Abstract
Bone adhesives are known as a promising fracture treatment material because they can quickly heal broken bones. However, the existing bone adhesives have the disadvantages of weak binding ability to bone tissue, non-absorption, or difficulty in curing under wet conditions, which limits their wide application in the field of bone tissue engineering. In this study, the raw material of magnesium phosphate bone adhesive was modified, and the composite material of magnesium phosphate bone repair was prepared by the method of solid–liquid blending crosslinking. Mineral-organic composite, a new type of bone adhesive was prepared using calcined dolomite and montmorillonite as mineral components, phytic acid and gelatin as organic components. The compressive strength, porosity and bonding strength of the magnesium phosphate-based bone adhesive were analyzed by Fourier transform infrared spectroscopy, X-ray diffraction and scanning electron microscopy. When the dosage of dolomite is 8 wt% and the concentration of gelatin is 9 wt%, the adhesion strength of the bone adhesive is 2.04 MPa after 168 h placement. And the compressive strength of the bone adhesive was 6.66 MPa after 168 h placement. After the prepared bone adhesives were immersed in SBF solution for 14 and 21 days, EDS analysis showed that the accumulated material was bone-like hydroxyapatite, indicating that the prepared bone adhesives had good osteogenic activity. In addition, it was also found that the bone adhesive had fluid absorption ability and no cytotoxicity. So, conclusively it can be stated that such newly synthesized bone adhesive has significant medical potential.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Every year, there are countless incidents of bone fracture happen due to osteoporosis, bone diseases, cumulative strain, external forces, and other reasons [1]. Bone materials commonly used after fracture include metal implants, bone cement and bone adhesives (Table 1). Most broken bones can only be fixed by implanting mechanical fasteners, such as metal alloy plates or metal nails. However, the clinical application shows that implant fixation as a traditional treatment for fractures still exists a series of problems. A considerable number of implants that fix severe fractures are removed by a second surgery after the tissue is fully healed, which increases the patient's pain and risk of infection [2]. Bone cement has the characteristics of low biological cost, easy operation, sufficient strength and in-situ formability, and is a widely used bone substitute in fracture. However, a large amount of heat is usually released during the preparation of bone cement, which may lead to thermal necrosis of surrounding bone tissue [3]. The above problems have shifted the attention of medical researchers to bone adhesives that have the advantages of fast bonding, bonding small pieces of a broken bone, and no removal after bone healing. The idea of being able to glue bone fragments with a biocompatible adhesive remains highly attractive to orthopedic surgeons [4].
Developments in bone adhesives can roughly be classified into two groups: synthetic and biologically derived/inspired. Polymethyl methacrylate (PMMA) bone adhesive is the most classic synthetic bone adhesive. PMMA bone adhesives are commonly used in bone surgery for the fixation of implants such as hip and knee arthroplasty. However, their intrinsic adhesion to bone is very weak or even no adhesion (adhesion must be enhanced by various pretreatments or chemical modifications).Their lack of chemical interactions, significant heat generation and contraction, non-biodegradability, and toxicity of methyl methacrylate monomers are the drawbacks of this bone adhesive [5]. Other synthetic bone adhesives include polyurethane, lactic acid-methacrylate-based systems, glass ionomer adhesives, etc. These systems tend to have higher adhesion strength, but they usually exhibit relatively poor biocompatibility, and most of them are not biodegradable within the time range required by the clinic. Biologically derived/inspired bone adhesives refer to natural organisms, such as mussels, oysters, frogs and marine worms, which produce adhesion proteins and fix themselves on the matrix to make them still adhere underwater. Zhang et al. synthesized a biodegradable mussel-inspired hyperbranched poly (b-amino ester) (polydopamine-co-acrylate, PDA) and hydroxyapatite (HAp) enhanced adhesive [6]. Although the monocatechol modified adhesive has appropriate wet adhesion strength and high mechanical properties, its osteoconductive and osteogenic properties are not enough to support clinical applications.
In this content, Magnesium phosphate bone adhesive has been evaluated for its high initial strength and controllable degradation rate [7]. Although Magnesium phosphate bone adhesive has many merits, its setting time is so short and compressive and adhesive strength are poor that makes it difficult to use in practice [8]. Therefore, the clinical properties of MPC might be further be improved by the addition of bioactive materials. Dolomite is a sedimentary rock containing calcium and magnesium carbonates and is an important material for various industries such as the pharmaceutical industry, inorganic binders and concrete [9]. Calcined dolomite (CD) is used to transform this mineral into magnesium and calcium oxides to offer Ca2+ and Mg2+ ions that physiologically involved in bone metabolism [10]. Montmorillonite (MMT) is a layered silicate [(Na,Ca)0.33(Al,Mg)2Si4O10(OH)2·nH2O] with high specific surface area (up to 600 m2/g) and aspect ratio, which is already approved by the FDA as an additive in various medicinal products [11, 12]. Phytic acid is a natural organic molecule extracted from edible beans. Phytic acid is often used as a metal corrosion inhibitor because of its strong chelating ability to various metal ions due to its phosphate group. The biocompatibility of phytic acid has been verified [13, 14]. The combination of magnesium phosphate with phytic acid has also been studied as a bone binder [15]. Gelatin is a natural polymer product produced by the hydrolysis of collagen. It consists of peptides and proteins rich in amine and carboxylic acid groups. Its degradability, biocompatibility, cheapness, and high availability make it one of the most popular choices for tissue adhesives and it has been recognized as a GRAS (generally considered safe) material [16].
