Abstract
New fluorinated dendrimer-type block copolymers were applied to the dispersion of single-walled carbon nanotubes (SW-CNTs) and single-walled carbon nanotubes containing carboxy groups [(SW-CNT)-COOH] in water. Fluorinated block copolymer could disperse SW-CNTs more effectively in water, compared to that of the corresponding ABA triblock-type fluoroalkyl end-capped dimethylacrylamide oligomer [RF-(DMAA)n-RF]. Dynamic light-scattering (DLS) measurements and transmission electron microscopy (TEM) images show that SW-CNTs could be smoothly encapsulated into fluorinated copolymeric aggregates cores. Interestingly, it was demonstrated that SW-CNTs could be in part released from the fluorinated copolymeric aggregates/SW-CNTs composites or encapsulated into these composites with increasing the dispersion times. On the other hand, fluorinated block copolymer and RF-(DMAA)n-RF oligomer were not able to disperse well (SW-CNT)-COOH in water; however, ABA triblock-type fluoroalkyl end-capped acrylic acid oligomer was able to disperse quite effectively (SW-CNT)-COOH in water.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, there has been attractive interest in single-walled carbon nanotubes (SW-CNTs) due to exhibit unique physical and chemical properties such as a high flexibility, chemical stability, electrical conductivity, which result from the high ratio of length to diameter and π–π connections among adjacent carbons [1]. SW-CNTs have a variety of potential applications such as nanodevices, biochemistry, and reinforcing materials for polymer composites; however, they have a great deal of difficulties to obtain uniform dispersed distribution of SW-CNTs in aqueous and organic media, because SW-CNTs exhibit an extremely poor solubility in these media [2]. Hitherto, we have reported that ABA triblock-type fluoroalkyl end-capped oligomers could exhibit a wide variety of unique properties such as high solubility, surface active properties, biological activities, and nanometer size-controlled self-assembled molecular aggregates which cannot be achieved by the corresponding non-fluorinated and randomly fluoroalkylated ones [3]. Especially, these fluorinated oligomeric aggregates could provide suitable host moieties to interact with a variety of guest molecules such as organic dyes, low-molecular biocides, calcium carbonates, and fullerenes to afford stable fluorinated oligomeric aggregates/guest molecules nanocomposites [4]. In these fluorinated oligomeric aggregates/guest molecules nanocomposites, we have also succeeded in preparing fluorinated oligomeric aggregates/carbon nanotube composites possessing a dispersibility in water [5]. Therefore, the preparation of fluorinated oligomeric aggregates/carbon nanotubes composites possessing a higher dispersibility and stability in water is of particular interest from the developmental viewpoint of new fluorinated functional materials. Very recently, we have found that new fluoroalkyl end-capped dendrimer-type block copolymers can be prepared by the use of fluoroalkanoyl peroxide as a key intermediate [6]. It was also demonstrated that these fluorinated dendrimer-type block copolymers could form the new fluorinated molecular aggregates to have a higher dispersion ability for not only SW-CNTs and fullerenes but also magnetic nanoparticles in water, compared to that of the corresponding ABA triblock-type fluoroalkyl end-capped oligomers [6]. We now give a full account of the dispersion of SW-CNTs in water by the use of new fluorinated dendrimer-type block copolymer.
Experimental
NMR spectra and Fourier-transform infrared (FTIR) spectra were measured using JEOL JNM-400 (400 MHz) FT NMR SYSTEM (Tokyo, Japan) and Shimadzu FTIR-8400 FT-IR spectrophotometer (Kyoto, Japan), respectively. Molecular weights were measured using a Shodex DS-4 (pomp) and Shodex RI-71 (detector) gel permeation chromatography (GPC) calibrated with polystyrene standard using tetrahydrofuran (THF) as the eluent. Dynamic light-scattering (DLS) and static light-scattering (SLS) measurements were performed using Otsuka Electronics DLS-7000 HL (Tokyo, Japan). Ultraviolet-visible (UV-vis) spectra were measured using Shimadzu UV-1600 UV-vis spectrophotometer (Kyoto, Japan). Transmission electron microscopy (TEM) images were obtained using a JEOL JEM-1210—electron microscopy (Tokyo, Japan).
