Abstract
Barium titanate stannate (BaTi1−xSnxO3, 0 ≤ x ≤ 0.25) thin films were deposited directly on copper foil substrates via a chelate chemical solution process. The films were subsequently crystallized in a reducing atmosphere such that substrate oxidation was avoided and that the 2-valent state of tin could be stabilized. Despite the stabilization of the low-melting temperature SnO oxidation state at high temperatures, the final grain size was smaller with increased tin incorporation similar to other B-site substituted BaTiO3 films. Temperature and field-dependent dielectric measurements revealed a reduction in dielectric constant and dielectric tuning with increasing tin concentration. The reduction in permittivity with reduced grain size is consistent with the well-known trends for ceramic barium titanate and in combination with a defect-dipole model involving Sn acceptors, can be used to explain the experimental trends. Phase transition frequency dependence was studied and for compositions containing up to 25 mole percent tin. No phase transition dispersion was observed and thus no strong evidence of relaxor-like character. The phase transition became increasingly diffuse with deviation from Curie–Weiss behavior, but the observed transition temperatures agreed well with bulk reference data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Barium strontium titanate (BST) and barium titanate zirconate (BTZ) thin films have been widely investigated for applications in tunable electronics, [1–3] dynamic random access memory, [4, 5] and decoupling capacitors [6]. Barium titanate stannate (BTS) is a chemically similar perovskite solid solution of the ferroelectric barium titanate and the linear dielectric barium stannate. Additions of barium stannate to barium titanate are known to result in a phase transition pinching effect, where the cubic-tetragonal transition temperature decreases and the tetragonal-orthorhombic and orthorhombic-rhombohedral transition temperatures increase [7–9]. As a result, compositions containing approximately 10 mole percent and greater levels of BaSnO3 transition directly from cubic to rhombohedral. The phase transition pinching effect has important implications from a technological perspective in allowing for a flattened temperature response that is desirable for many device applications. Additionally, a transition to a diffuse phase transition with second order character at a 10 mole percent level followed by relaxor behavior with clear departure from Curie–Weiss transition behavior and a frequency dispersive Tmax at the 20 mole percent level are observed [10–12]. While a wealth of information exists on BTS in bulk form, [7–11, 13] it has garnered considerably less attention in the thin film community. Of the BTS films studied, all display peak permittivities limited to less than 650, [14–20] and diminished dielectric tunability. These two features likely result from fine grain dimensions and limited processing temperatures [21]. Decreased grain dimensions may stem from two sources: (i) low-processing temperature and (ii) incorporation of tin into the precursor gel, which may alter nucleation behavior or otherwise limit diffusion and densification and coarsening.
Recent advancements in base-metal/complex oxide thin film processing have been afforded by a successful marriage of bulk ceramic controlled atmosphere firing schedules and thin film deposition techniques. This was first demonstrated for ferroelectric films by Dawley et al. in the chemical solution deposition (CSD) of BST on nickel tapes [22, 23]. Using similar techniques it was shown that device quality perovskites of the barium titanate family could be deposited and processed on copper foil substrates [6, 24, 25]. While successful for preparing high permittivity dielectrics in contact with base-metal substrates, the temperature response of permittivity for pure barium titanate may be too sharp for many capacitor applications. To tailor the temperature response, zirconium and hafnium substitutions for titanium are routinely used in the bulk, and have been explored in thin films to pinch the phase transitions and achieve X7R characteristics. While successful in shifting the permittivity peak to lower temperatures and increasing transition diffuseness for thin film embodiments, a permittivity decrease was observed as the substituent level increased [24, 25]. This permittivity decrease was attributed to a grain size reduction [24]. What is required is a solid solution combination that provides the necessary phase transition shift, while allowing for the solid-state diffusion that supports grain growth. We have recently shown that the addition of fluxing agents including boron [26] and barium borate [27] can enhance dielectric properties by enabling grain growth and improving film crystallinity. We propose that the use of tin oxide, specifically with Sn2+, i.e., SnO during densification, may achieve the desired fluxing effect as well as shift the phase transition temperature, thereby simplifying the solution preparation procedures.
