Abstract
A good deal of information regarding the synthesis and optical properties of chemically grown (Cd1−xSnx)S (x = 0.01–0.40) thin film has been reported. The growth of these films depends on various preparative parameters and deposition conditions. The reactant concentration, pH, deposition temperature, and rate of agitation were found to influence significantly the quality and thickness of the films. The photoconductive studies have been done with the help of rise and decay curves. Lifetime, mobility, and trap depth are calculated for observed parameters. Band gap measurements for the prepared films have been done with the help of optical transmission spectra using UV-VIS-IR spectrophotometer (190–1100 Å). Interesting results of PC rise and decay studies, optical absorption, and transmission spectra have been presented and discussed. Mobility is influenced significantly by Sn composition. The action spectra showed displacement in the absorption edge towards lower energy side by the addition of Sn mole content. The energy gap decreased from 2.1 to 1.9 eV for x = 0.01 and 1.85 eV for x = 0.02.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today new materials play an important role in the field of electrical, electronic, magnetic, optical, medical, and communication engineering. Thin films of pure and doped CdS had been extensively studied [1–10]. The development of low-cost optoelectronic devices requires more and more understanding of optical properties. The Sn-doped Cadmium chalcogenides are potentially promising materials in many devices such as current controlled devices, switching devices, and photoconductive cells [2, 4, 6]. (Cd1−xSnx)S is one of such series that has been chosen for the investigations because this system as such has been scantily studied. The two binary compounds carry technological importance. The energy gaps of the two component semiconductors are such that a large fraction of the solar spectrum (=1.7 eV) could be utilized [6]. It has therefore been proposed to synthesize the (Cd1−xSnx)S thin films by a simple chemical growth process developed in our laboratory and to report here the compositional effect on optical characteristics and photoconductive (PC) studies of the above films.
Although several workers have used a variety of techniques [7, 8, 11] to prepare pure and doped CdS films, chemical bath deposition (CBD) method is often preferred for its simplicity [9, 10, 12]. It is observed that a lesser amount of work has been done so far in preparing Sn-doped CdS films for characterization of its photoconductive properties. Keeping in view the above aspects, further work on these films is desirable. In the present paper photo response properties of these films deposited by CBD method at high temperature (74 °C) is reported. The amount of each constituent has been carefully adjusted to obtain stable and homogeneous solution. Films of these materials require a more comprehensive study of the material properties. Therefore the present work is an extension of the earlier study [1, 2, 13], and it is aimed at improving the material characterization and also to further investigate the applicability of a band model approach to the description of this material.
Experimental details
Film preparation
The films were prepared on substrates of commercial quality microscopic glass slide of dimensions 24 × 75 mm. The substrates were previously degreased in nitric acid for 48 h, cleaned in ultrasonic cleaner with distilled water, and then allowed to dry in air. Such cleaned slides were dipped vertically into a mixture of solution of 1 M Cadmium Sulfate, 1 M Stannous Chloride, 1 M Thiouria, Triethanolamine, and 30% aqueous ammonia (All AR grade 99.9% pure). The solution was prepared in triple distilled water and pH value of the mixture was approximately 11. The prepared solution was stirred with the help of homogenizer for 2 min. All the depositions were made at a temperature of 74 °C in a water bath for 1.25 h. The deposition of films is based on precipitation followed by condensation. In the beginning when precipitation started, stirring was done. After that, the deposition was made in the static condition. After depositions the films were cleaned by flushing with distilled water and then dried in open atmosphere at room temperature.
The deposition process is based on the controlled precipitation of Cd2+ and Sn2+ ions simultaneously and is made energetically favorable by interaction of the ions from the solution with the substrate surface. Obviously under suitable conditions of temperature, pH, concentration, deposition time, nature of the substrate, etc., deposition of good quality thin films of mixed composition would result. Sn was ad-mixed up to 40% of the CdS content (in order to check a comparatively higher photo response in the visible region) and the highest photo response was found for (Cd0.99Sn0.01)S.
