Abstract
Experimental results obtained on the preparation of hydrophobic silica aerogels by ambient pressure drying method using the sodium silicate precursor with the variation of solvent exchanging process, are reported. The silica hydrogel was prepared by passing the 1.12 specific gravity sodium silicate through the Amberlite (TM) 120 Na+ resin and addition of 1 M ammonium hydroxide to silicic acid. The gel was kept in an oven for 3 h to strengthen the gel. Solvent exchange was carried out with ethanol and hexane for 36 h each followed by 24 h silylation using 20% hexamethyldisilazane (HMDZ) in hexane. Unreacted HMDZ was washed with hexane by keeping the gel in hexane for 24 h. Solvent was decanted and the gel was dried for 24 h by keeping the gel at 50 °C for 6 h, at 150 °C for 12 h and at 200 °C for 6 h. The low density (0.06 g/cm3), highly porous (96.9%), highly hydrophobic (contact angle of 160°), low thermal conductivity (0.07 W/m K) aerogels were obtained for the process of three times exchange with ethanol and three times exchange with hexane in 36 h each, followed by silylation with 20% HMDZ in hexane and two times washing with hexane in 24 h. FTIR studies showed the increase in the intensity of the Si–H and C–H bands of the aerogels with the increase of solvent exchanging times because of increase in silylation for more times of solvent exchange processes. It was found from the TG–DTA studies that the hydrophobicity of the aerogels retained up to the temperature of 325 °C. Water absorption studies show that the aerogels were remained hydrophobic up to 4 months when the aerogels were placed over the water as well as for up to 60 h in a 90% humid atmosphere. SEMs of the aerogels reveal that the pore sizes of the silica network increased, so the percentage of optical transparency decreased with the increase in exchange times with ethanol and hexane.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Silica aerogels consist of more than 90% air and less than 10% solid silica in the form of highly cross-linked network structure. These materials are unique with low density (0.03 g/cm3), large surface area (≅ 1,000 m2/g), small pores (≅ 50 nm), low thermal conductivity (≅ 0.02 W m−1 K−1), and low sound velocity (≅ 100 m s−1) [1–4]. Hence silica aerogels have several applications such as Cerenkov radiation detectors in nuclear reactors and high-energy Physics [5–8], inertial confinement fusion (ICF) targets [9] containers for liquid rocket propellants [10], adsorption and catalytic supports [11].
So far, the synthesis of silica-based aerogels has been accomplished mainly through the controlled condensation of small colloidal particles produced by sol-gel processing in alcoholic and aqueous solutions followed by supercritical drying process. A highly desirable goal in aerogel synthesis is the elimination of the supercritical drying process, the most expensive and risky aspect of the process, i.e., drying with siloxanes at high temperatures and pressure with flammable solvent evacuation which is a risky method for commercialization. Therefore, interests exists in methods of preparing the silica aerogels at ambient pressures using low cost precursors such as sodium silicate. A few research reports are available in the literature on the preparation of silica aerogels at atmospheric pressure using surface modification prior to drying [12–22]. In the present paper, effect of solvent exchange process for the exchange of water in the hydrogels in the preparation of hydrophobic silica aerogels using ethanol, hexane, silylation with 20% HMDZ in hexane with sodium silicate precursor followed by drying for 24 h at 50 °C for 6 h, at 150 °C for 12 h, and at 200 °C for 6 h is reported.