The current investigation presents a methodology for depositing BTS thin films onto base-metal copper substrates via CSD. In doing so, the structure-property relationships are discussed in the context of developing a dielectric/substrate embodiment offering large grain size and an engineered temperature-dependent dielectric response sufficient for use in embedded passive technologies.
Experimental procedure
Dielectric compositions of Ba(TixSn1−x)O3 with (0 ≤ x ≤ 0.25) were prepared using a CSD technique. Precursor solutions containing barium, titanium, and tin were prepared via a chelate chemistry [28, 29]. Tin n-butoxide and titanium isopropoxide were combined and reacted with 2,4 pentanedione and diethanolamine as a chelating agents in a 1:2 ratio. Barium acetate was separately dissolved in glacial acetic acid and the two precursors were combined in an equimolar ratio, where solution stoichiometry was controlled by the constituent masses. The resulting solution was 0.3 M as referenced to barium concentration. The solution was spin cast onto 18-μm thick copper foils at 3,000 RPM for 30 s. The films were subsequently placed on a hotplate at 250 °C for 7 min and 30 s for solvent evaporation and gel consolidation. This process was repeated five times, resulting in films with an as-fired thickness of approximately 625 nm.
Films were heated at 30 °C/min to 900 °C, held for 30 min for crystallization and subsequently cooled to 150 °C before removal from the furnace. To protect the copper substrate from oxidation during the high-temperature crystallization anneal and to stabilize the 2-valent state of tin, a sufficiently reducing atmosphere must be employed. Figure 1 shows the pO2—temperature equilibrium phase diagram for each of the metal species, oxygen, and the metal oxides [30]. The atmosphere-temperature space available to reduce copper and oxidize barium, titanium, and tin while stabilizing the SnO phase is highlighted. To tailor the atmosphere to the required conditions an equilibrium reaction established by hydrogen, oxygen impurities in carrier gases, and water vapor was achieved by flowing nitrogen from a liquid source and hydrogen containing forming gas through an H2O bubbler at room temperature. Upon heating to 900 °C, this mixture results in an atmosphere containing a pO2 of 10−13 atm with a total pressure of 1 atm, which is sufficient to oxidize all film species and protect the copper substrate in addition to stabilizing the Sn2+ state. The pO2 was monitored in situ with a solid-state oxygen sensor (Australian Oxytrol Systems) to ensure proper conditions. A reoxidation anneal at 550 °C and 10−7 atm O2 for 30 min was used to minimize electrically active point defects resulting from the low pO2 and high-temperature crystallization anneal [31, 32]. We note that this reoxidation anneal should promote conversion of residual Sn2+ to Sn4+ and remove the potential for acceptor-like behavior of Sn on the B-site. 40 nm thick platinum top electrodes (∼10−4 cm2) were deposited through a shadow mask via DC magnetron sputtering to define metal-insulator-metal capacitor structures.
Phase assemblage in the film/foil stack was studied with a Bruker AXS D-5000 X-ray diffractometer equipped with a Hi-Star area detector. The film microstructures were characterized by atomic force microscopy (AFM) using a CP Research Thermomicroscopes Scanning Autoprobe in contact mode and by field-emission scanning electron microscopy (FE-SEM) with a JEOL 6400F instrument. Dielectric properties were studied as functions of electric field and temperature using a HP 4192 impedance analyzer and a modified MMR Technologies cryogenic temperature stage. Permittivity—field measurements were conducted at room temperature from −15 to 15 volts at 10 kHz with a 0.05 V oscillator. Phase transition behavior was studied by measuring the permittivity and loss tangent of the films from 100 K to 450 K at a 5 K/min ramp rate and zero DC bias with oscillator conditions ranging from 1 to 100 kHz and 0.05 V.