Measuring instruments
An incandescent bulb of 100 W and 200 W was used for PC rise and decay studies. For PC studies coplanar electrodes (1.5 mm wide and 24 mm long at a separation of 2 mm) were formed by applying carbon and silver ink to the surface of the film as ohmic contact material. The photocurrents were measured by exposing the total area of the film. A digital nanometer (DNM-121), scientific, Roorkee and an ESCORT multimeter (3136A) were used for measuring lower range and higher range photocurrent, respectively. For the study of optical absorption spectra, a CHEMITO, Double beam (UV-VIS) spectrophotometer (SPECTRASCAN-UV-2600) was used.
Results and discussion
The deposits are smooth, well adherent to the substrate, uniform, densely packed, and reproducible over the entire range of the composition parameter x. The color of the sample was found to change from an orange yellow to pale yellow and finally to greenish yellow. Thick (dense) depositions were observed for lower concentration of Sn and it decreases for higher concentration. The uniform and dense structure of thin films is influenced by the texture of films.
PC rise and decay studies
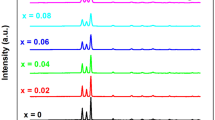
The PC rise and decay curves of some representative (Cd1−xSnx)S films prepared at 74 °C are shown in Fig. 1. The general nature of rise and decay curves is almost similar. The maximum photocurrent was observed for 0.99: 0.01 combinations of CdS to SnS. It is observed that films show a fast rise and decay of photocurrent. The rise and decay time is of the order of minutes. The measurement was made by exposing the film to light radiations and simultaneously recording the dark current for a time period. Rise and decay curves of photocurrent are governed by the trapping states and recombination centers lying in the forbidden zone of a photoconductor [13]. The general nature shows a fast rise in the beginning followed by saturation. The latter sets in when recombination becomes dominant. Solid solutions have variable energy gap depending upon the composition, hence photo-conducting (PC) properties also change with composition.
The observed rise in curve is resultant of two competing processes, the injection of charge carriers into the conduction band and the re-trapping. If the trap density is large enough, most of the charges injected into conduction band are trapped into trapping centers, which reduce the photocurrent. The filled traps will be emptied after the exciting radiation is switched off at a rate depending upon their cross section and ionization energy. A long tail in the decay curves appear because of slow release of charge carriers from deep traps [10].
The decay law can analyze the photocurrent decay curves
where I(ph)m is the maximum Photocurrent just before the light is interrupted, p = 1/τ is the probability of escape of a carrier from the traps per second which is given by the relation,
where S is a frequency factor equal to 1010/s [4] and τ is the photocurrent decay time and E is the trap depth.
Lifetime and mobility
The instantaneous value of lifetime (τ) is calculated from slope of initial state of the photoconductivity decay, using relation:
where Δσst is static photocurrent and tan δ is the slope of initial state of the photoconductivity decay. Mobility is calculated from the formula
and
where, V, applied voltage and L, electrode spacing.
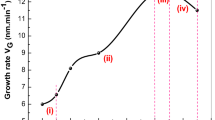
Using relation (3), (4), and (5) Photoconductivity gain, lifetime (τ), and mobility (μ) were calculated. Trap depth can also be obtained by the analysis of decay curves i.e., by peeling off the hyperbolic curves observed in present cases into proper number of exponentials. The values of Idc, Ipc, (Ipc/Idc), trap depths, lifetime, and mobility for films prepared at 74 °C are summarized in Table 1. From the table, it is observed that the values of lifetime and mobility both increase in the presence of impurities, which may be a main cause of photosensitization. The plot of mobility versus Sn composition is shown in Fig. 2.
Optical absorption spectra
The spectral absorbance and transmittance of (Cd1−xSnx)S film deposited on amorphous glass substrates prepared at 74 °C was recorded in the wavelength range 400–800 nm at room temperature and are displayed in Figs. 3 and 4. It can be observed from the figure that, a broad peak in the region 450–510 nm is seen which is associated to the presence of Sn. The optical properties of the films are determined from the transmission spectra. The values of optical absorption coefficient (α) are of the order of 106 (m−1) for near edge absorption given by α = −ln T/t, where ‘T’ is transmission and ‘t’ is the thickness of the film. The values of α have been calculated at different wavelengths from the transmission spectra. The plots of absorption co-efficient (α) verses photon energy (hν) for two typical representation samples (x = 0.01, 0.02) are shown in Fig. 5 in the range 1–3 eV. Steep rise of absorption co-efficient (α) leads to narrow photosensitivity. A steep absorption rise was observed on the shorter wavelength side. These optical data were analyzed in terms of the expression for near-edge optical absorption in semiconductors. The thickness of the films was determined by the weight difference method in which area and weight of the film were measured. The thickness was obtained by assuming bulk density of materials [14].