Experimental procedure
Hydrophobic silica aerogels were prepared in three steps: (1) preparation of silica hydrogels, (2) solvent exchange and surface modification (silylation) of gels and (3) drying the surface modified gels. The chemicals used were 1.36 specific gravity sodium silicate, ammonium hydroxide, Amberlite (TM) (TM trade mark of Rohm and Haas) 120 Na+ form resin, ethanol, hexane (LOBA, India) and hexamethyldisilazane (HMDZ, Sigma Aldrich, Germany). Systematic preparation of ambient pressure dried silica aerogels was shown in Fig. 1. In the first step, 1.36 sp. gr. sodium silicate solution was diluted to 1.12 sp. gr. solution and passed through the Amberlite resin to get silicic acid. 0.1 mL of 1 M NH4OH was added to the 25 mL of silicic acid and a hydrogel formed in 10 min. The gel was kept in the oven for 30 min to strengthen the silica network. In the second step, the water present in the pores of the gel was exchanged with 25 mL ethanol for 25 mL of gel by placing the gel in ethanol for 36 h at 50 °C by keeping the gel in oven. The ethanol in the gel was exchanged with 25 mL of hexane by keeping the gel in hexane for 36 h at 50 °C. Hexane was decanted and the gel was kept in 25 mL of 20% HMDZ in hexane (5 mL HMDZ + 20 mL hexane) for 25 mL of gel for 24 h at 50 °C for surface modification. Unreacted HMDZ in the gel was exchanged with 25 mL of hexane for 24 h. Various exchanging times with solvents are given in Table 1. If the water in the gel was exchanged with hexane directly for 36 h without ethanol exchange, that aerogel named as sample 1 as mentioned in the table. Whereas, the water of gel was exchanged with 25 mL ethanol for one time or two times or three times in 36 h followed by one time hexane exchange in 36, 24 h silylation and one time hexane exchange in 24 h, those aerogels named as sample 2, 3, and 4, respectively. In the similar way according to the number of times of hexane exchange in 36 h before silylation and in 24 h after silylation, the names of the aerogels are given as shown in the table. In the third step, the hexane was decanted and the silylated gels were dried for 24 h by varying the temperature of 50 °C for 6 h, at 150 °C for 12 h, and at 200 °C for 6 h. The silica aerogels were cooled to room temperature and used for characterization.
Characterization
The percentage of volume shrinkage of the aerogels was calculated from the volumes of the gel and aerogel. The bulk density (ρb) of the aerogel was measured using the known volume of the aerogel and its mass by a microbalance of 10−5 g accuracy. The refractive index (η) [23], porosity and pore volume of the aerogels were determined with the formulae:
where ρ s is the skeletal density of the silica aerogels.
The hydrophobicity of the aerogels was tested by measuring the contact angle (θ) with water droplet of 0.5 cm diameter placed on the aerogel surface with contact angle meter (USA) as well as using the formula [24]:
where I is the height of the water droplet and W is the base width of the droplet touching the aerogel surface. The I and W were measured with travelling microscope.
The thermal conductivity of the aerogel was measured from the Thermal conductivity meter (C-T meter, France) by sandwiching the thermocouple with uniform samples.
The surface chemical modification of the aerogels was studied using the Fourier Transform Infrared spectroscopy (FT-IR, Perkin-Elmer) which gave the information about the various chemical bondings such as –OH, Si–OH, Si–O–Si, Si–C, and C–H. The optical transmission of the samples was measured using the UV–Visible spectrophotometer at 750 nm.
The thermal stability of the aerogels in terms of retention of hydrophobicity was estimated from the thermo gravimetric and differential thermal analysis (TG–DTA, SDT Model 2960 TA Universal Instruments, USA) as well as by heating the samples in the furnace at different temperatures and putting the cooled samples on the surface of the water. The retention of hydrophobicity (water repelling property) in terms of time was also judged from the absorption of water by the aerogels for various time intervals by putting the aerogels over the water as well as from humidity chamber (Remi Company, India). The micro structure of the silica aerogels were observed with Scanning Electron Microscope (Model Philips XL-30 SEM analyzer).
Results and discussion
Ambient pressure dried hydrophobic silica aerogels with pore volume and pore sizes in the range of supercritical dried aerogel was prepared by silylation before drying of well-dispersed siliconoxide via sol-gel method. Many potential advantages inherent in the use of sol-gel techniques in preparing hydrophobic aerogels are strongly dependent on the solvent exchange, silylation and drying methods. Even though supercritical drying prevents the silica network collapse induced by capillary forces arising from the liquid–vapor interface leading to material with low-density and high-porosity aerogels with suitable properties for many applications, but supercritical process has numerous drawbacks due to the extreme conditions used. Therefore, a simple method of ambient pressure drying method for hydrophobic silica aerogels has been reported [13]. It is based on the use of silylation on the surface of inorganic gel, prevents the irreversible shrinkage of the porous structure during drying. The silica aerogels produced by super critical drying are hydrophobic for little time as a result of esterification reaction occurring between surface Si–OH groups and alcohol inside the autoclave [25]. However such aerogels retain the hydrophobicity for short time or an hour when exposed to humid atmosphere. The loss of hydrophobicity is due to the chemical reaction of the pore surface Si–OCH3 groups with water. Therefore the durable hydrophobic aerogels are generally produced by surface modification method [26]. In the present work the hydrophobic aerogels were prepared by surface modification with 20% HMDZ in hexane during the solvent exchange process of the silica hydrogels, prepared from sodium silicate. The concept of using cheap raw material water glass based gels is the gels experience highest degree of monolithicity because of these wet gels had the highest stiffness (shear modulus) and large pore size (permeability) [27]. The water in the hydrogels was exchanged with ethanol and then with hexane before surface chemical reaction with HMDZ. Surface modification of the silica gel with HMDZ has shown in the following reaction.