Results and discussion
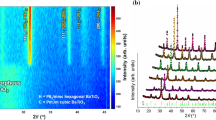
θ−2θ X-ray diffraction patterns for each composition are shown in Fig. 2. Diffraction peaks consistent with a perovskite phase and the copper substrate are identified in each composition. No additional peaks attributable to either tin oxide or copper oxide can be identified, suggesting that tin was fully incorporated into the lattice and that the reductive conditions were sufficient to protect the copper substrate. To verify that tin was incorporated into the lattice, lattice parameter measurements were conducted by collecting diffraction patterns of the 001, 002, and 003 peaks and calculating the lattice constant using a Nelson–Riley extrapolation [33] with the results shown in Fig. 3. The linear lattice parameter increase is consistent with the incorporation of the larger Sn ion on the perovskite B-site and indicates full barium stannate solubility for films containing up to 25% BaSnO3.
Figure 4 shows 3 μm × 3 μm topographic AFM scans for each composition. A grain size decrease is observed, particularly for the composition containing 25% mole percent barium stannate. The average grain size was quantified using a linear intercept method with the results shown in Fig. 5 illustrating the grain size—composition relationship. The error bars indicate a 95% confidence interval in the measured grain diameters [34]. It should be noted that the decrease is not as pronounced as was observed in the BTZ [24] and barium titanate hafnate [25] compositions. This is particularly evident at the 25 mole percent level where the tin substituted samples had an average grain size of 95 nm and the zirconium and hafnium substituted films were 78 nm and 76 nm, respectively. This difference suggests that the SnO stabilization was successful in either limiting the grain size reduction by fluxing the system, or that the Sn did not change the nucleation and growth characteristics to the extent of Zr and Hf, which are suspected to inhibit grain growth via their refractory nature and to promote nucleation from the amorphous gel.
The temperature dependence of permittivity and loss tangent is shown in Fig. 6. Two distinct trends can be identified with increasing tin level: (i) the phase transition temperature, Tmax, decreases and (ii) the maximum permittivity, εmax, decreases. The phase transition temperature was plotted versus composition in Fig. 7 along with values from the bulk work of Smolenskii et al. [9]. The trend of transition temperature and composition is virtually identical in both the film and bulk systems further indicating complete solid solution formation. A permittivity decrease was expected given the grain size reduction, however the degree observed was more pronounced than in the Ba(Ti,Zr)O3 and Ba(Ti,Hf)O3 systems as shown in the maximum permittivity versus average grain diameter plot in Fig. 8, compared with a pure BaTiO3 series [21]. For compositions containing 5 mole percent and less BaSnO3 a trend of permittivity and grain size is identical to the pure system. For films containing 10 mole percent tin and greater, however, the trend deviates and there is a much greater permittivity reduction.
Phase transition temperature as a function of BaSnO3 level for films on copper and bulk ceramics [9]
Permittivity at the phase transition as a function of average grain size for BaTi1−xSnxO3 and pure barium titanate [21]
The permittivity and loss tangent field dependence was measured at room temperature as shown in Fig. 9. A similar trend of decreasing low-field permittivity with increasing tin level is identified. It is important to note that the high-field permittivity saturates to the same value for each composition. This indicates that the intrinsic lattice response is identical and that the extrinsic ferroelectric response is dampened by the tin addition resulting in diminished dielectric tunability. Though many factors can reduce the nonlinear dielectric response, there are two in the current system that are believed to predominate. The first is a scaling effect associated with an average grain size decrease. The second is an increase in the number and/or strength of domain wall pinning sites. The Ba(Ti,Zr)O3 [24] and Ba(Ti,Hf)O3 [25] systems showed an identical trend of decreasing permittivity with grain size as the pure BaTiO3 system and the Ba(Ti,Sn)O3 system does not, suggesting that the second contributor is dominant in films containing greater than 5% tin. One proposed mechanism is the presence of Sn2+ on the normally 4+ valent B site, which could allow for ionic compensation by oxygen vacancies as shown in Kröger–Vink notation [35] in Eq. (1). This is a reasonable assumption given the low-saturating loss tangents at high fields for each composition.