Materials
SW-CNT [purity >50%: diameter: 1–2 nm, length: 0.5–100 μm; impurities: other nanotube, amorphous carbon, carbon-coated metal nanoparticles] and SW-CNT containing carboxy groups [(SW-CNT)-COOH: purity: 80–90%; the coverage of carboxy groups on the side wall of SW-CNT is 3–6 atomic %; diameter: 4–5 nm; length: 500–1,500 μm; impurities: other nanotube, amorphous carbon, carbon-coated metal nanoparticles (5–10%)] were purchased from Sigma-Aldrich Japan Corp. (Tokyo, Japan).
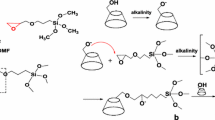
Dendrimer-type copolymer containing fluoroalkyl segments [RF-(TRIV-Si)n-RF-block-RF-(DMAA)q-RF] was prepared according to our previuosly reported method [6]. ABA triblock-type fluoroalkyl end-capped oligomers were prepared by the reactions of fluoroalkanoyl peroxides with the corresponding monomers according to our previously reported method [7].
Dispersion of SW-CNT in water by the use of RF-(TRIV-Si) n -RF-block-RF-(DMAA) q -RF
To an aqueous solution of RF-(TRIV-Si) n -RF-block-RF-(DMAA) q -RF; RF = CF(CF3)OC4F9; 4.0 g/dm3: 2 mL] were added SW-CNTs (2.0 mg). The mixture was stirred with a magnetic stirring bar at 30 °C for 3 days. The aqueous solution thus obtained was centrifuged for 30 min (1000 rpm), and then the residual transparent black SW-CNTs solution was filtered through a 1.2-μm filter membrane to obtain a similar transparent solution. The relative amounts of dispersed SW-CNTs in water were estimated by the optical density at 500 nm (UV-vis. spectra) with the use of the molar absorption coefficient (ε) of SW-CNTs-o-dichlorobenzene solution reported by Smalley et al. [8] and the relative amounts of dispersed SW-CNTs were summarized in Fig. 1.
UV-vis spectra of aqueous solutions of fluorinated polysoaps [RF = CF(CF3)OC4F9] in the presence of SW-CNTs. Concentration of fluorinated polysoaps: 4.0 g/dm3 (2 mL); Stirring conditions: 30 °C/3 days; Used SW-CNT: 2 mg. (a) RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF in the presence of SW-CNTs. Dispersed SW-CNTs: 129 μg/mL, (b) RF-(DMAA) n -RF in the presence of SW-CNTs. Dispersed SW-CNTs: 22 μg/mL, (c) RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF
The amounts of dispersed (SW-CNT)-COOH in water with ABA triblock-type fluorinated oligomer were also determined under similar conditions.
Results and discussion
Single-walled carbon nanotubes (2 mg) were added to an aqueous solution of dendrimer-type fluoroalkyl end-capped block copolymer [RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF; RF = CF(CF3)OC4F9; Mn = 7840, 4 g/dm3: 2 mL] illustrated in Scheme 1. The mixture was stirred with a magnetic stirring bar at 30 °C for 3 days. The aqueous solution thus obtained was centrifuged, and then the residual SW-CNTs solution was filtered through a 1.2-μm filter membrane to obtain a transparent black solution. We have also studied on the dispersion of SW-CNTs in water by the use of the corresponding ABA triblock-type fluoroalkyl end-capped dimethylacrylamide oligomer [RF-(CH2CHCONMe2)n-RF [RF-(DMAA)n-RF]: RF = CF(CF3)OC4F9; Mn = 6480, 4 g/dm3: 2 mL] under similar conditions, for comparison. The relative amounts of dispersed SW-CNTs in water were estimated by the optical density at 500 nm with the use of the molar absorption coefficient (ε) of SW-CNTs-o-dichlorobenzene solutions reported by Smalley et al. [8]. The original RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF and RF-(DMAA) n -RF have no absorbance around 500 nm, and the relative amounts of dispersed SW-CNTs were summarized in Fig. 1.