In this study, a bone adhesive has been made of bone glue phytic acid and calcined dolomite containing calcium and magnesium ions. Based on strong chelation compatibility between phytic acid and calcium and magnesium ions, it is expected that the prepared adhesive would have strong compressive and tensile performance. Phytic acid and gelatin can be cross-linked based on ionic bond. By introducing MMT and CD into the magnesium phosphate-based adhesive, the CD can improve the adhesive strength of the bone adhesive, while the rigid structure of MMT can improve the compressive strength of the bone adhesive after curing. MMT can also promote biomineralization and help the formation of bone at defects. The optimized mineral bone adhesive has strong adhesion to broken bone, and its biocompatibility and biodegradation have also been verified. Accounting all these facts, it can be assumed that crosslinked phytic acid-gelatin associated with magnesium phosphate, calcined dolomite and montmorillonite would be a promising healing material for repairing fractured bones.
Materials and methods
Materials
Natural dolomite and MMT were used from Qingyang, Anhui Province. Natural dolomite is calcined in a muffle furnace for 4 h at 900 °C. The MMT is purified by centrifugation and deposition. Gelatin and phytic acid were purchased from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China), and Macklin reagent, respectively. Magnesium phosphate pentahydrate was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Magnesium phosphate is obtained by calcination of magnesium phosphate trihydrate at 400 °C for 4 h. Hela cells were a gift from a friend in biology at school. Trypsin (0.25%) and CCK-8 kit were purchased from Shanghai Titan Technology Co., LTD., and the complete culture medium was purchased from Hangzhou Nuoyang Biotechnology Co., LTD.
Preparation of magnesium phosphate-based bone adhesive
Magnesium phosphate-based bone adhesive consists of a powder phase and a liquid phase. The powder phase was homogeneously mixed with calcined magnesium phosphate pentahydrate and CD or MMT. Then powder phase was milled by the help of an agate mortar (Sinopharm Chemical Reagent Co., Ltd). The liquid phase was prepared from deionized phytic acid (PA) and gelatin (Gel). By adding deionized water and different concentrations of gelatin into 25% phytic acid solution, the liquid phase with gelatin concentration of 5 wt%, 7 wt%, 9 wt%, 11 wt% and 13 wt% was obtained, denoted as MP-PA-Gel-X-CD-8 (X was gelatin concentration and dolomite addition amount was 8 wt%). By adding different amounts of dolomite to magnesium phosphate, the solid component with different amounts of dolomite content was denoted as MP-PA-Gel-9-CD-Y (Y is gelatin concentration, gelatin concentration was 9 wt%). The ratio of powder to liquid was 1.25 g/mL. Finally, according to the amount of montmorillonite added, the bone adhesive was denoted as MP-PA-Gel-9-CD-8-MMT-Z (Z is the amount of montmorillonite added).
Performance analysis and characterization of bone adhesive
Compressive strength and porosity
The compressive strength was measured using a Digital Push & Pull Tester (SHAHE). The mixtures were stirred for 2 min to obtain homogenous pastes and then cast into the organic glass mold, naturally cured without adding external force. Bone adhesive was de-molded after reaching the final setting, then placed in a centrifugal tube cured in a shaking water bath (SHA-B, Changzhou) at a constant temperature of 37 °C, 100% humidity, and 120 r/min for 2 days. The setting reaction was stopped by immersing the cement in anhydrous ethanol, then dried and polished uniformly on both sides to measure. Three measurements were performed for each group, and the outcomes were recorded as means ± standard deviation (means ± SD).
The porosity of the prepared cement was determined after being cured for 2 days using the immersion method. Briefly, the samples were immersed in absolute ethanol until it was saturated. The cement was weighed before and after the immersion in alcohol named M1 and M2 respectively. Then the suspended weight of the sample soaked with ethanol was named M3. The porosity (P) was calculated using the formula:
All samples were triplicated in the experiment.
Adhesive strength
Grind the cattle bones into a cylindrical shape with a diameter of 0.7 cm and a length of 1 cm. The configured bone adhesive is applied to the surface of the bovine bone to join the two pieces together. The final solidification of the bone adhesive occurred 1 min after the internal stirring began. Test the adhesive strength was noted after 1 h, 24 h and 168 h using Digital Push & Pull Tester (SHAHE). Three measurements were performed for each group, and the results were recorded as means ± standard deviation (means ± SD).
