Abstract
The miscibility and melting properties of binary crystalline blends of poly(ethylene 2,6-naphthalate)/poly(trimethylene terephthalate) (PEN/PTT) have been investigated with differential scanning calorimetry (DSC). The glass transition and cold crystallization behaviors indicated that in PEN/PTT blends, there are two different amorphous phases and the PEN/PTT blends are immiscible in the amorphous state. The polymer–polymer interaction parameter, \( \chi _{12}\), calculated from equilibrium melting temperature depression of the PEN component was −1.791 × 10−5 (300 °C), revealing miscibility of PEN/PTT blends in the melt state.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Poly(trimethylene terephthalate) (PTT) has been recently introduced as a commercial polymer, joining the other linear aromatic polyesters, poly(ethylene terephthalate) (PET) and poly(butylene terephthalate) (PBT). It combines the desirable physical properties of PET (strength, stiffness, toughness, and heat resistance) with the processing advantages of PBT (low melt and mold temperatures, rapid crystallization, and faster cycles), while retaining basic polyester benefits of dimensional stability, electrical insulation, and chemical resistance [1]. These characteristics make PTT suitable for uses in fibers, films, packing and engineering thermoplastic markets [1]. Compared to PTT, poly(ethylene 2,6-naphthalate) (PEN) contains a naphthalene ring in its backbone making it more rigid than PTT. It shows superior performance, such as low permeability toward oxygen and carbon dioxide, resistance to ultraviolet light, hot-fill capability [2–5]. Thus, PEN has found for a variety of applications, such as films, magnetic tapes, and packaging [6, 7].
Polymer blending is one of the most effective techniques to develop new materials with properties better than those of the original polymers. Blends of PEN and PET have been more widely studied because of their commercial importance [8–12]. It has been found that an originally immiscible PEN/PET blend can turn into a homogeneous single-phase blend [8]. The compatibilization effect was attributed to the fine homogenization of the phases during the preparation of the blend, as well as to the occurrence of transesterification taking place between the PET and PEN components [13–17].For the PET/PEN blends having the transesterification level of above 10%, all the blends quenched from molten state exhibited the same dynamic mechanical behavior in the glass transition region showing a single glass transition temperature T g [15]. The research by Mutsumasa et al. revealed that the melt rheological behavior of the PEN/PET blends was very similar to that of the two parent polymers. The melt viscosity of the blends was between that of PEN and that of PET [16]. As to the crystallization behavior of PEN/PET blends, Shi and Jabarin evaluated the isothermal crystallization kinetics in terms of the Avrami equation. The Avrami exponent (n) is different for PET, PEN, and the blends, indicating different crystallization mechanisms, were occurring in blends than those in virgin PET and PEN [18]. By the isothermal DSC tests along with Avrami analysis of PEN/PET blend, Rwei reported that PET’s crystallization is significantly retarded when blended with PEN [9]. Bang et al. found that the crystallization rate of PEN/PET blend depended on the sequence distribution of polymer chains, which was determined by the level of transesterification rather than the composition change of separated phases [12].
PTT can crystallize easily without the presence of any nucleating agent [19]. However, the PEN has a disadvantage of slow crystallization, which makes PEN undesirable for injection molding application under conventional processing conditions [20]. Therefore, it is also expected that blending of PEN and PTT will offer an interesting route to combine the complementary properties of both polymers.
Although PEN/PET blends have been extensively investigated, there are few papers about PEN/PTT blends. The purpose of this article is to investigate miscibility and the nature of melting behavior in PEN/PTT blends. Differential scanning calorimetry (DSC) was employed to study the miscibility and melting properties of the blends. The polymer–polymer interaction parameter \( \chi _{12}\) and interaction energy density B were calculated from the equilibrium melting depression of PEN using the Nishi–Wang equation.
Experimental
Materials
PEN in pellet form with an intrinsic viscosity (IV) of 0.57 dL/g was obtained from Teijin Chemical. PTT (Corterra CP509205, Shell Chemicals) in pellet form with an IV of 0.92 dL/g was used in this study.