It is hypothesized that for compositions containing 5% tin and less that the reoxidation anneal was successful in filling oxygen vacancies and changing Sn2+ to Sn4+ and thus has allowed for the expected grain size and permittivity trend. Kinetic limitations in samples containing 10 and 25% tin likely resulted in materials with a high concentration of Sn2+–V ••O defect complexes. These defect complexes could then pin domain wall motion and limit the extrinsic ferroelectric contributions to permittivity. This ability of defect dipoles to pin domains and cause aging effects in ferroelectric perovskites is well known [36].
Both Ba(Ti,Zr)O3 and Ba(Ti,Hf)O3 showed the onset of relaxor behavior as the substituent concentration increased [24, 25]. Figure 10 shows the compositional and temperature dependence of permittivity and loss tangent at 1, 10, and 100 kHz. As the tin level increases, there is an increase in the phase transition diffuseness. While relaxor behavior is typically observed at the 20 mole percent level in Ba(Ti,Sn)O3 ceramics, [10, 11] no phase transition temperature frequency dispersion is observed in the thin films, even at the 25 mole percent level. There are two possible explanations for the observed behavior. First, the 25% level is close to the minimum concentration necessary to observe dispersion in the bulk, and it is possible that a higher level is necessary, especially in systems with such fine grains. Second, the likelyhood of Sn2+ remaining in the lattice may alter the onset and stability of polar-microregions.
Chemical solution deposited BTS thin films were prepared with compositions containing ranging from 0 to 25 mole percent tin. A processing atmosphere was chosen such that the Sn2+ state of tin was stabilized and resulted in a minimization of grain size reduction in comparison with BTZ and barium titanate hafnate. X-ray diffraction patterns reveal single-phase films suggesting tin was fully incorporated into the lattice. A decrease in phase transition temperature and maximum permittivity was observed as the tin level increased. The decrease in permittivity was attributed to a combination of a grain size effect in addition to incomplete stabilization of Sn4+, which resulted in defect-dipole formation and limited extrinsic ferroelectric contributions to the dielectric constant. Frequency-dependent measurements did not reveal phase transition frequency dispersion. It is hypothesized that the 2-valent state of tin may sufficiently alter the dielectric response such that relaxor behavior is not observed.
References
Tombak A, Maria JP, Ayguavives F, Jin Z, Stauf GT, Kingon AI, Mortazawi A (2002) IEEE Microw Wirel Co 12:3. doi:https://doi.org/10.1109/7260.975716
Pervez NK, Hansen PJ, York RA (2004) Appl Phys Lett 85:4451. doi:https://doi.org/10.1063/1.1818724
Ghosh D, Laughlin B, Nath J, Kingon AI, Steer MB, Maria JP (2006) Thin Solid Films 496:669. doi:https://doi.org/10.1016/j.tsf.2005.09.025
Kingon AI, Maria JP, Streiffer SK (2000) Nature 406:1032. doi:https://doi.org/10.1038/35023243
Hofer C, Hoffmann M, Boettger U, Waser R (2002) Ferroelectrics 270:179. doi:https://doi.org/10.1080/00150190211242
Ihlefeld J, Laughlin B, Hunt-Lowery A, Borland W, Kingon A, Maria J-P (2005) J Electroceram 14:95. doi:https://doi.org/10.1007/s10832-005-0866-6
Jonker GH (1955) Philips Tech Rev 17:129