Characterization
The MP-PA-Gel-X-CD-Y-MMT-Z samples were cured for 8 h at 37 °C and ground to powders. X-ray powder diffraction (XRD) was detected by Empyrean-type XRD produced by PNAlytical company in the Netherlands. The test was performed using Ni filter. The sample was excited by Ka-ray (λ = 0.1541 nm) generated by Cu target to generate diffraction. The working voltage was 40 kV, the working current was 40 mA, the step size was 0.0262, and the scanning speed was 0.02°/s. The diffraction angle range of magnesium phosphate/CD bone adhesive system was selected to be 10–80°. The XRD characterization results of the samples were analyzed by MDI Jade.
Fourier transform infrared spectroscopy (FT-IR) The Nicolet 6700 FT-IR produced by Thermonic Co., Ltd.was used to test the samples prepared by potassium bromide tableting at room temperature under infrared lamp irradiation. The measured infrared wavelength range was 4000 to 400 cm−1. In order to analyze the effect of gelatin concentration and calcined dolomite addition on the molecular group of bone adhesive.
The morphology of samples was observed through a field emission scanning electron microscope (FE-SEM, HITACHI Regulus 8100, Japan) at an acceleration voltage of 15 kV. The related elemental distribution was analyzed with energy-dispersive X-ray spectroscopy (EDS, Oxford Ultim Max 65, Britain). After setting for 2 days, samples were removed from the constant-temperature water bath and soaked in alcohol for 8 h to prevent hydration, then were dried and vacuum coated with gold to observe.
In vitro studies
Degradation experiment
After hardening for 48 h, the initial weights of the adhesive were recorded as W0. Then, the adhesives were soaked into Tris–HCl solution (pH 7.4) at a ratio of 20 mL g−1 (solution volume/adhesive weight) and constantly shaken in a water bath at 37 °C for 28 d. At the indicated time points (1, 3, 7, 14, 21, 28, 35, 42 and 56 d), the adhesives were removed from the solution, rinsed with deionized water, and dried at 37 °C for 8 h. The new weights of the adhesives were recorded as W1. The weight loss ratio (T) was calculated using equation:
Cell culture
Cells were cultured and expanded under 37 °C, a relative humidified atmosphere of 95% air and 5% CO2. The medium was ordinarily refreshed every 2 days. When the cells reached an appropriate density according to each experimental scale, they have subcultured with 0.25% (w/v) trypsin-ethylene diamine tetra-acetic acid (EDTA) solution (Gibco). For cell vitality, the cells were planted in a basic medium and replaced with extract solutions when the density was perfect in the next day.
Cell proliferation assay
In vitro cytocompatibility of the materials was evaluated by the cell counting kit-8 (CCK-8) assays. The bone adhesive obtained by air-drying the composite solution in Petri dishes (growth area 19.5 cm2) was sterilized by placing the Petri dishes with films under ultraviolet light for 1.5 h. All samples were placed in 96-well plates and pre-wet with culture medium prior to cell seeding. Then, cells were seeded on the surfaces of all samples at a density of 1 × 104, and the plates were incubated for 24 h. The culture medium was refreshed every day. At the indicated time points, 10 µL CCK-8 solution was added to each well and incubated for 2 h at 37 °C. Finally, 100 µL culture medium was transferred to 96-well plates and the optical density value measured using a microplate reader at 450 nm.
Results and discussion
Physical and chemical properties of magnesium phosphate-based bone adhesive
By mixing the bone adhesive material in vitro and moving it to the section of the polished bovine femur, the adhesion strength of the magnesium phosphate bone adhesive material to the bovine femur after adding gelatin and calcined dolomite were investigated (Fig. 1). The adhesive strength of bone adhesive materials increased with the increase of placement time, and the adhesive strength reached the maximum at 168 h (2.02 MPa). This might be because the action sites of metal ions between bone adhesive materials and bone sections were increased with time, and the evaporation of water in bone adhesive materials was conducive to the curing and bonding of bone adhesive materials in bone sections. With the increase of gelatin concentration, the adhesive strength of bone adhesive materials increased first and then decreased, which might be because the crosslinking effect between gelatin and phytic acid became stronger and stronger with the increase of gelatin concentration. Then with the increase of calcined dolomite, more and more calcium and magnesium sources were provided by dolomite, which increases the chelation between phytic acid and Ca2+, Mg2+.