Sample preparation
The PEN/PTT blends with compositions ranging from 90/10 to 10/90 in weight ratio were prepared by melt blending. Before blending, both polymers were dried at 150 °C for 5 h in an air oven to remove moisture to minimize the possibility of hydrolysis. The samples of PEN/PTT blends with compositions ranging from 90/10 to 10/90 in weight ratio were prepared in a 30-mm co-rotating twin screw extruder (TE-34) with an L/D of 28, a barrel temperature ranging from 235 to 295 °C, and a screw speed of 80 rpm. The strand from the extruder was quenched in a water bath and cut into chips.
Characterizations
The measurement of the thermal behavior of PEN, PTT, and their blends with various compositions was conducted with a differential scanning calorimeter (DSC) (Perkin-Elmer Pyris 1 DSC) equipped with a liquid nitrogen cooling system. Both temperature and heat flow were calibrated by a standard sample of indium. The heat of fusion of indium (28.5 J/g) was used to calibrate the thermal response of the calorimeter. All samples were dried in vacuum at 50 °C for 10 h before the measurements. To avoid uneven thermal conduction of the samples, which may cause different amounts of broadening and shifting of the peak positions, the aluminum pans were always filled with the same quantity of specimen, about 5 mg. The samples were sealed in the aluminum pans and heated above 295 °C for 2 min to eliminate previous thermal histories. For the determination of glass transition temperatures, the samples were cooled to 10 °C at a rate of 300 °C/min to attain the completely amorphous state.
The glass transition T g, cold crystallization (T cc), and melting temperatures (T m) were determined from the second heating scan at a rate of 10 °C/min over the range 0–300 °C. To observe the equilibrium melting point (T m 0) of PEN, PTT, and PEN/PTT blends, the samples were first melted at 295 °C for 2 min under nitrogen atmosphere to eliminate the crystalline residues. Then, they were subsequently quenched at a rate of 300 °C/min to the desired crystallization temperature (T c). After isothermal crystallization for 10 h at T c, the samples were immediately heated up from T c to 295 °C at a rate of 10 °C/min. The peak temperature of the endotherm was considered as the melting point of the sample.
Results and discussion
Miscibility
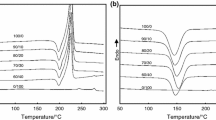
The miscibility level and the crystalline characteristics of the blends were studied by DSC. Figure 1 shows the DSC thermograms for the melt-quenched PEN/PTT blend samples with T g, T cc, and T m temperatures against composition. T g is indicated by an arrow. The values of T g, T cc, and T m temperatures are presented in Table 1.
The T g can provide useful information on blend miscibility. One of the most commonly used criteria for establishing the phase behavior in amorphous binary polymer blends is the presence of one or more T gs. If the blend is one-phase, a single T g lying between the values for each component is detected and characterizes the mixture. If the blend is two-phase, then two T gs are observed close to or matching those of the two components. Thus, for an immiscible blend, two T gs generally appear on the DSC scan, whereas for miscible blends or copolymers, only one T g is observed [21].
In Fig. 1, the T g values of virgin PEN and PTT are 123.4 and 47.2 °C, respectively. For PEN-rich blends (90/10, 80/20, 70/30), a single T g was observed, but they are almost identical to that of virgin PEN (Table 2, T g2). For PTT-rich blends (40/60, 30/70, 20/80, 10/90), the single T g value for each sample is also almost identical to that of virgin PTT (Table 2, T g1). For the blends with compositions of 60/40 and 50/50, two separated glass transitions can be found and they are almost identical to those of virgin components (Table 2). It can be speculated that the lack of another T g for the above PEN-rich blends (or PTT-rich blends) is due to the low PTT (or PEN) level. The measurement of T g provides evidence that PEN and PTT are immiscible in the amorphous phase.
In addition, the immiscibility of the PEN/PTT blends in the amorphous phase could be evidenced by another thermal behavior, cold crystallization. For the blends with compositions of 90/10, 80/20, 70/30, 30/70, 20/80, and 10/90, only one cold crystallization exotherm peak T cc was found. For PEN-rich blends (90/10, 80/20, 70/30), the T cc decreased slightly with the PTT content (Table 2, T cc2), indicating that PTT made the cold crystallization of PEN faster. While for PTT-rich blends (30/70, 20/80, 10/90), the T cc increased slightly with the PEN content (Table 2, T cc1), which indicated that PEN made the cold crystallization of PTT slower. However, two cold crystallization exotherm peaks were found for the blends with compositions of 60/40, 50/50, and 40/60, which indicated that the PEN and PTT chains acted independently as they separated into two phases. Thus, the lack of the low-temperature T cc for PEN-rich blends (90/10, 80/20, 70/30) and high-temperature T cc for PTT-rich blends (30/70, 20/80, 10/90) could also be attributed to the low PTT or PEN level.
