Abstract
This study is concerned with investigation of forming Ti fiber reinforced TiAl3 composite by infiltration-in situ reaction. The as-cast material was obtained by pressing molten pure Al into a preform which was composed of Ti particles and Ti fibers. Based on the differential scanning calorimetry (DSC) result, in situ reaction samples were obtained by heating as-cast materials to 660, 950, and 1300 °C, and held for 1 h, respectively. The microstructure evolution of in situ reaction samples was analyzed by scanning electron microscope and Energy dispersive X-ray (EDX). In addition, the phase composition of products was inspected by X-ray diffraction (XRD). Experiment results show that TiAl3 was formed initially, which was the unique product between Ti and Al. While at high temperature, products of Ti fibers and Al were complex, and TixAl1−x (0.25 < x < 0.75) compounds were formed around Ti fibers. Finally, TiAl3 decomposed, and oxidation occurred. The mechanism of in situ reaction between Ti and Al in this system was discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Titanium aluminide compounds (Ti3Al, TiAl, TiAl3) have been investigated extensively as potential high temperature structural materials [1, 2]. This is due to their outstanding engineering performances, such as excellent acid/alkali corrosion resistance, high specific modulus and strength [3]. Especially TiAl3 has many attractive characteristics such as low density (~3.3 g/cm3), high hardness and high melting temperature (~1390 °C). However, the intrinsic low tensile ductility at room temperature and poor high temperature strength has limited its application [4, 5]. Alloying with fourth-period transition elements (such as Cr, Mn, Fe and Co) and reinforcing with particles (such as TiC, TiB2, TiN) which have not resulted in great improvement of ductility at room temperature [6–8].
Recently, the introduction of long fiber reinforcements in these compounds might overcome the disadvantage [9–11]. The reinforcement is often silicon carbide (SiC/C) monofilaments with a thin carbon protective coating. However, above 800 °C, the carbon protective coating of the continuous reinforcement tends to react with matrix during consolidation, leading to deterioration of mechanical and physical properties of resulting composites [12, 13]. It was stated by Zhang et al. [14] that TiNb fiber could be introduced into titanium aluminide matrix by using a hot-pressed fabrication technology. On the other hand, He and Hu [15] studied a Ti alloy matrix composite with Ti fiber reinforcement and investigated the interface reactant between fibers and the matrix.
The present study suggests a new way to fabricate TiAl3 matrix composite (Ti fiber as a reinforcement) by infiltration-in situ reaction technology. The microstructure evolution, and phase formation were analyzed, and reaction mechanism was investigated.
Materials and experimental methods
Ti fiber reinforced TiAl3 composite which was prepared by infiltration-in situ reaction method. Ti particles (38 μm in diameter), Ti fibers (120 μm in diameter), and pure Al were used as raw materials. The nominal composition of Ti particles and Ti fibers is shown in Table 1. The stoichiometric ratio of Ti particles to Al was about 1:3, and the volume fraction of Ti fibers was about 35%.
Ti fibers were coated by Ti particles, and coated fibers were aligned in a steel mold. Molten pure Al was pressed into the mold, and then the mold cooled rapidly, subsequently, the as-cast material was obtained.
Ti and Al (Ti fiber and Al, as well as Ti particle and Al) in situ reaction sequence was studied by differential scanning calorimetry (DSC). The as-cast sample was heated to 1400 °C, at a rate of 10 °C/min, in an STA 449C DSC. Based on the DSC result (there were exothermic peaks at 660, 950, and 1300 °C), as-cast samples were heated to 660, 950, and 1300 °C, and held for 1 h at different temperature, respectively. Reaction temperatures were accurate to ±5 °C.
An S-4700 scanning electron microscopy (SEM) was used for morphology examination. Energy dispersive X-ray (EDX) was used to identify and calculate the approximate elemental composition of products. X-ray diffraction (XRD) was performed using Philips X’pert X-ray diffraction device for phase analysis, and the patterns were compared with standard spectra from powder diffraction files.
Results and discussion
As-cast composite
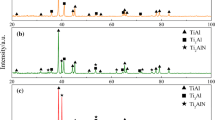
Figure 1 shows the SEM microstructure and the corresponding XRD pattern of as-cast composites. The micrograph indicates that a thin reaction layer (an average thickness of 2 μm) was formed between Ti fiber and Al (Fig. 1a). EDX result shows that this product was TiAl3 (the atomic ratio of Ti:Al was about 1:3), and a thin layer (~ 2 μm) of TiAl3 phase was also formed between Ti particles and Al.
Formation of TiAl3 was also confirmed by XRD (Fig. 1b). TiAl3 diffraction pattern was observed besides the Ti and Al patterns (they are raw materials), which indicates the interface reactant of Ti and Al was formed.
DSC curve
Figure 2 shows the DSC curve of as-cast materials. An exothermic peak at about 653 °C was recorded. Subsequently, an endothermic peak at about 656 °C was detected, which indicates the melting of Al. Following the endothermic peak, a sharp exothermic peak at about 660 °C can be observed. By heating the as-cast material to high temperature, other two exothermic peaks can be seen (at about 950 and 1300 °C, respectively).