The cohesion strength of magnesium phosphate bone cement is higher than 0.92 MPa, but the bonding strength between magnesium phosphate bone cement and bone tissue is 0.1–1.0 MPa [17], mainly because the bonding strength between magnesium phosphate bone cement and bone tissue is not high. The bonding process is a complex process involving physics and chemistry, mainly involving the interaction between the adhesive and the bonded object, such as wetting and adhesion, chemical bonding and interdiffusion of interface elements, etc. Magnesium phosphate bone cement materials interact with bone tissue primarily through mechanical occlusion, and the existence of chemical bonds is also controversial. Through the weak acid environment provided by phytic acid, the calcined dolomite providing Ca2+ and Mg2+ was added to the magnesium phosphate system, that is, the additives similar to the groups such as PO43−, Ca2+ and OH− in the bone tissue were added to the magnesium phosphate system, so as to improve the adhesive strength between the bone adhesive and the bone tissue [17].
Bone adhesive need to bear certain forces in the process of treatment. Figure 2A reveals the influence of different concentrations of gelatin and the amount of calcined dolomite on the compressive strength of bone adhesive. With the increase of gelatin concentration, the compressive strength of bone adhesive showed a trend of first increasing and then decreasing. When the gelatin concentration was 9 wt%, the compressive strength touched the maxima of 6.66 MPa. At the same time, the amount of dolomite also has an impact on the compressive strength. When the amount of dolomite was increased to 8 wt%, the compressive strength reached the maxima. Gelatin could form an ionic bond-based crosslinking with phytic acid, which became more pronounced with increasing gelatin concentration, consequently, the overall compressive strength of bone adhesive was increased. Keeping the gelatin concentration at constant, the effect of calcined dolomite weight percentage on compressive strength was studied. When the amount of dolomite added is small, phytic acid can only chelate with a small amount of Ca2+ and Mg2+ released by CaO and MgO, resulting in low compressive strength of bone adhesive. Likewise, when the amount of dolomite is high, more Ca2+ and Mg2+ will chelate with phytic acid, thus improving the compressive strength of the corresponding bone adhesive. However, with the further increase of calcined dolomite content, the compressive strength of the material decreases.
The compressive strength of bone adhesive has a certain relationship with the porosity of the material. With the increase of porosity, the compressive strength of the material will decrease accordingly [18]. Figure 2B shows the effects of gelatin concentration and calcined dolomite addition on the porosity of bone adhesive. The results showed that the effect of porosity on the properties of bone adhesive corresponded to the effect of compressive strength as shown in Fig. 2A. Compressive strength and porosity are invertedly correlated, which is consistent with literature reports [19]. Bone adhesive with lower gelatin concentration and higher dolomite addition would cause incomplete reaction and looser structure, resulting in higher porosity and lower compressive strength. When the concentration of gelatin is low, the amount of calcined dolomite has an obvious effect on the porosity of the material, but the overall porosity is high, about 20.5%. When the concentration of gelatin was 9 wt% and the amount of dolomite was 8 wt%, the porosity of bone adhesive was the lowest, which was about 14.17%. However, with the increase of gelatin concentration, the reaction between gelatin and solid powder is more complete, so that the structure of the bone adhesive is closer, the porosity is correspondingly lower, and the compressive strength is improved to a certain extent. With the further increase of gelatin concentration, the porosity of the material increases.
Interaction of magnesium phosphate-based bone adhesive
Figure 3 represents the plausible interactions of bone adhesive consist of crosslinked phytic acid—gelatin associated with Ca2+, Mg2+, and Si4+ generated from calcined dolomite, magnesium phosphate and montmorillonite respectively. Phytic acid is strongly acidic and has strong chelating ability. It can produce insoluble compounds with Ca2+, Mg2+, Fe3+, Zn2+ and other metal ions, as well as form complexes with proteins. Human bones contain Ca2+ and Mg2+, so phytic acid chelates with Ca2+ and Mg2+ in bones. The calcined dolomite is composed of magnesium oxide and calcium oxide, which will generate magnesium hydroxide and calcium hydroxide after they are dissolved in water, and then provide Ca2+ and Mg2+ to the system. Phytic acid can just combine with these Ca2+ and Mg2+ to chelate, which may further enhance the mechanical properties of bone adhesive [15]. Phytic acid is also thought to be a rich, non-toxic and naturally available crosslinker. It can be used as an alternative to expensive natural crosslinkers. When gelatin is dissolved in water, the large molecular chains of proteins stretch out and phytic acid, as a small molecular additive, can enter the molecules of gelatin. Phytic acid and gelatin mainly interact with two polar groups, amino (–NH2) and hydroxyl (–OH), to form a cross-linking reaction based on ionic bond [20, 21]. In addition, studies have found that gelatin may also have coordination with Ca2+ and Mg2+, and there are –C=O and –NH2 groups carrying lone pairs of electron on the amide bond of gelatin molecules. Both of them have the potential to provide unpaired electrons to Ca2+ and Mg2+, thus forming coordination bonds [22]. The release of silicon ions in montmorillonite can also promote the regeneration of bone tissue. Si2+ stimulation of angiogenesis is an important part of bone regeneration, the newly formed blood vessels provide sustainable nutrients and oxygen to bone fragments, support the bone repair process, and the vascular system enables osteoblast differentiation to produce solid bone formation.