Surface chemical reaction:

HMDZ is a good silylating agent because presence of two Si(CH3)3 groups which contain six CH3 groups and can replace the H of the surface Si–OH in the inert media. HMDZ reacts with water and ethanol but not with hexane and hexane cannot replace the water completely as it is immiscible with water. Therefore, it is necessary the pore water should be replaced with ethanol which is soluble in water and then the ethanol should be replaced with hexane as it is miscible in hexane.
Table 1 shows the number of times ethanol and hexane exchanged in 36 h and their respective properties were described in Table 2. From Table 2, it was came to know that the percentage of volume shrinkage, density, thermal conductivity, refractive index, and percentage of optical transmission of the aerogels decreased and pore volume, percentage of porosity and contact angle of the aerogels are increased with increasing the number of times of exchanging the water of the hydrogel in 36 h with ethanol followed by hexane for 36 h by keeping the silylation time and percentage of HMDZ in hexane constant at 24 h and 20, respectively. As we found that the aerogel (sample 1) obtained with direct replacement of water with hexane had very high density (0.3 g/cm3) and hydrophilic (contact angle of 65°). With increasing the number of times of exchange with ethanol followed by hexane in 36 h each from 0 to 4, the density of the aerogels decreased (0.06 g/cm3) for three times and further it was found that there was not any change in the density of the aerogels for >3 times of solvent exchange with ethanol and hexane. For complete surface chemical modification of the gels with HMDZ, the pore water should be completely exchanged with ethanol and then hexane. Even impartial exchange of the water with ethanol followed by hexane leads the incomplete surface silylation, in the silica gels resulted the dense and less hydrophobic aerogels. It is interesting to note that how the silylation procedure is capable of preserving at least partially the gel structure derived from the use of increasing times of solvent exchanges. During drying of silylated gels for 24 h, first the gels under go shrinkage to minimum volume and expand to its original volume [18], “Spring Back”. In the impartial silylated gel, when they reach to minimum volume during the drying, a part of surface which had Si–OH groups condensed and form Si–O–Si which was a irreversible [28] and the part of surface that covered with Si–O–Si(CH3)3 as they came together did not undergo condensation because of inert CH3 groups and they repelled each other so spring back to higher volumes. Hence the gel expanded non-uniformly making the gel unable to reach its original volume resulting in a more dense aerogel. It was found that with increase of solvent exchanging times, the surface chemical modification increased, and during the drying the percentage of spring back increased. In the case of effective surface modification a complete recovery of the gel during the last stages of drying has previously observed [12]. It was found that three times ethanol followed by three times hexane exchange in 36 h each making the gel of 100% spring back to reach to its original volume resulted low density and highly hydrophobic silica aerogels.
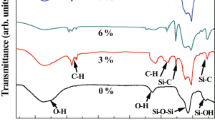
To find out the silylation in the aerogels of various exchange processes, FTIR spectra of the sample 1(A), sample 6(B), sample 10(C), sample 11(D) are shown in Fig. 2. FTIR indicates that along with Si–O–Si and O–H absorption bands at 1,100 and 3,600, 1,600 cm−1, respectively for unmodified aerogel sample there were additional absorption bands at 2,900 and 1,450 cm−1 related to C–H and 1,350, 840 cm−1 related to Si–C bands for the modified gels [29, 30]. It was found that the intensity of the O–H band decreased and the Si–C, C–H bands increased from A to D because number of Si(CH3)3 groups attached to the silica gel surface increased indicating the better surface modification. These facts can also seen to be reflected in the contact angle studies depicted in Table 2.
The water absorption studies were carried out by placing the A, B, C, and D aerogels on the water as well as in the humidity chamber with 90% humidity at 30 °C. The aerogels were removed at different intervals from the water and humidity chamber to find the percentage of weight increase in the aerogels as shown in Figs. 3 and 4 respectively. It was found from the figures that the percentage of weight increase is very high in A than D aerogel. Since, when the aerogels were kept over the water, there is a 75% (i.e., 1 g of aerogel increased to 1.75 g) (hydrophilic) and 0.5% (i.e., 1 g of aerogel increased to 1.05 g) (hydrophobic) weight increase for A and D aerogels, respectively in 4 months. Whereas, in the humidity chamber, it was found that there is a 80% (i.e., 1 g of aerogel increased to 1.8 g) and 0.5% (i.e., 1 g of aerogel increased to 1.05 g) weight increase for A and D, respectively in 60 h. This shows that the A aerogel is hydrophilic because most of its surface has covered with OH groups [26] and whereas the D aerogel is hydrophobic as its surface has –Si(CH3). The Si(CH3)3 groups of HMDZ replace H of the surface OHs of silica cluster and they form a layer on the surface which is non-hydrolyzable and hydrophobic as shown below.
