyde into hydrocinamaldehyde and another one leading through the initial hydrogenation of the C=O bond to cinnamyl alcohol followed by the rapid hydrogenation into 3-phenylpropanol (Fig. 4).
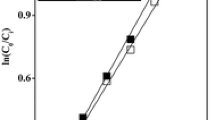
The catalytic results reported in Table 3 showed similar activities and different selectivity values between the different nanostructured Pd/HNT catalysts. The Pd/HNT ex situ catalyst exhibits a higher selectivity in hydrocinnamaldehyde than the Pd/HNT in situ catalyst. XPS analysis did not detect the presence of bromide while 1.4 at.% N and 15–19 at% C were detected on nanostructured Pd/HNT catalysts. As shown by Di Gregorio et al. [37], bromide coming from the CTAB surfactant can selectively poison (100) facets modifying selectivity. Herein, the absence of Br can rule out such a poisoning effect on selectivity. The atomic percentages of C and N would correspond to the elimination of 85% of the cetyltrimethylammonium moieties coming from CTAB during the washing procedure. Due to its cationic character, the remaining cetyltrimethylammonium species are more probably spread onto the titanate support without being in close contact with the Pd particles minimizing its impact on selectivity. Therefore, the selectivity change is more related to the relative proportions of (100) and (111) facets of the nanostructured catalyst. Indeed, the selective hydrogenation of α,β-unsaturated aldehydes is a structure-sensitive reaction whose selectivity depends on the relative proportion of (100) or (111) facets [32]. Since cubes expose preferentially (100) facets, icosahedra, (111) facets, and rods, a mixture of lateral (100) facets and (111) extremities, a rough calculation showed that the Pd/HNT ex situ catalyst would be composed of 2/3 of (111) sites and 1/3 of (100) sites, while Pd/HNT in situ would mainly expose 90% of (111) sites. This calculation should be considered cautiously since the Pd/HNT in situ catalyst mainly presents deformed icosahedra particles. However, even taking into account this point, the Pd/HNT in situ catalyst would present a very high proportion of (111) sites. According to Delbecq et al. [32, 33], these sites would be more selective for the hydrogenation into saturated alcohols. Since the Pd/HNT in situ catalyst is formed of smaller Pd particles, its dispersion value will be higher than for the Pd/HNT ex situ catalyst (6.1 vs. 2.5%). This would lead to a TOF value 2.5 times higher for the Pd/HNT ex situ catalyst (0.89 s−1 vs. 0.35 s−1). Since this latter catalyst exposes a higher proportion of (100) sites, this result is in agreement with previous studies showing a higher intrinsic hydrogenation activity of Pd(100) sites [36].
Results were also compared to an isotropic Pd/HNT catalyst. Even if the lower surface-to-volume ratio of the isotropic catalyst led obviously to much higher activity, comparison of TOF values showed that the intrinsic activity per site is only 30% higher than for the Pd/HNT ex situ catalyst. Moreover, the selectivity in PP is the lowest among all the studied catalysts. This result is in agreement with those acquired for nanostructured Pd catalysts since, due to its small size, the isotropic catalyst will expose a higher amount of corner, defect, or edge sites. These more open sites would present similar selectivity properties to those expected for (100) sites.
Conclusions
Palladium nanostructured particles can be prepared using a seeding-mediated approach leading to well-defined morphologies. Hydrogenotitanate (HNT) nanotubes elaborated by hydrothermal treatment of TiO2 particles were used as support for these nanostructured Pd particles. The growth of the Pd nanoparticles was performed either in solution followed by impregnation on the HNT support (ex situ) or from Pd isotropic particles (“seeds”) first impregnated on titanate and contacted with a “growth” solution (in situ). Different Pd morphologies were obtained depending on the method of preparation. Moreover, the titanate support partly hindered the growth of the nanostructured particles and induced the formation of only deformed icosahedra particles. The catalytic results reveal that the selectivity is influenced by the shape of the Pd nanostructured particles with a higher selectivity into saturated alcohols when the proportion of (111) Pd sites increases. Further work is in progress in order to explore in detail the relationship between the exposition of Pd(111) planes and the variations of selectivity.