Characterization of magnesium phosphate-based bone adhesive
SEM analysis
Figure 4 shows SEM images of magnesium phosphate-bone adhesive prepared by varying gelatin concentrations (8 wt% calcined dolomite) (Fig. 4A) and amount of calcined dolomite (9 wt% gelatin) (Fig. 4B) at magnification of 20.0 and 50.0 k. It can be seen that with the increase of gelatin concentration, the structure of bone adhesive gradually becomes firmer first and then looser. This may be because with the increase of gelatin concentration, solid particles bond through the ionic bond crosslinking of gelatin and phytic acid, making solid particles start to connect together, the porosity of materials decreases, and the surface of bone adhesive becomes smoother. Moreover, the size of the formed block material has increased significantly, and the solid particles are connected to each other into pieces, which made the adhesion more compact, and thus improved the adhesion strength and compressive strength of bone adhesive. However, with the further increase of gelatin concentration, a large number of solid particles condense and accumulate together, creating a large gap between each other. This may be because the gelatin concentration is too high, which makes the reaction between the solid phase and the liquid phase too fast. When the two contact with each other, the bone adhesive immediately solidifies. In addition, the reaction rate is too fast, so that some of the solid components are not in contact with the liquid component and are coated by the solid particles, which leads to the agglomeration and increase the size of particles.
SEM images of bone adhesive formed by A different gelatin concentrations (calcined dolomite(CD) content: 8 wt%): a^ & a*: 5 wt%, b^ & b*: 7 wt%, c^ & c*: 9 wt%, d^ & d*: 11 wt%, e^ & e*: 13 wt%, B (A) different CD content (gelatin concentration: 9 wt%): a^ & a*: 5 wt%, b^ & b*:8 wt%, c^ & c*: 11 wt%, d^ & d*: 14 wt%, e^ & e*: 17 wt%; [^ &* signify the resonance 20.0 and 50.0 k respectively]
Similarly, when different amounts of dolomite are added, the structure of bone adhesive is also gradually compact and then loose. This may be because when the amount of dolomite is small, dolomite can provide less calcium and magnesium sources, but more Mg2+ in free form, so that the chelation reaction rate of phytic acid and metal ions is too fast, so that the solid and liquid components react quickly. The rapid condensation of solid components results in loose connections between particles and obvious boundaries. With the increase of dolomite addition, it can be seen that the size of the formed particles increases significantly, and the particles are connected into a complete block solid with a smooth surface and a dense structure, indicating that phytic acid reacts completely with Mg2+ and Ca2+ provided by calcined dolomite.
However, with the further increase of dolomite added, it can be seen that solid particles of different sizes are formed on the surface of the bone adhesive, and there is a large gap between the particles, which may be due to the excessive content of MgO and CaO provided by calcined dolomite. On the one hand, the solid and liquid components react incomplete, thus forming solid particles of different sizes; On the other hand, the solid phase component reacts too quickly with the liquid phase component, resulting in part of the solid phase group divided into components that are not in contact with the liquid phase and are coated by the solid particles formed by the reaction. Excessive calcined dolomite hinders the formation of interlocking crystals, resulting in the dispersion of solid particles and loose structure, so its mechanical properties are obtained so poor. A comprehensive analysis of the microscopic morphology of bone adhesive shows that the structure of MP-PA-Gel-9-CD-8 bone adhesive is the densest, which is consistent with the result that it has the best mechanical properties.
XRD analysis
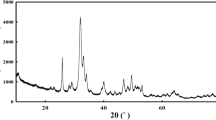
The crystal types of bone adhesive with different concentrations of gelatin were characterized. The characteristic diffraction peaks at 2θ = 42.91° and 2θ = 62.29° belong to the (200) crystal plane and (220) crystal plane of MgO, respectively (Fig. 5). With the increase of gelatin concentration, the diffraction peaks gradually become sharp, and the intensity of MgO diffraction peaks is the least obvious when gelatin concentration is 9 wt%. This indicates that phytic acid and gelatin have the strongest effect on Ca2+ and Mg2+ provided by calcined dolomite, so the corresponding MgO diffraction peak intensity is the least obvious. 2θ = 37.93° is the (101) crystal plane of Mg(OH)2 [23]. The production of magnesium hydroxide is mainly due to the reaction of magnesium oxide with water. However, the XRD pattern only shows the diffraction peak of MgO, but does not show the diffraction peak of CaO. This may be because compared with calcium oxide, magnesium oxide is more difficult to dissolve in water. Therefore, in bone adhesive, CaO reacts with water to generate calcium hydroxide and then releases Ca2+ to interact with phytic acid and gelatin, while only a part of MgO dissolves in water to generate magnesium hydroxide. Mg2+ is then released to interact with phytic acid and gelatin.
