Abstract
Flow-through capacitor (FTC) with carbon nanotube (CNT) electrode was employed to remove NaCl from saltwater solution. According to the investigation by transmission electron microscopy (TEM) and nitrogen adsorption, the BET specific surface area of CNT electrodes decreased with increasing nanotube diameter, which had a narrow distribution in each sample. Besides, removal of NaCl per unit weight of CNT electrode increased linearly with the BET specific surface area of CNT electrodes. As a result, CNT electrode with the smallest nanotube diameter had the best removal characteristic, with removal efficiency up to 95% and energy-consumption of 3.0 Wh L−1, and the process of regeneration could be carried out easily in a short time. Comparing removal experiment with charge/discharge test, it was concluded that FTC based on the same electro-adsorption theory as double layer capacitors. The cyclic voltammograms of CNT electrode in salt water showed that the current occurred by adsorption and desorption of ions in the range of −0.3–0.9 V, which directly explained the reason why CNT electrode could be used to remove NaCl.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water resources crisis is one of the biggest resources crises in this century in the world. High recoveries for brackish and sea water purification are the important ways to solve this crisis. The FTC is a newly developing technology that shows promise in this regard [1]. A major difference in the operation of this technology, as compared to other technologies, is that the water purification device is cost-cutting, energy-saving and environment-friendly. FTC works by electro-adsorption of ions on a charged electrode surface. Early versions used activated carbon as electrode [2–4], but activated carbon electrode has such defects as high resistivity, high energy-consumption and low strength. As far as highly conductive carbon black is concerned, the strength of carbon black electrode is low and the effective pore volume which ions could enter is small. CNTs have a novel structure, low resistivity, high strength, and high stability. So CNTs can form the highly conductive net in the electrode, and simultaneously the strength of the electrode can be highly increased. Moreover, CNT electrode has more effective pores due to the meso-pore structure of CNTs. The paper authors had patented using CNTs in the fabrication of FTC [5], which have extended the purification ability of FTC to the saltwater solution [6–8]. In this paper, the influences of nanotube diameter of CNTs on removal of NaCl from salt water were investigated.
Experimental
Preparation of CNT electrodes
CNTs synthesized by the catalytic decomposition of methane, were supplied by Shenzhen Nanotech Port Co., Ltd. (China). Raw CNTs were purified and modified to increase the effective surface area [6, 7]. The mixture of CNTs and binder powders in a weight ratio of 80:20 was pressed under 25 MPa pressure at 150 °C, where the binders were made up of phenolic resin (90%) and urotropine (10%), and then the tablet was carbonized at 850 °C for 2 h in nitrogen atmosphere. Finally, CNT electrode with high strength was obtained after natural cooling [8]. CNT electrodes with nanotube diameters of 1∼2 nm, 2∼10 nm, 10∼20 nm, 10∼30 nm and 40∼60 nm, were marked as sample A, B, C, D, and E respectively. Each electrode was 115 mm in length, 75 mm in width and 15 g in mass.
Removal of NaCl
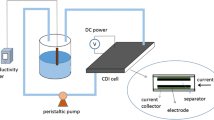
FTC apparatus was shown in Fig. 1. The saltwater solution was passed through the capacitor at a flow rate of 10 ml/min, where the initial concentration was 5000 mg/l and the number of electrode tablet was 20. NaCl was removed by applying a direct voltage of 1.0 V between the electrodes. On the other hand, for regeneration of the CNT electrodes, a reverse voltage of 1.0 V was applied to both electrodes to desorb and recover concentrated saltwater solution. A CyberScan CON 200 conductivity meter was employed to directly get the data of the salt concentration.
Results and discussion
CNTs with different nanotube diameters were observed by TEM of JEOL JEM-2010, and powdered samples were dispersed in absolute ethanol by ultrasonication for 10 min in a KQ-250B ultrasonic bath. As shown in Fig. 2, the nanotube diameter, which increased from sample A to E, had a narrow distribution in each sample and the nanotube diameter of sample A was the smallest of all, about 1.8 nm. CNT electrodes, characterized by nitrogen adsorption at 77 K with Micromeritics ASAP 2010, presented type IV nitrogen adsorption isotherms, shown in Fig. 3, with a hysteresis loop typical of a mesoporous material where desorption requires definitively higher energy than adsorption [9]. According to nitrogen adsorption isotherms, the BET specific surface area could be obtained in Table 1, which showed that BET specific surface area of CNT electrode decreased with increasing nanotube diameter.