Si(CH3)3 covering on the silica surface for A and D samples:

From the chemical reaction as well as silylation of silica surface with HMDZ, it was known that all the OH groups were silylated in the D aerogel hence they are highly hydrophobic where as A aerogel is partially silylated leading to hydrophilic nature. The –Si(CH3)3 groups attached to the surface via oxygen bridges –O–Si(CH3)3 thus causes better withstanding of hydrophobic covering against water. The water penetration in the pores due to capillary action is resisted by the hydrophobic surface alkyl groups. So the percentage of weight increase of the aerogels when they placed on the water and in humid atmosphere for longer timings, in D is lesser than A aerogel as shown in Figs. 3 and 4. And also as shown in Table 2 that the contact angle of the aerogels increased from the sample 1 to sample 11 because the number of Si(CH3)3 attached on the surface of silica increased. Hence more number of –Si(CH3)3 attached to the surface show high contact angle due to its better hydrophobic covering.
Figure 5(a) and (b) shows the SEM of aerogels of three-dimensional nanoporous structures for A and D aerogels, respectively. It was found from the SEM microstructures that the pore size and particle sizes are less in the A (sample 1) than D (sample 11) aerogel. The optical transparency of the aerogel decreased from sample 1 to 11 as shown in Table 2. This is because in sample 1 due to low surface modification, there is an intensive condensation of surface –OH groups in the pores leading to the formation of small pores in the silica network, so they are transparent where as in sample 11, due to repulsion of surface Si(CH3)3 groups, the pore size and particle size increases resulted less transparency of the aerogels.
The thermal stability of the aerogels in terms of retention of hydrophobicity was estimated from the thermogravimetric and differential thermal analysis (TG-DTA) as well as heating the aerogels at different temperatures in the furnace and putting the cooled samples over the water surface. The retention of the hydrophobicity (water repelling property) was judged from the absorption of water by the aerogels. Figure 6 shows the TG-DT analysis of the A, B, C, and D aerogels in the oxygen atmosphere up to 600 °C. The TGA and DTA studies in the oxygen atmosphere revealed that all the modified gels are thermally stable up to the temperature of 325 °C and above the 325 °C, the weight of the samples decrease due to the oxidation of the methyl groups leaving the silica network. This fact can clearly seen as there is sharp exothermic peak in the DTA when the temperature is raised above 300 °C. The sharpness of the peak increased from A to D aerogels because of increase in number of methyl groups from A to D aerogels. It can be also seen in the TGA curves as the weight loss in A is less than D aerogel because of less surface modification in A than D aerogel so less number of methyl groups that undergo oxidation than the D aerogel. The aerogels that heated up to 300 °C the aerogels did not absorb the water (hydrophobic) where as the aerogels heated up to 350 °C absorbed the water as aerogels became hydrophilic.
Conclusions
The method reported in the present work is an easy and reproducible way to obtain a highly hydrophobic and porous aerogels. The ambient pressure dried hydrophobic silica aerogels were prepared using the sodium silicate precursor by varying the solvent exchanging process with ethanol and hexane. The surface modification was carried out with 20% HMDZ in hexane. The solvent exchange process was carried out from 0 to 4 times of ethanol and hexane in 36 h each and 24 h of silylation and 24 h of washing the unreacted HMDZ with hexane. It was found that the density and volume shrinkage decreased from 0.300 to 0.06 g/cm3 with increasing the solvent exchanging times with ethanol and hexane from 1 to 3 times and remained constant for >3 times of solvent exchange. From the contact angle studies it was found that the hydrophobicity and durability of hydrophobicity of the aerogels increased from sample 1 to 12 samples because of increasing the surface modification. It was also confirmed from the FT-IR studies that the intensity of the Si–C and C–H bands increased from sample 1 to sample 12. TGA–DTA studies shows that the retention of hydrophobicity in the aerogels is up to the temperature of 325 °C and above this temperature the aerogels become hydrophilic. From the TG–DTA it was also found that the weight loss and sharpness of the exothermic peak in the sample 12 is more than the sample 1 because of more methyl oxidation in the sample 12 than the sample 1. The optical transparency of the sample decreased from sample 1 to 12 because of increase in pore sizes in the silica network as seen in the SEMs of the aerogels.