In addition, the characteristic diffraction peaks at 2θ = 13.5°, 2θ = 18.8° and 2θ = 29.34° are attributed to the (111) crystal plane, (021) crystal plane and (113) crystal plane of MgHPO4·3H2O [23]. Magnesite is produced when magnesium phosphate interacts with phosphoric acid or acid phosphate, such as (Ca(H2PO4)2). Phytic acid can chelate calcium and magnesium sources provided by calcined dolomite, and there is also Ca2+ and Mg2+ in bone, so phytic acid can chelate Ca2+ and Mg2+ in bone. During these chelation, phosphoric acid (H3PO4) is further combined with magnesium phosphate to form the hydrated form of MgHPO4·3H2O [24]. It can be seen that the increase of gelatin concentration has little effect on the binding of phytic acid and magnesium phosphate, so the diffraction peak intensity of phosphor magnesite is basically unchanged. In addition, the diffraction peak of the new product is not detected in the XRD pattern, which may be because the crystallinity of the substance is too low, so it is not detected. It is also possible that the amount of material produced is too low, so the peak intensity is not significant.
XRD analysis was conducted on bone adhesive with different amounts of calcined dolomite, and the results were shown in the figure above (Fig. 6). The characteristic diffraction peaks at 2θ = 42.91° and 2θ = 62.29° are (200) crystal plane and (220) crystal plane of MgO, respectively. When the addition of calcined dolomite is 8 wt%, the diffraction peak intensity of magnesium oxide is the least obvious, indicating that the phytic acid and gelatin react fully with the calcium and magnesium sources provided by calcined dolomite. This is consistent with the above infrared analysis results. In addition, the characteristic diffraction peak of 2θ = 39.41° is the (101) crystal plane of Mg(OH)2. As can be seen from the figure above, the diffraction peak of Mg(OH)2 gradually becomes sharp with the increase of the addition of calcined dolomite, indicating that the reaction is close to saturation at this time, and the calcined dolomite provides too much calcium and magnesium sources, leading to incomplete reaction. When the addition amount of calcined dolomite is 8 wt% (that is, MP-PA-Gel-9-CD-8), the diffraction peak of magnesium hydroxide does not appear, which may be because phytic acid and gelatin completely react with Ca2+ and Mg2+, which is the least obvious consistent with the diffraction peak of MgO mentioned above.
In addition, there is only Mg(OH)2 diffraction peak in the XRD pattern, while Ca(OH)2 diffraction peak is very weak or even absent. The characteristic diffraction peaks of 2θ = 13.62° and 2θ = 29.48° are attributed to the (111) crystal plane and (113) crystal plane of the magnesite, respectively. It can be seen that the overall diffraction peak of the magnesite does not change much, which may be because the reaction between phytic acid and magnesium phosphate has reached saturation, so the addition of calcined dolomite has little influence on the magnesite. There is still no new crystal surface in the XRD pattern, which may be related to the low content of new products or low crystallinity.
FT-IR analysis
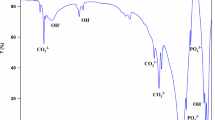
FT-IR is widely used in the study of functional groups of samples. The above figure shows the FT-IR spectra of MP-PA-Gel-X-CD-8 bone adhesive after adding different concentrations of gelatin (Fig. 7). The five spectra show strong similarity in the FT-IR band of main characteristics. The peaks at 595 and 1096 cm−1 are attributed to the vibration peak of PO43− [25], among which 1096 cm−1 is the infrared vibration absorption peak of phosphate (PO42−) in phytic acid [26]. About 798 cm−1 is the C–H out-of-plane deformation vibration of substituted aromatic ring in phytic acid [27], so the presence of phytic acid can be preliminarily determined. The peak around 1384 cm−1 is the characteristic absorption peak of gelatin [26], and 1550 cm−1 is the N–H bending vibration in the gelatin amide II band [28]. It can be seen from the figure that the stretching vibration peak is enhanced with the increase of gelatin concentration, which proves that gelatin is one of the raw materials for preparing bone adhesive. The O–H vibration peaks at around 1636 and 3455 cm−1 [3] may be caused by the incomplete drying of the bone adhesive, or by the water absorption of the material during the experiment, or by the binding water of the new formation.
The vibration peak of O–H at about 1636 cm−1 is gradually enhanced with the increase of gelatin concentration. Therefore, it can be excluded that the bone adhesive was not completely dried and the material is exposed to air and absorbed water during the experiment. Instead, the hydroxyl group in the bone adhesive increased after the addition of gelatin. The increase in the width of the hydroxyl absorption peak at 3455 cm−1 may also be related to gelatin, where the increase in gelatin concentration introduced a large number of hydroxyl groups. The peak at 3695 cm−1 may be due to the chelation of phytic acid with metal calcium and magnesium ions [29]. As can be seen from the figure, although the absorption and vibration peak at 3695 cm−1 is not obvious, it can still be seen that the chelation of bone adhesive is stronger when the gelatin concentration is 9 wt%.