As shown in Fig. 1, RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF and RF-(DMAA) n -RF oligomer were able to disperse SW-CNTs in water. This finding suggests that these fluorinated block copolymer and oligomer should provide the suitable host moieties to interact with SW-CNTs in water. The DLS measurements at 30 °C showed that the size (number−average diameter) of molecular assemblies formed in aqueous solutions of RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF could increase from 20 to 254 nm by dispersion of SW-CNTs. A similar tendency for the increase of the size (from 11 to 146 nm) was also observed in the dispersion of SW-CNTs in water with RF-(DMAA)n-RF oligomer. More interestingly, RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF was able to disperse SW-CNTs quite effectively about six times compared to that of RF-(DMAA)n-RF oligomer under similar conditions.
An extremely higher dispersibility of SW-CNTs thus obtained would be due to the architectures of self-assembly of fluorinated dendrimer-type block copolymers in aqueous solutions. It is well-known that fluoroalkyl end-capped dimethylacrylamide homooligomers [RF-(DMAA) n -RF] can form the nanometer size-controlled self-assembled molecular aggregates imparted by the aggregation between end-capped fluoroalkyl segments in oligomers in aqueous and organic media [7(a), 9]. Therefore, it is suggested that our present fluorinated dendrimer-type block copolymers could also form the molecular aggregates resembling micelles derived from the aggregations of end-capped fluoroalkyl segments in block copolymers in aqueous solutions. We have measured the molecular weights of RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF copolymer in water by SLS measurements at 30 °C. These results were shown in Table 1.
As shown in Table 1, the molecular weights of RF-(DMAA) n -RF homooligomeric aggregates determined by SLS and GPC measurements were 569500 and 6480, respectively. This finding indicates that fluorinated molecular aggregate formed by RF-(DMAA) n -RF oligomer in aqueous solutions are considered to consist of around 88 fluorinated oligomeric molecules. Similarly, the molecular weight (199800) of RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF copolymer determined by SLS was higher than that (7840) by GPC. Thus, fluorinated dendrimer-type copolymer should form the self-assembled molecular aggregates, which consists of around 25 fluorinated block copolymeric molecules in aqueous solutions, and in particular, the aggregations of end-capped fluoroalkyl segments in block copolymers are strongly involved in establishing the molecular aggregates as shown in Fig 2.
Thus, such new fluorinated dendrimer-type block copolymers should open new development in the higher dispersion of SW-CNTs, which in general exhibit an extremely poor dispersibility in water, through the encapsulation of SW-CNTs into the fluorinated dendrimer-type copolymeric aggregates in aqueous solutions. Especially, fluorinated dendrimer-type block copolymers should form the more suitable aggregates cores for the encapsulation of SW-CNTs, compared to that of traditional ABA block-type fluorinated RF-(DMAA)n-RF oligomer.
We studied in detail on the dispersion of SW-CNTs in water by the use of RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF copolymer and RF-(DMAA)n-RF oligomer, and these results were shown in Fig. 3.
As shown in Fig. 3, RF-(TTRV-Si) n -RF-block-RF-(DMAA) q -RF copolymer was more effective in the dispersion of SW-CNTs in water, compared to that of RF-(DMAA)n-RF oligomer. The amounts of dispersed SW-CNTs in water were increased with the increase of the concentration of block copolymer from 0.5 to 2.5 g/dm3. However, unexpectedly, the amounts of dispersed SW-CNTs were found to decrease by the increase of the concentration of block copolymer from 2.5 to 3.0 g/dm3. Similarly, unexpected results were obtained in the cases of the concentrations of the block copolymer from 3.5 to 4.0 g/dm3 and from 4.0 to 5.0 g/dm3. TEM images show that SW-CNTs could be smoothly encapsulated into dendrimer-type block copolymeric aggregate cores to afford the corresponding fluorinated aggregates/SW-CNTs composites in each concentration of block copolymer, especially above the concentration of 3.0 g/dm3 (block copolymer) (see Fig. 4).
In addition, we studied on the relationship between the amounts of dispersed SW-CNTs in water and the dispersion time, and these results were shown in Fig. 5.