The glass transition and cold crystallization behaviors indicated that in PEN/PTT blends, there are two different amorphous phases and the PEN/PTT blends are immiscible in the amorphous state. An immiscibility for PEN/PET blend has also been found [8]. However, the copolymers of PET and PEN can be formed by transesterification during the melt mixing, and after a certain transesterification has been achieved, the miscible PEN/PET blends can be obtained [15]. In this study, the PEN/PTT blends were prepared at 295 °C for less than 3 min to avoid transesterification. Thus, the lack of copolymers formed by transesterification may result in the immiscible thermal behavior.
Melting behavior
From the subsequent melting thermograms after cold crystallization in Fig. 1, only one melting point was found for PEN/PTT blends with compositions of 90/10, 80/20, 70/30, and 10/90. However, two melting points around 270 and 226 °C were found for the blends with compositions of 20/80, 30/70, 40/60, 50/50, and 60/40. The higher one T m2 is the melting point of PEN crystal and the lower one T m1 is the melting point of PTT crystal. Because the DSC themograms showed two distinct melting peaks for some blends, it could be concluded that PEN and PTT crystallites coexisted in the blends. They did not form the cocrystals due to their different chemical structures. Figure 2 shows the observed T m values for PEN, PTT, and their blends versus blend composition dependence.
It can be found that the T m of each component in the blends decreases with increasing content of the other component. The similar melting-point depression phenomena have also been reported in other binary crystalline polymer blends, such as PET/PTT, PEN/PBT, PBT/PAr(I-100), and PBSU/PEO, etc. [22–24]. The lowered melting point of the crystalline component with respect to the pure polymer is usually a result of thermodynamically favorable interactions in blends [8]. Thus, the melting-point depression is also used extensively to evaluate the miscibility of polymer blends. There are two basic origins of melting-point depression: morphological and thermodynamic [25]. Morphological variables such as lamellar thickness and crystal perfection can profoundly affect the melting point.
To confirm the miscibility of the two polymers in the melt-state, the polymer–polymer interaction parameter is determined from T m 0 depression method [26]. The T m 0 was determined by Hoffman–Weeks extrapolation method using Eq. 1 [27, 28]:
where T m and T m 0 are the experimental melting point and equilibrium melting point for the crystals produced at T c in the blend, respectively. \( \gamma\) is the thickening ratio between the initial thickness of a chain-folded lamella and the final lamellar thickness.
Figure 3 describes the procedure of determining T m 0 of PEN and its PEN/PTT blends according to the Hoffman–Weeks equation. In this procedure, the measured T m of samples crystallized at T c was plotted against T c. Data of 60/40, 50/50, 40/60, 30/70, and 20/80 blends were omitted due to the obvious coexistence of PEN and PTT crystals that was analyzed by DSC (Fig. 1). Thus, the samples used in Fig. 3 contained the PEN crystals only. Each piece of experimental data was obtained by isothermal crystallization for 10 h. An increase in T m with the T c can be observed. By extrapolation of T m ∼ T c plot to T m = T c line, the value of T m 0 can be obtained from the intersection at the T m = T c line and the \( \gamma\) value can be obtained from the slope of T m ∼ T c line. The values of T m 0 and \( \gamma\) are listed in Table 2.
The equilibrium melting point of PEN is 292.5 °C, which is lower than those previously reported in the literature, 300 °C by Buchner et al. [29], 480 °C by Cheng and Wunderlich [30], and 337 °C by Papageorgiou et al. [31].
It can be found in Table 2 that the thickening ratio \( \gamma\) decreased with the increase of PTT content. This indicated that the PEN crystals became less stable due to the smaller lamellar thickness that resulted in the melting point decrease. It is obvious that the addition of the PTT makes the molecular transport of the PEN segments more difficult and limits the thickening of the PEN crystals; therefore, it could cause a depression of the corresponding melting points.