In order to understand the characteristics of microstructure evolution and phase transformation during in situ reaction, as-cast materials were heated to three different temperatures of 660, 950, and 1300 °C (based on the DSC result), and held for 1 h, respectively.
In situ reaction composite
When the as-cast material was heated to 660 °C and held for 1 h, the SEM image was shown in Fig. 3. It is obvious that the thickness of reacting layer around Ti fiber increased. At the same time, the reacting layer thickness also increased between Ti particles and Al.
The extent of reaction increased with increasing temperature. Figure 4a is the SEM image of as-cast sample was held at 950 °C for 1 h, in which there were four sequential layers around Ti fiber. An expanded view of the interface between Ti fiber and Al was shown in Fig. 4b, which marked with A, B, C, D, and E in different atomic number contrast. The element concentrations at different positions were measured by EDX, as illustrated in Fig. 5. From “A” to “E”, Ti and V decreased, while Al increased.
Chemical composition of different positions in Fig. 4
While at this stage, Ti particles eliminated, and lumpy shaped particles were formed. Higher magnification SEM photographs of the matrix in Fig. 4a was shown in Fig. 4c. EDX indicates that these particles were TiAl3. Some white products were observed around these lumpy particles. These white products were composed of Ti, Al, and O (Ti 27.55%, Al 53.03%, O 19.4%), which implies that oxidation occurred at this temperature.
At 1300 °C, the thickness of reacting layers around Ti fiber increased (Fig. 6a), and the element concentrations at different positions (Fig. 6b) were shown in Fig. 7. From “F” to “J”, Ti and V decreased, while Al increased.
Chemical composition of different positions in Fig. 6
TiAl3 in the matrix decomposed, and islands TiAl3 phases and rodlike shape particles (Ti 42.91%, Al 26.81%, O 30.27%) formed. The higher magnification SEM photograph was shown in Fig. 6c.
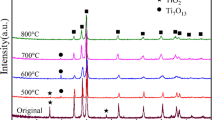
Figure 8 shows XRD patterns of in situ reaction samples. When the sample was held at 660 °C for 1 h, there were TiAl3 and Ti phases (mainly Ti fiber from SEM observation) without Al diffraction peaks. With an increase in temperature, at 950 °C, Al2O3 and Ti4O7 XRD patterns were generated, besides the formation of TiAl3. At 1300 °C, the intensity of TiAl3 peaks decreased, but the relative intensity of TiO2 and Al2O3 increased.
In addition, it is noted that multiphase products of Ti–Al compounds (besides TiAl3) around Ti fiber are observed by SEM, but they have not been detected in XRD patterns, which suggests the quantities of them are below the resolution of the instrument.
Thermodynamic analyses
It is clear from the aforementioned results that two former exothermic peaks in the DSC curve can be considered as a big exothermic peak, which was interrupted by the melting of Al. Because the exothermic reaction began before the melting of Al, but the reaction rate was slow and the heat was consumed by the melting of Al, the total heat was dropped. On the other hand, the activity of Al increased quickly after Al melted, and the reaction between Ti and Al was promoted. When the heat covered the melting of Al, the second exothermic peak appeared. Therefore, the exothermic reaction of Ti and Al preceded the melting of Al, and the melting temperature of Al was at about 656 °C.
The possible products at whole temperature range can be evaluated by comparing their free energy change (∆G). Using thermodynamic data, the ∆G of Ti–Al intermetallics can be plotted as function of temperature. Figure 9 shows this relationship between ∆G and temperature, ∆G curves for TiAl2 and Ti2Al5 are lower than others, but the formation of them is based on the formed TiAl [16]. Both ∆G curves for Ti3Al and TiAl are straight lines, but they have different sloping ratio so that a cross point can be appeared (the cross point at about 700 K). ∆G of TiAl3 is more negative than Ti3Al and TiAl in the temperature range of 200–1600 K, indicating that the formation of TiAl3 is easier than direct formation of Ti3Al and TiAl. Thus, the product at 660 °C was TiAl3.
The third broad exothermic peak (at about 950 °C) shown by the DSC curve is possible due to the formation of multiphase products (Ti–Al compounds), and the formation of oxidation.
TiAl3 was the unique product between Ti particle and Al. While multiphase products of Ti–Al compounds were formed between Ti fiber and Al. The possible reaction between Ti fiber and Al is shown below.
According to Kattner [17], ∆G for reaction 2 is that ∆G < 0, and the reaction can proceed.
The different products between two couples (Ti fiber and Al couple, Ti particle and Al couple), it is related to the content of Al in different parts. For Ti particle and Al couple, the amount of Al was high, Al diffused continuously along the grain boundaries of TiAl3 and to be reaction interface, and reaction 1 proceeded constantly, therefore, TiAl3 was the unique product. For Ti fiber and Al couple, the amount of Al around Ti fiber was limited, Al was consumed soon and reaction 2 began. Al in TiAl3 is a very active element, it diffused along grain boundaries and formed TixAl1−x (0.25 < x < 0.75) compounds [18]. In addition, because of O existence, Al2O3 and Ti4O7 were detected.