To investigate the performance of CNT electrodes with different nanotube diameters, the charge/discharge tests of the electrodes were performed in 5000 mg/l NaCl solution by the DC-5 battery testing instrument. Generally, the specific capacitance (C S) of the electrodes was calculated by the equations:
According to Faraday Law, the theoretical removal amount per unit weight of CNT electrode (W theo) was calculated as follows:
In the same time, the real removal amount per unit weight of CNT electrode (W real) was evaluated by experimental data as the following equation:
Efficiency of removal process (η) was given as:
where Q was the quantity of electricity, E was the voltage, i was the discharge current, t was the discharge time, C was the electrostatic capacitance, G was the mass of electrode, M was molecular weight of NaCl, F was Faraday constant, V was the volume of fresh water, and C real was the concentration of fresh water.
The experimental data in Table 2 showed that real removal data highly corresponded with theoretical removal data, with the efficiency more than 90%. It could be concluded that FTC using CNT electrodes was very efficient and it based on the same electro-adsorption theory as double layer capacitors. Besides, it could be seen that the real removal amount per unit weight of CNT electrode increased with the BET specific surface area of CNT electrodes. The relationship between S BET and W desal was shown in Fig. 4. The fair correlation could be suggested that removal of NaCl was mainly dependent on the BET specific surface area of CNT electrode.
Generally, the electrostatic capacity of double layer capacitors depends on the surface area of the electrode interface, as follows [4, 10]:
where ε is the permittivity of solution, δ is the distance between the electrode and center of ion, and S is the surface area of the electrode interface. So the removal characteristic of the electrode increased with the surface area of the electrode interface. As a result, sample A with the highest surface area has the best removal characteristic of all. The removal and regeneration curve of sample A in Fig. 5 showed that NaCl removal efficiency was up to 95% and the process of regeneration could be carried out easily in a short time. According to the principle of FTC, energy-consumption (W) of removal process was calculated as follows:
where P was the output power of regulated power supply, V was the volume of salt water treated, and t was the applied time. Energy-consumption of sample A calculated was about 3.0 Wh L−1. In the same condition, NaCl removal efficiency of activated carbon electrode, with BET specific surface area of 1434.4 m2 g−1, was only about 80%, and energy-consumption calculated was about 6.1 Wh L−1, which was twice more than that of sample A.
The cyclic voltammograms of sample A was investigated with a CHI660A electrochemical workstation by a three electrode-system, including a CNT electrode, a platinum wire counter electrode and a saturated calomel reference electrode, and the current signals were obtained at the optimum conditions in 5000 mg/l NaCl solution using a potential scan from −0.5 V to 1.2 V with the scan rate of 10 mV/s and then reversing the order. It was known to all that the achievement of box-shaped cyclic voltammograms over a wide range of scan rates was the ultimate goal in electrochemical double-layer capacitors. Fig. 6 showed that sample A was very stable in the range of −0.3 V–0.9 V. Due to application of potential difference, hydrogen was created when the potential was less than −0.3 V and oxygen was created when the potential was over 0.9 V. In the range of −0.3 V–0.9 V, the current occurred by adsorption and desorption of ions, which directly explained the reason why CNT electrode could be used to remove NaCl.
Conclusions
It was efficient enough to remove NaCl from saltwater solution using the FTC with CNT electrodes. The nanotube diameter of CNTs had a great effect on removal of NaCl. The amount of removal was mainly dependent on the BET specific surface area of CNT electrode, which decreased with increasing nanotube diameter, and thus CNT electrode with the smallest nanotube diameter had the best removal characteristic of all. Though there are a few problems remaining to be solved, our results show a promising technique for removal of NaCl from saltwater solution with CNT electrode.
References
Andelman M (1993) US5192432
Andelman M (1998) Filtr Separat 35:345
Noack A (2002) DE1003457
Oda H, Nakagawa Y (2003) Carbon 41:1037
Shi L, Xie J, Liu H, Yang H, Li C, Zhu W (2003) CN1463927A
Zhang DS, Dai K, Fang JH, Shi LY, Wen Y, Liu JQ (2005) J Funct Mater (Ch) 36:282
Zhang DS, Shi LY, Fang JH, Dai K, Li XK (2006) Mater Chem Phys 97:415
Zhang DS, Shi LY, Fang JH, Dai K, Liu JQ (2006) Mater Chem Phys 96:140
Frackowiak E, Delpeux S, Jurewicz K, Szostak K, Cazorla-Amoros D, Béguin F (2002) Chem Phys Lett 361:35
Nishino A (1996) J Power Sources 60:137
Acknowledgements
The authors acknowledge the supports of the National High Technology Research and Development Program (863 Program) of China (2002AA302302) and Shanghai NanoScience & Techology Special Project (0223nm001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, D., Shi, L., Fang, J. et al. Influence of diameter of carbon nanotubes mounted in flow-through capacitors on removal of NaCl from salt water. J Mater Sci 42, 2471–2475 (2007). https://doi.org/10.1007/s10853-006-1293-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-006-1293-z