As shown in Fig. 5, the amounts of dispersed SW-CNTs in water by using block copolymer were found to become extremely higher than that of RF-(DMAA)n-RF oligomer in each dispersion time. Especially, the amounts of dispersed SW-CNTs by using block copolymer increased with increasing the stirring times from 1 to 4 days; however, the amounts of dispersed SW-CNTs decreased with the increase of stirring times from 4 to 6 days. Such similar results were observed in the cases of the increase of stirring times from 7 to 8 days, from 8 to 9 days, and from 9 to 10 days. These interesting findings in Figs. 3 and 5 are not clarified in detail at the present time; however, one though is as follows (see Fig. 6): that is, fluorinated dendrimer-type copolymeric aggregates could provide suitable host moieties to interact with SW-CNTs as guest molecules (Fig. 6a). SW-CNTs should be in part released (to Fig. 6c) from the fluorinated aggregates/SW-CNTs composites (Fig. 6b) or encapsulated (from Fig. 6c) into these composites with increasing the dispersion times. These release and encapsulation behaviors of SW-CNTs would be due to the flexible architectures formed by the aggregation of terminal fluoroalkyl segments in our present fluorinated dendrimer-type block copolymer.
(SW-CNT)-COOH possesses carboxy groups on the sidewall of SW-CNTs. Thus, it is strongly expected that self-assembled fluorinated oligomeric aggregates formed by ABA triblock-type fluoroalkyl end-capped acrylic acid oligomers [RF-(CH2CHCOOH)n-RF; RF = fluoroalkyl groups [RF-(ACA)n-RF)]] should interact more effectively with (SW-CNT)-COOH as a guest molecule through the intermolecular hydrogen bonding between the carboxy groups in both (SW-CNT)-COOH and RF-(ACA)n-RF oligomers, compared to that of block copolymer or RF-(DMAA)n-RF oligomer. In fact, we have studied on the dispersion of (SW-CNT)-COOH in water by the use of RF-(ACA)n-RF oligomer, fluorinated block copolymer and RF-(DMAA)n-RF oligomer, and these results were shown in Fig. 7.
As shown in Fig. 7, UV-vis. spectra of dispersed (SW-CNT)-COOH solutions in water show that the relative amounts of dispersed (SW-CNT)-COOH estimated by the optical density at 500 nm were found to increase effectively in the case of RF-(ACA)n-RF oligomer [RF = CF(CF3)OC4F9; Mn = 2550].
Additionally, we summarized the amounts of dispersed (SW-CNT)-COOH and SW-CNTs in water by the use of RF-(ACA)n-RF oligomer, fluorinated block copolymer, and RF-(DMAA)n-RF oligomer including their composites size under similar conditions, and these results were shown in Table 2.
As shown in Table 2, the amounts of dispersed (SW-CNT)-COOH in water were estimated to be 136, 76, and 69 μg/ml, respectively, and the highest dispersibility was obtained by the use of RF-(ACA)n-RF oligomer. On the other hand, fluorinated block copolymer was the most effective for the dispersion of SW-CNTs in water. DLS measurements show that the size of self-assembled molecular aggregates formed by RF-(ACA)n-RF oligomer could increase effectively from 11 to 112 nm and 116 nm by the dispersion of (SW-CNT)-COOH and SW-CNTs in water, respectively, as well as fluorinated block copolymer and RF-(DMAA)n-RF oligomer. TEM images also show that (SW-CNT)-COOH should be smoothly encapsulated as a guest molecule into fluorinated acrylic acid oligomeric aggregate cores (Fig. 8a). In contrast, (SW-CNT)-COOH could be tightly encapsulated into fluorinated block copolymeric aggregate cores (Fig. 8b), because dendrimer-type block copolymeric aggregate cores consist of fine networks in water.
In this way, it was verified that RF-(ACA)n-RF oligomer was able to disperse (SW-CNT)-COOH quite effectively in water, although this oligomer failed to disperse SW-CNTs effectively in water. This finding indicates that intermolecular hydrogen bonding between the carboxy groups in both oligomer and SW-CNTs could afford the good dispersibility of (SW-CNT)-COOH in water. In order to clarify this dispersion characteristic with RF-(ACA)n-RF oligomer, we have studied in detail on the dispersion of (SW-CNT)-COOH in water by the use of RF-(ACA)n-RF. We also studied on the dispersion of (SW-CNT)-COOH in water by the use of fluorinated block copolymer under similar conditions, for comparison. These results were shown in Fig. 9.