When the temperature reached 1300 °C, the temperature at the reactive interface rose over the melting point of TiAl3 (1390 °C), due to high exothermic heat [19], and local TiAl3 phases melted, leading to islands of TiAl3 existence in the matrix.
Accompanied with TiAl3 decomposition, some rod-shaped products formed. EDX reveals that these were Ti and Al oxides, which were detected as TiO2 and Al2O3 by XRD. The microstructure and composition of oxidation products are very complex and the reader is referred to previous publications for a more detailed description [20–22]. Since endothermic heat of TiAl3 decomposition is smaller than exothermic heat of oxidation formation, the fourth sharp exothermic peak is owing to the formation of Al2O3 and TiO2.
Combining both metallographical and thermoanalytical results, the possible reaction mechanism of Ti fiber reinforced TiAl3 composite could be separated into three stages.
First stage, TiAl3 is generated. Below and at the melting point of Al, the product of Ti fiber and Al couple and the product of Ti particle and Al couple are TiAl3.
Second stage, Ti–Al compounds are formed. With the increasing temperature, the content of TixAl1−x (0.25 < x < 0.75) will be rearranged according to phase equilibrium through elements diffusion.
Last stage, TiAl3 decomposes and oxidation occurs. TiAl3 is an unstable phase at higher temperature, accompany with the decomposition of TiAl3, TiO2, and Al2O3 are formed.
A model of the infiltration-in situ reaction mechanism of Ti fiber reinforced TiAl3 composite is shown in Fig. 10.
Conclusions
The reaction product between Ti particle and Al was TiAl3 under a particular range of holding temperature. TiAl3 decomposed when reaction temperature at 1300 °C.
The reaction product between Ti fiber and Al were complex. TiAl3 was formed at Ti fiber and Al interface initially, when the as-cast material was heated to 950 °C, multiphase Ti–Al compounds were formed due to interaction between Ti and TiAl3.
At high temperature (>950 °C) oxidation occurred. Al2O3, Ti4O7, and TiO2 were formed.
Ti fiber reinforced TiAl3 composite has been prepared by infiltration-in situ reaction. The reaction among Ti fiber, Al, and Ti particle can be separated into three stages: TiAl3 generated and formed a layer; multiphase Ti–Al compounds formed; TiAl3 decomposed, and oxidation occurred.
References
Clemens H, Kestler H (2000) Adv Eng Mater 2:551
Wu XH (2006) Intermetallics 14:1114
Djanarthany S, Viala J-C, Bouix J (2001) Mater Chem Phys 72:301
Colinet C, Pasturel A (2002) Intermetallics 10:751
Wang T, Zhang JS (2006) Mater Chem Phys 99:20
Milman YV, Miracle DB, Chugunova SI, Voskoboinik IV, Korzhova NP, Legkaya TN, Podrezov YN (2001) Intermetallics 9:839
Hsu CJ, Chang CY, Kao PW, Ho NJ, Chang CP (2006) Acta Mater 54:5241
Prakash U, Buckley RA, Jones H, Sellars CM (1992) J Mater Sci 27:2001. doi:https://doi.org/10.1007/BF01117910
Kiyoshi M, Kanryu I, Masami S, Masao I, Masakazu K, Isamu Y (2006) Mater Sci Eng A 428:175
Mileiko ST, Povarova KB, Korzhov VP, Serebryakov AV, Kolchin AA, Kiiko VM, Starostin MY, Sarkissyan NS, Antonova AV (2001) Scripta Mater 44:2463
Sanguinetti Ferreira RA, Arvieu C, Quenisset JM (2005) Scripta Mater 53:329
Sanguinetti Ferreira RA, Arvieu C, Guillaume B, Quenisset JM (2006) Composites Part A 37:1831
Lü XH, Yang YQ, Ma ZJ, Liu CX, Chen Y (2006) Trans Nonferrous Met Soc China 16:77
Zhang QC, He GY, Wu JS (2000) Mater Sci Technol 8(4):77
He GY, Hu SP, Chu SJ, Li LF, Zhang TX, Cai XZ (1996) Chin J Nonferrous Met 6(4):110
Mark A, Didier de F (1992) Phys Rev B 46(9):5055
Kattner UR, Lin JC, Chang YA (1992) Metall Trans 23A:2081
Liu Y, Huang BY, He YH (2000) Trans Nonferrous Met Soc China 10(1):29
Jinkeun O, Sung G, Sunghak L, Nack JK (2003) J Mater Sci 38:3647. doi:https://doi.org/10.1023/A:1025637600400
Kovács K, Perczel IV, Josepovits VK, Kiss G, Réti F, Deák P (2002) Appl Surf Sci 200:185
Camille YJ, William EL, Evan C (2006) Intermetallics 14:54
Zollinger J, Lapin J, Daloz D, Combeau H (2007) Intermetallics 15:1343
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y.M., Xiu, Z.Y., Wu, G.H. et al. Study on Ti fiber reinforced TiAl3 composite by infiltration-in situ reaction. J Mater Sci 44, 4258–4263 (2009). https://doi.org/10.1007/s10853-009-3618-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-009-3618-1