The bone adhesive with different amounts of calcined dolomite was characterized by infrared (Fig. 8). 570 cm−1 corresponds to the vibration peak of ν4 (P–O) of the free state PO43−, and 778 cm−1 is the deformation vibration outside the C–H plane of the substituted aromatic ring in phytic acid. The vibration peak of 1083 cm−1 is HPO42− the stronger ν4 (P–O) stretching vibration peak. 1382 and 1544 cm−1 are the characteristic absorption peaks of gelatin, and 1643 and 3440 cm−1 belong to the vibration peaks of O–H. The peak at 3693 cm−1 is the absorption peak of phytic acid chelating with calcium and magnesium ions in calcined dolomite. As can be seen from the figure, when the amount of calcined dolomite is 8 wt%, the intensity of the absorption peak is strong, indicating that phytic acid chelating with calcium and magnesium sources provided by calcined dolomite is the strongest at this time, and then the peak intensity gradually decreases with the increase of the amount of calcined dolomite.
In addition, the cross-linking reaction between phytic acid and gelatin in MP-PA-Gel-9-CD-8 bone adhesive was also explored. FT-IR spectra of phytic acid, gelatin and MP-PA-Gel-9-CD-8 bone adhesive were shown in Fig. 9. The effective crosslinking between phytic acid and gelatin is the result of ionic bond crosslinking between phytic acid anion O=P–OH group and gelatin cationic -NH2 group [30]. As shown in Fig. 9, phytic acid has an absorption peak at 3380 cm−1, corresponding to the characteristic O–H band of phytic acid [21]. The wide and strong absorption peak at 3278 cm−1 in curve b can be attributed to the N–H stretching vibration and O–H stretching vibration of gelatin [31], and the peak at 3455 cm−1 is the vibration peak of O–H (curve c). After the cross-linking reaction between phytic acid and gelatin, the tensile vibration peaks of O–H and N–H moved significantly to 3455 cm−1, which also indicated that the gelatin and phytic acid in the prepared magnesium phosphate- based bone adhesive had a cross-linking reaction based on ionic bond [32].
Studies on biological activity in vitro
Solution absorption performance
Studies have shown that montmorillonite can improve the biocompatibility of bone adhesive. Therefore, this study added an appropriate amount of montmorillonite on the basis of magnesium phosphate-based bone adhesive to explore the effects of the addition of montmorillonite on the biological activity of bone adhesive. The trend of absorbent capacity of MP-PA-Gel-9-CD-8-MMT-1.4 bone adhesive in deionized water and Tris–HCl solution is basically the same (Fig. 10), showing first increase and then basically maintain the same situation. The water absorption rate of bone adhesive in deionized water was 18.3% after 2 h and 25.4% after 12 h. The increase trend was not obvious after that, especially after 24 h, the water absorption rate basically remained at 26%, indicating that the bone adhesive had basically reached saturation state at this time. The difference is that the absorbance of the bone adhesive in Tris–HCl solution is less than that in deionized water. As can be seen from the figure, in the Tris–HCl solution, the water absorption rate of the bone adhesive was 9.8% at 2 h, 18.7% at 12 h, and basically remained unchanged after that. It can be found through experiments that MP-PA-Gel-9-CD-8-MMT-1.4 bone adhesive has a certain absorptive capacity in both deionized water and Tris–HCl solution, which indicates that this bone adhesive has a certain pore structure inside and can absorb certain blood and tissue fluid in vivo. Thus promoting the absorption of nutrients is needed for bone repair.
In vitro degradation performance
Biodegradability is one of the important properties of bone adhesive. The degradability also has a direct impact on the application prospect of the materials. This section explores the degradation performance of three materials, MP-PA, MP-PA-Gel-9-CD-8 and MP-PA-Gel-9-CD-8-MMT-1.4, after soaking in Tris–HCl buffer solution for different time (Fig. 11). As can be seen from the figure, for all materials, the weight loss rate of bone adhesive gradually increases with the extension of soaking time. Relatively speaking, the weight loss rate of MP-PA bone adhesive is relatively low, and it is always the lowest at all test time points. When calcined dolomite and montmorillonite are added, the degradation rate of bone adhesive is significantly accelerated. For MP-PA-Gel-9-CD-8-MMT-1.4, MP-PA-Gel-9-CD-8 and MP-PA, the degradation rate increased rapidly during 0–28 days, and the weight loss rate reached 40.5%, 33.2% and 30.1%, respectively. The degradation rate decreased after 28 days.