As shown in Fig. 9, RF-(ACA)n-RF oligomer was able to increase the amounts of dispersed (SW-CNT)-COOH with increasing the stirring times from 1 h to 1 day, and almost constant values (ca. 135 μg/ml) were obtained above 1 day. This dispersion characteristic behavior in RF-(ACA)n-RF oligomer is quite different from that of fluorinated block copolymer, indicating that the dispersion of (SW-CNT)-COOH with RF-(ACA)n-RF oligomer would be depend on the intermolecular hydrogen bonding interaction. Thus, the release of (SW-CNT)-COOH from the fluorinated nanocomposites illustrated in Fig. 6 [from (b) to (c)] could not be occurred due to the hydrogen bonding interactions related to carboxy groups.
In conclusion, dendrimer-type fluoroalkyl end-capped block copolymer was able to disperse SW-CNTs quite effectively in water. On the other hand, the corresponding ABA triblock-type RF-(DMAA)n-RF oligomer was not able to disperse SW-CNTs in water, effectively. DLS measurements and TEM images of fluorinated block copolymer showed that SW-CNTs could be smoothly encapsulated into fluorinated block copolymeric aggregate cores as guest molecules to increase their aggregate core size. On the other hand, dendrimer-type fluorinated block copolymer was not effective for the dispersion of (SW-CNT)-COOH in water; however, (SW-CNT)-COOH could be more effectively dispersed into water by the use of RF-(ACA)n-RF oligomer.
References
(a) Niyogi S, Hamon MA, Hu H, Zhao B, Bhowmik P, Sen R, Itkis ME, Haddon RC (2002) Acc Chem Res 35:1105; (b) Khabashesku VN, Margrave JL (2004) Encycl Nanosci Nanotechnol 1:849
(a) Ham HT, Choi YS, Chee MG, Chung IJ (2006) J Polym Sci: Part A: Polym Chem 44:579; (b) Tomonari Y, Murakami H, Nakashima N (2006) Chem Eur J 12:4027; (c) Cotinuga I, Picchioni F, Agarwal US, Wounters D, Loos J, Lemstra PJ (2006) Macromol Rapid Commun 27:1073; (d) Kim J-B, Premkumar T, Lee K, Geckeler KE (2007) Macromol Rapid Commun 28:276
(a) Sawada H (1996) Chem Rev 96:1779; (b) Sawada H, Matsumoto T, Nakayama M (1992) Yuki Gosei Kagaku Kyokaishi 50:592; (c) Sawada H, Kawase T (2001) Kobunshi Ronbunshu 58:147; (d) Sawada H, Kawase T (2001) Kobunshi Ronbunshu 58:255; (e) Sawada H (2000) J Fluor Chem 101:315; (f) Sawada H (2000) J Fluor Chem 105:219
(a) Sawada H (2007) Prog Polym Sci 32:509; (b) Sawada H (2007) Polym J 39:637
Sawada H, Shindo K, Iidzuka J, Ueno K, Hamazaki K (2005) Eur Polym J 41:2232
Yoshioka H, Suzuki M, Mugisawa M, Naitoh N, Sawada H (2007) J Colloid Interface Sci 308:4
(a) Sawada H, Yoshino Y, Ikematsu Y, Kawae T (2000) Eur Polym J 36:231; (b) Sawada H, Gong Y-F, Minoshima Y, Matsumoto T, Nakayama M, Kosugi M, Migita T (1992) J Chem Soc Chem Commun 537
Bahr JL, Mickelson ET, Bronikowski MJ, Smalley RE, Tour JM (2001) Chem Commun 2:193
Sawada H (2003) J Fluor Chem 121:111
Acknowledgement
Thanks are due to Asahi Glass Co., Ltd. for supply of C4F9OCF(CF3)C(=O)F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sawada, H., Naitoh, N., Kasai, R. et al. Dispersion of single-walled carbon nanotubes in water by the use of novel fluorinated dendrimer-type copolymers. J Mater Sci 43, 1080–1086 (2008). https://doi.org/10.1007/s10853-007-2329-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-2329-8