The equilibrium melting-point data in PEN/PTT blends can be analyzed by the Nishi–Wang equation based on the Flory–Huggins theory, that is used for the treatment of the melting-point depression observed in the case of binary amorphous/crystalline polymer blends [26, 32]:
where the subscripts 1 and 2 denote the amorphous and crystalline components, respectively; T m 0 and T m are the equilibrium melting points of the pure crystalline component and its blends; V u is the molar volume of the polymer repeating unit; m is the degree of polymerization; R is the universal gas constant; \( \Updelta H_u\) is the perfect crystal heat of the crystalline component; \( \phi\) is the volume fraction of the component in the blend; and \( \chi _{12}\) is the polymer–polymer interaction parameter. Because the degree of polymerization m is very large for polymers and the entropy of mixing could be negligible, the equation can be simplified as Nishi–Wang equation:
It is well known that the melting point of a polymer is affected not only by thermodynamic factors but also by morphological parameters such as the crystal thickness; the equilibrium melting point of the polymer is used here to separate morphological from thermodynamic effects in analysis of melting-point depression. Eq. 3 showed that a melting point depression would yield a negative \( \chi _{12}\) value for the blend, which indicated a miscibility of the blends. According to the Nishi and Wang formulation, the interaction energy density B characteristic of the polymer pair can be defined as [26]
Substituting Eq. 4 into Eq. 3 yields a linear relationship between \( {{\left( {{1 \mathord{\left/ {\vphantom {1 {T_{\text{m}} }}} \right. \kern-\nulldelimiterspace} {T_{\text{m}} }} - {1 \mathord{\left/ {\vphantom {1 {T_{\text{m}} }}} \right. \kern-\nulldelimiterspace} {T_{\text{m}} }}^0 } \right)} \mathord{\left/ {\vphantom {{\left( {{1 \mathord{\left/ {\vphantom {1 {T_{\text{m}} }}} \right. \kern-\nulldelimiterspace} {T_{\text{m}} }} - {1 \mathord{\left/ {\vphantom {1 {T_{\text{m}} }}} \right. \kern-\nulldelimiterspace} {T_{\text{m}} }}^0 } \right)} {\phi _1 }}} \right. \kern-\nulldelimiterspace} {\phi _1 }} \) and \( {{\phi _1 } \mathord{\left/ {\vphantom {{\phi _1 } {T_{\text{m}} }}} \right. \kern-\nulldelimiterspace} {T_{\text{m}} }}\):
Eq. 5 was used to plot the melting-point depression and to give the interaction energy density value. According to DSC analysis in Fig. 1, in construction of the plots using Eq. 5, we assume that for blends with PTT contents of less than 30%, PEN is considered to be the crystalline component, whereas PTT is considered to be the amorphous component rejected from the crystalline phase. The following values were used with Eq. 5: for PEN, V PEN = 182.4 cm3/mol, ΔH PEN = 182.4 J/g [33]; for PTT, V PTTu = 149.6 cm3/mol [23]. Figure 4 shows the plot of Eq. 5 for the PEN/PTT blends, assuming the melting point of PEN was depressed by the addition of PTT component. A straight line fitted with the least-square method was obtained.
\( {{\left( {{1 \mathord{\left/ {\vphantom {1 {T_{\text{m}} }}} \right. \kern-\nulldelimiterspace} {T_{\text{m}} }} - {1 \mathord{\left/ {\vphantom {1 {{T_{\text{m}}} ^0 }}} \right. \kern-\nulldelimiterspace} {{T_{\text{m}}} ^0 }}} \right)} \mathord{\left/ {\vphantom {{\left( {{1 \mathord{\left/ {\vphantom {1 {T_{\text{m}} }}} \right. \kern-\nulldelimiterspace} {T_{\text{m}} }} - {1 \mathord{\left/ {\vphantom {1 {{T_{\text{m}}} ^0 }}} \right. \kern-\nulldelimiterspace} {{T_{\text{m}}} ^0 }}} \right)} {\phi _1 }}} \right. \kern-\nulldelimiterspace} {\phi _1 }}\) versus \( {{\phi _1 } \mathord{\left/ {\vphantom {{\phi _1 } {T_{\text{m}} }}} \right. \kern-\nulldelimiterspace} {T_{\text{m}} }}\) from the melting-point depression of PEN
From the slope value of the fitting straight line in Fig. 4, the interaction energy density B = −5.72 × 10−4 J/cm3 and the polymer–polymer interaction parameter \( \chi _{12} = - 1.791 \times 10^{ - 5}\) (the processing temperature 300 °C) can be obtained. The very small negative values of B and \( \chi _{12}\) for the PEN/PTT system indicate that the polymer pair can form a thermodynamically stable miscible mixture at temperatures above the melting point.