References
Fricke J, Emmerling A (1992) In: Reisfield R, Jorgensen CK (eds) Chemistry, spectroscopy and applications of sol-gel glasses, Springer series, Structure and bonding, vol 77. Springer, Berlin, p 371
Mulder CAM, Van Lierop JG (1986) In: Fricke J (ed) Aerogels. Springer, Berlin, p 68
Attia YA (1994) Mater Technol 9:1
Hrubesh LW (1998) J Non-Cryst Solids 225:335
Carlson PJ, Johansson KE, Norrloy JK, Pingot O, Tavernier S, Van Den Bogert F, Van Luncker L (1979) Nucl Instrum Methods 160:407
Buzykaev AR, Danilyuk AF, Ganzbur SK,Gorodtskaya TA, Kolachev GM, Kravchenko EA, Mikerov VI, Minakov GD, Onuchin AP, Shamov AG, Tayursky VA (1998) J Non-Cryst Solids 225:381
Sumiyoshi T, Adachi I, Enamoto R, Iijima T, Suda R, Yokoyama M, Yokogava H (1998) J Non-Cryst Solids 225:369
Tilloston TM, Hrubesh LW, Simpsom RL, Lee RS, Swansiger RW, Simpson LR (1998) J Non-Cryst Solids 225:358
Kim K, Jang KY, Upadhye RS (1991) J Am Ceram Soc 78:1987
Pajonk GM, Teichner SJ (1985) In: Fricke J (ed) Proceedings of the first international symposium on aerogels, Wurzburg, Germany, p 193
Pajonk GM (1991) Appl Catal 72:217
Prakash SS, Brinker CJ, Hurd AJ, Rao SM (1995) Nature 374:439
Hurd AJ (1995) J Non-Cryst Solids 190:264
Yang HS, Choi SY, Hyun SH, Park HH, Hong JK (1997) J Non-Cryst Solids 221:151
Haereid S, Dahle M, Lima S, Einarsrud MA (1995) J Non-Cryst Solids 186:96
Haereid S, Nilsen E, Einarsrud MA (1996) J Porous Mater 2:315
Smith DM, Stein D, Anderson JM, Ackerman W (1995) J Non-Cryst Solids 186:104
Deshpande R, Smith DM, Brinker CJ (1992) US patent, Applic SNPCT/US 94, 05105
Lee CJ, Kim GS, Hyun SH (2002) J Mater Sci 37:2237
Venkateswara Rao A, Parvathy Rao A, Kulkarni MM (2004) J Non-Cryst Solids 350:224
Parvathy Rao A, Venkateswara Rao A, Pajonk GM (2005) J Sol-Gel Sci Technol 36(3):285
Parvathy Rao A, Pajonk GM, Venkateswara Rao A (2005) J Mater Sci 40:3481
Buzeyskaev AK, Damilyuk AF, Ganzbur SF, Kravchenko EA, Onuchin AP (1999) Nucl Instrum Methods Phys Res A 433:396
Bekerman JJ (1958) Surface chemistry, theory and applications, 2nd edn. Academic Press Inc., New York, p 343
Brinker CJ, Scherer GW (1990) Sol-gel science. Academic Press, San-Diego, p 536
Yokogawa H, Yokoyama M (1995) J Non-Cryst Solids 186:23
Venkateswara Rao A, Nilsen E, Einarsrud MA (2001) J Non-Cryst Solids 296:165
Venkateswara Rao A, Pajonk GM, Parvathy NN, Elaloui E (1994) In: Attia YA (ed) Sol-gel processing and applications. Plenum Publications, New York, p 237
Yoldas BE (1984) J Non-Cryst Solids 63:145
Hering N, Schriber K, Reidel R, Lichtenberger O, Woltersodorf J (2001) Appl Organomater Chem 15:879
Acknowledgements
The authors are highly thankful to the Department of Science and Technology (DST), New Delhi, for funding this work under the project No. SP/S2.CMP-01/2002. A. Parvathy Rao and Poonam M. Shewale are thankful for providing the fellowships in the project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parvathy Rao, A., Venkateswara Rao, A., Pajonk, G.M. et al. Effect of solvent exchanging process on the preparation of the hydrophobic silica aerogels by ambient pressure drying method using sodium silicate precursor. J Mater Sci 42, 8418–8425 (2007). https://doi.org/10.1007/s10853-007-1788-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-1788-2