Novosiltsev NS, Khodakov AL (1956) Sov Phys Tech Phys 1:306
Smolenskii GA, Isupov VA (1954) Zh Tekh Fiz 24:1375
Mueller V, Beige H, Abicht HP (2004) Appl Phys Lett 84:1341. doi:https://doi.org/10.1063/1.1649820
Yasuda N, Ohwa H, Asano S (1996) Jpn J Appl Phys, Part 1 35:5099. doi:https://doi.org/10.1143/JJAP.35.5099
Smolensky GA (1970) J Phys Soc Jpn 28(Suppl):26
Mueller V, Beige H, Abicht HP, Eisenschmidt C (2004) J Mater Res 19:2834. doi:https://doi.org/10.1557/JMR.2004.0386
Kuo YF, Tseng TY (1999) Electrochem Solid-State Lett 2:236. doi:https://doi.org/10.1149/1.1390795
Yoon KH, Park JH, Jang JH (1999) J Mater Res 14:2933. doi:https://doi.org/10.1557/JMR.1999.0392
Zhai JW, Shen B, Yao X, Zhang LY (2004) J Am Ceram Soc 87:2223. doi:https://doi.org/10.1111/j.1151-2916.2004.tb07495.x
Zhai J, Shen B, Yao X, Zhang L (2004) Mater Res Bull 39:1599. doi:https://doi.org/10.1016/j.materresbull.2004.05.010
Halder S, Victor P, Laha A, Bhattacharya S, Krupanidhi SB, Agarwal G, Singh AK (2002) Solid State Commun 121:329. doi:https://doi.org/10.1016/S0038-1098(02)00017-0
Nakagauchi R, Kozuka H (2007) J Sol-Gel Sci Technol 42:221. doi:https://doi.org/10.1007/s10971-007-0772-2
Song S, Zhai JW, Yao X (2007) J Sol-Gel Sci Technol 44:75. doi:https://doi.org/10.1007/s10971-007-1604-0
Ihlefeld JF, Vodnick AM, Baker SP, Borland WJ, Maria J-P (2008) J Appl Phys 103(7):074112
Dawley JT, Clem PG (2002) Appl Phys Lett 81:3028. doi:https://doi.org/10.1063/1.1516630
Dawley JT, Ong RJ, Clem PG (2002) J Mater Res 17:1678. doi:https://doi.org/10.1557/JMR.2002.0247
Ihlefeld JF, Borland W, Maria J-P (2005) J Mater Res 20:2838. doi:https://doi.org/10.1557/JMR.2005.0342
Ihlefeld JF, Borland WJ, Maria J-P (2008) Thin Solid Films 516:3126. doi:https://doi.org/10.1016/j.tsf.2007.08.096
Ihlefeld JF, Borland WJ, Maria J-P (2008) Scr Mater 58:549. doi:https://doi.org/10.1016/j.scriptamat.2007.11.008
Ihlefeld JF, Borland WJ, Maria J-P (2007) Adv Funct Mater 17:1199. doi:https://doi.org/10.1002/adfm.200601159
Hoffmann S, Waser R (1999) J Eur Ceram Soc 19:1339. doi:https://doi.org/10.1016/S0955-2219(98)00430-0
Schwartz RW, Clem PG, Voigt JA, Byhoff ER, Van Stry M, Headley TJ, Missert NA (1999) J Am Ceram Soc 82:2359
Barin I (1995) In: Thermochemical data of pure substances VCH. Weinheim, New York
Yang GY, Dickey EC, Randall CA, Barber DE, Pinceloup P, Henderson MA, Hill RA, Beeson JJ, Skamser DJ (2004) J Appl Phys 96:7492. doi:https://doi.org/10.1063/1.1809267
Ihlefeld JF, Losego MD, Collazo R, Borland WJ, Maria JP (2008) J Mater Sci 43:38. doi:https://doi.org/10.1007/s10853-007-2135-3
Nelson JB, Riley DP (1945) Proc Phys Soc Lond 57:160. doi:https://doi.org/10.1088/0959-5309/57/3/302
ASTM E 112-96 (2003) In: Standard test methods for determining average grain size. ASTM International, West Conshohocken, PA
Kröger FA, Vink HJ (1956) Solid State Phys 3:307
Jaffe B, Cook WR, Jaffe HLC (1971) In: Piezoelectric ceramics. Academic Press, London, New York
Acknowledgement
The authors wish to acknowledge the financial support of E.I. Du Pont de Nemours and Company.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ihlefeld, J.F., Borland, W.J. & Maria, JP. Synthesis and properties of barium titanate stannate thin films by chemical solution deposition. J Mater Sci 43, 4264–4270 (2008). https://doi.org/10.1007/s10853-008-2618-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2618-x