Conclusion
The miscibility and melting behavior in PEN/PTT blends have been investigated. The glass transition and cold crystallization behaviors indicated that in PEN/PTT blends, there are two different amorphous phases and the PEN/PTT blends are immiscible in the amorphous state. The addition of the PTT could cause a depression of the corresponding melting points of PEN. The polymer–polymer interaction parameter \( \chi _{12}\) and interaction energy density B were calculated from the equilibrium melting depression of PEN using the Nishi–Wang equation. The negative values of \( \chi _{12}\) and B indicate that PEN/PTT blends are thermodynamically miscible in the melt state.
References
Dangayach K, Chuah H, Gergen W, Dalton P, Smith F (1997) ANTEC Conf Proc 2:2097
Buchner S, Wiswe D, Zachmann HG (1989) Polymer 30:480
Cakmak M, Wang YD, Simhambhatla M (1990) Polym Eng Sci 30:721
Kim SH, Kang SW, Park JK, Park YH (1998) J Appl Polym Sci 70:1065
Nakamae K, Nishino T, Tada K, Kanamoto T, Ito M (1993) Polymer 34:3322
Van Den Heuvel CJM, Klop EA (2000) Polymer 41:4249
Uchida YN (1994) Mater Technol 137:63
Shi Y, Jabarin SA (2001) J Appl Polym Sci 81:11
Rwei SP (1999) Polym Eng Sci 39:2475
Lee SC, Yoon KH, Park IH, Kim HC, Son TW (1997) Polymer 38:4831
William GK, Bryan BS (2001) Polymer 42:8703
Hyo JB, Jong KL, Kwang HL (2000) J Polym Sci Part B: Polym Phys 38:2625
Mano JF, Denchev Z (2002) Diffus Defect Data Pt A Diffus Forum 206–207:135
Andresen E, Zachmann HG (1994) Colloid Polym Sci 272:1352
Aoki Y, Li L, Amari T, Nishimura K, Arashiro Y (1999) Macromolecules 32:1923
Kyotani M, Pudjiastuti W, Saeed A (1999) J Macromol Sci Phys B 38:197
Ihm DW, Park SY, Chang CG, Kirn YS, Lee HK (1996) J Polym Sci Polym Chem 34:2841
Shi Y, Jabarin SA (2001) J Appl Polym Sci 81:23
Chen M, Chen CC, Ke KZ, Ho RM (2002) J Macromol Sci Phys B 41:1063
Xia G, Ruigang L, Ying Y, Manna J, Haishan B (2003) Macromol Mater Eng 288:372
Mishra SP, Deopura BL (1985) Makromol Chem 186:641
Yishan Y, Kyung-Ju C (1997) Polym Eng Sci 37:91
Ting LL,Woo EM (2004) Polym Int 53:1813
Liu AS, Liau WB, Chiu WY (1998) Macromolecules 31:6593
Cheung YW, Stein RS (1994) Macromolecules 27:2512
Nishi T, Wang T (1975) Macromolecules 8:909
Hoffman JD, Davis GT, Lauritzen JI Jr (1976) Treatise on solid state chemistry. Plenum Press, New York
Hoffman JD, Weeks JJ (1962) J Res Natl Bur Stand Sect A 66:13
Buchner S, Wiswe D, Zachmann HG (1989) Polymer 30:480
Cheng SZD, Wunderlich B (1988) Macromolecules 21:789
Papageorgiou GZ, Achilias DS, Karayannidis GP (2004) Polym Int 53:1360
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, New York
Rahman MH, Nandi AK (2002) Macromol Chem Phys 203:653
Acknowledgements
This work was supported by the Scientific Research Foundation of Huizhou University (No.C506.0201) and the Science Development Project of Huizhou (No. 2007P48). The authors gratefully acknowledge the financial support and wish to express their thanks to the referees for critically reviewing the manuscript and making important suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liang, H., Xie, F., Wu, W. et al. Miscibility and melting properties of poly(ethylene 2,6-naphthalate)/poly(trimethylene terephthalate) blends. J Mater Sci 43, 2739–2744 (2008). https://doi.org/10.1007/s10853-008-2496-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2496-2