References
Tessonnier JP, Pesant L, Ehret G, Ledoux MJ, Pham-Huu C (2005) Appl Catal A Gen 288:203
Singh UK, Vannice MA (2001) Appl Catal A Gen 213:1
Ponec V (1997) Appl Catal A Gen 149:27
Sivakumar T, Mori T, Kubo J, Morikawa Y (2001) Chem Lett 30:860
Sivakumar T, Krithiga T, Shanthi K, Mori T, Kubo J, Morikawa Y (2004) J Mol Catal A Chem 223:185
Xia Y, Yang P, Sun Y, Wu Y, Mayers B, Gates B, Yin Y, Kim F, Yan H (2003) Adv Mater 15:353
Bavykin DV, Friedrich JM, Walsh FC (2006) Adv Mater 18:2807
Panpranot J, Kontapakdee K, Praserthdam P (2006) J Phys Chem B 110:8019
Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K (1998) Langmuir 14:3160
Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K (1999) Adv Mater 11:1307
Du GH, Chen Q, Che RC, Yuan ZY, Peng LM (2001) Appl Phys Lett 79:3702
Seo DS, Lee JK, Kim H (2001) J Cryst Growth 229:428
Zhu Y, Li H, Koltypin Y, Hacohen YR, Gedanken A (2001) Chem Comm 2616
Yuan ZY, Zhou W, Su BL (2002) Chem Comm 1202
Chen Q, Du GH, Zhang S, Peng LM (2002) Acta Cryst B 58:587
Zhang Q, Gao L, Sun J, Zheng S (2002) Chem Lett 31:226
Wang YQ, Hu GQ, Duan XF, Sun HL, Xue QK (2002) Chem Phys Lett 365:427
Chen Q, Zhou W, Du G, Peng LM (2002) Adv Mater 14:1208
Ma R, Bando Y, Sasaki T (2003) Chem Phys Lett 380:577
Sun X, Li Y (2003) Chem Eur J 9:2229
Tian ZR, Voigt JA, Liu J, Mckenzie B, Xu HJ (2003) J Am Chem Soc 125:12384
Yang J, Jin Z, Wang X, Li W, Zhang J, Zhang S, Guo X, Zhang Z (2003) Dalton Trans 3898
Yuan ZY, Su BL (2004) Coll Surf A 241:173
Tsai CC, Teng H (2004) Chem Mater 16:4352
Thorne A, Kruth A, Tunstall D, Irvine JTS, Zhou W (2005) J Phys Chem B 109:5439
Sikhwivhilu LM, Coville NJ, Naresh D, Chary KVR, Vishwanathan V (2007) Appl Catal A Gen 324:52
Xiong Y, Chen J, Wiley B, Xia Y, Aloni S, Yin Y (2005) J Am Chem Soc 127:7332
Piccolo L, Valcarcel A, Bausach M, Thomazeau C, Uzio D, Berhault G (2008) Phys Chem Chem Phys 10:5504
Lim B, Xiong Y, Xia Y (2007) Angew Chem Int Ed 46:7157
Xiong Y, Xia Y (2007) Adv Mater 19:3385
Berhault G, Bausach M, Bisson L, Becerra L, Thomazeau C, Uzio D (2007) J Phys Chem C 111:5915
Delbecq F, Sautet P (1995) J Catal 152:217
Delbecq F, Sautet P (2002) J Catal 211:398
Kochkar H, Lakhdhar N, Berhault G, Bausach M, Ghorbel A (2009) J Phys Chem C 113:1672
Nikoobakht B, El-Sayed MA (2003) Chem Mater 15:1957
Berhault G, Bisson L, Thomazeau C, Verdon C, Uzio D (2007) Appl Catal A Gen 327:32
Di Gregorio F, Bisson L, Armaroli T, Verdon C, Lemaitre L, Thomazeau C (2009) Appl Catal A Gen 352:50
Kochkar H, Turki A, Bergaoui L, Berhault G, Ghorbel A (2009) J Colloid Interf Sci 331:27
Benlekbir S, Epicier T, Bausach M, Aouine M, Berhault G (2009) Phil Mag Lett 89:145
Perez-Juste J, Liz-Marzan LM, Carnie S, Chan DYC, Mulvaney P (2004) Adv Funct Mater 14:571
Berhault G, Bausach M (2008) Mater Res Symp Proc 1087:V06-06
Gallezot P, Richard D (1998) Catal Rev Sci Eng 40:81
Acknowledgement
This work has been supported by the “Action Intégrée Franco-Tunisienne du Ministère des Affaires Étrangères et Européennes Français et du Ministère de l’Enseignement Supérieur de la Recherche Scientifique et de la Technologie Tunisien”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jabou, K., Kochkar, H., Berhault, G. et al. Preparation and catalytic activity of nanostructured Pd catalysts supported on hydrogenotitanate nanotubes. J Mater Sci 44, 6677–6682 (2009). https://doi.org/10.1007/s10853-009-3632-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-3632-3