Band gap measurement
The optical data were analyzed from the following classical relation (Tauc relation)
where ‘hν’ is the photon energy, Eg is the band gap energy, A and n are constant. A is related to the effective masses associated with the valence and conduction bands. For allowed direct transitions, the value of n is equal to 1 and for forbidden direct transition n is equal to 3. For indirect allowed transitions n is equal to 4 and for indirect forbidden transition n is equal to 6 [13]. Figure 6 shows plot of (αhν)2 against photon energy (hν) of Sn-doped CdS film. Since the variation of (αhν)2 with photon energy (hν) for the film is a straight line, it indicates that the involved transition is a direct one. Band gap energy Eg was determined by extrapolating the straight-line portion to the energy axis for zero absorption coefficient (α). The energy band gap decreased typically from 2.1 to 1.9 eV as Sn composition in the solution is increased from 0% to 1% and then it decreases to 1.85 eV when Sn composition is increased to 2%. Energy band gap is found to decrease due to increasing concentration of Sn. The films show potential for light detection. Their photosensitivity domain can be tuned by controlling the crystallites size (Table 2).
Conclusions
Chemical deposition of (Cd1−xSnx)S thin film from an alkaline medium is made possible and higher mobility at carrier concentration values obtained. The optical spectra revealed a high co-efficient of absorption and a shift in absorption edge to a longer wavelength side with corresponding band-to-band type of transition. The decrease of the direct energy gaps by increasing Sn contents is probably due to the reduction of Sn vacancies in these films. High Photoconductive to dark conductive ratio of (Cd1−xSnx)S thin films on glass substrate with carbon contact is observed. No significant increase in the above ratio is observed with silver contact. The band gap was found to be 1.8 eV, which is comparable to the values reported earlier [1].
References
Roy P, Srivastava SK (2006) J Phys D Appl Phys 39:4771. doi:https://doi.org/10.1088/0022-3727/39/22/006
George PJ, Juarez AS, Nair PK (1996) Semicond Sci Technol 111:1090. doi:https://doi.org/10.1088/0268-1242/11/7/021
Sim LY, Kazmereski LL, Clak AH, Ireland PJ, Mortom DW (1978) J Vac Sci Technol 15:265. doi:https://doi.org/10.1116/1.569567
Chandra T, Bhushan S (2004) J Mater Sci (Springer) 39:6303
Deshmukh LP, More BM, Holikatti SG, Hankare P (1994) Bull Mater Sci 17:455. doi:https://doi.org/10.1007/BF02757889
Jodgudri SA, Mohite UK, Godave KM, Lokhande CD (1994) Ind J Pure Appl Phys 32:772
Sharma TP, Patidar D, Saxena NS, Sharma K (2006) Ind J Pure Appl Phys 44:125
Bhosale CH, Uplane MD, Patil PS, Lokhande CD (1994) Ind J Pure Appl Phys 32:269
Ubale AU, Sangwar VS, Kulkarni DK (2007) Bull Mater Sci 30:147
Bhushan S, Mukherjee M, Bose P (2001) Radiat Eff Defects 153:367
Chopra KL (1969) Thin film phenomena. McGraw-Hill, New York, p 14
Narayanan KL, Vijay Kumar KP, Nair KGM, Rao GVN (1997) Bull Mater Sci 20:287
Soliman LI, Afify HH, Battisha IK (2004) Ind J Pure Appl Phys 42:12
Sze SM (1981) Physics of semiconductor devices, vol 16. Wiley, New York, p 849
Acknowledgements
Authors are thankful to the Bhilai Institute of Technology, Durg management for providing all financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, R., Kumar, R. Compositional effect on optical characteristics of solution grown (Cd1−xSnx)S thin films. J Mater Sci 43, 5972–5976 (2008). https://doi.org/10.1007/s10853-008-2733-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2733-8