There are two main processes of bone adhesive in Tris–HCl buffer solution, the first is degradation of bone adhesive in Tris–HCl buffer solution, and the second is the reverse deposition of ions in Tris–HCl buffer solution on the surface of bone adhesive [33]. Therefore, 28 days ago was the degradation process of bone adhesive in Tris–HCl buffer solution, and 28 days later, ions in Tris–HCl buffer solution began to reverse deposit on the surface of bone adhesive. After 56 days of immersion, the weight loss rate of MP-PA-Gel-9-CD-8 and MP-PA-Gel-9-CD-8-MMT-1.4 reached 36.5 and 45.6%, respectively, which was higher than that of MP-PA (35.4%). In conclusion, the in vitro degradation experiments of these three bone adhesives show that the degradation rate of bone adhesive can be controlled by adding certain minerals, and it also indicates that the prepared magnesium phosphate/CD component and magnesium phosphate/CD/montmorillonite three group bone adhesive have better in vitro degradation performance.
In vitro osteogenic activity
The morphologies of bone adhesive soaked in SBF solution for 14 days and 21 days were analyzed (Fig. 12a, b). As can be seen from the figure, after 14 days of soaking in SBF buffer solution, the surface of MP-PA-Gel-9-CD-8-MMT-1.4 bone adhesive began to deposit rod-like and globular particles. Under low magnification (10.0 k), small and loose particles could be observed. Even the particles deposited at high multiples are relatively small, possibly because the deposition time is too short.
After the bone adhesive was immersed in the SBF buffer solution for 21 days (Fig. 12b), the particle size deposited on the surface of the bone adhesive began to become larger in the low magnification (10.0 k) image, with a large number of massive particles piled together. At high magnification (20.0 and 50.0 k), it can be seen more directly that a large number of small size particles began to deposit together and gradually joined into sheets, forming a relatively thick sedimentary layer on the surface of the bone adhesive, indicating that the above rod and spherical particles gradually mature.
In order to further investigate the composition of spherical and massive particles deposited on the surface of MP-PA-Gel-9-CD-8-MMT-1.4 bone adhesive, EDS analysis was performed on the surface of the bone adhesive mineralized for 14 and 21 days, respectively. When applied to bone, the synthetic biomaterial is required to form hydroxyapatite on the bone surface, which is beneficial for bone repair and growth. This bone-like apatite can be reproduced in SBF simulated body fluids. Figure 12c, d shows EDS spectra of MP-PA-Gel-9-CD-8-MMT-1.4 bone adhesive after 14 and 21 days of immersion in SBF buffer. By comparing the two figures, it can be seen that after 21 days of mineralization, the contents of magnesium and phosphorus significantly decreased, while the contents of carbon increased, and the Ca/P of the bone adhesive after 21 days of soaking in SBF buffer was 1.89, which was close to the Ca/P of bone apatite. Therefore, combined with the results of FT-IR, SEM and EDS, it was found that the prepared magnesium phosphate-based bone adhesive can induce the formation of bone-like apatite, so it has good osteogenic activity in vitro.
In order to further analyze the formation of new substances, the prepared soaked in SBF solution for 21 days and not soaked bone adhesive were compared with infrared analysis (Fig. 13). As mentioned above, 566 cm−1 is the vibration peak of PO43−, 1042 cm−1 is the infrared vibration absorption peak of phosphate (PO42−) in phytic acid, 1408 cm−1 is attributed to the stretching vibration of CO32−, 1654 and 3269 cm−1 are respectively the vibration peak of O–H. The peak at 2971 cm−1 is the C–H stretching vibration of gelatin. As can be seen from the figure, the infrared vibration absorption peak of MP-PA-Gel-9-CD-8-MMT-1.4 (Fig. 13b) bone adhesive at 566 cm−1 after 21 days of immersion in SBF solution shifted to the left compared with that of unsoaked bone adhesive (Fig. 13a). The P–O stretching vibration peak of PO43− was found at 566 cm−1, indicating that new sediments were generated on the surface of the bone adhesive after 21 days of mineralization.
In addition, MP-PA-Gel-9-CD-8-MMT-1.4 bone adhesive is soaked in SBF solution for 21 days, and the characteristic peak of carbonate (CO32−) appeared near 1408 cm−1, while the characteristic peak did not appear here in the bone adhesive without soaking. This indicates that new substances are generated during the process of soaking the bone adhesive in SBF [34]. This may be due to the formation of calcium carbonate or acidic hydroxyapatite during the soaking process. In summary, after 21 days of soaking in SBF solution, phosphoric acid was shifted and the absorption peak of carbonate was also found, which indicated that new substances might be formed to a certain extent, and the products contained phosphate and carbonate, indicating that new substances might be formed.
Evaluation of biocompatibility in vitro
In vitro biocompatibility test is an important aspect of biomaterials. The ideal bone adhesive should not be toxic or antagonistic. It can be evaluated by the toxicity of cells in vitro, which is one of the basic properties of bone adhesive. The results of in vitro biocompatibility by indirect method are shown in Fig. 14. The cytotoxicity of MP-PA-Gel-9-CD-8, MP-PA-Gel-9-MMT-1.4, MP-PA-Gel-9-Cd-8-MMT-1.4 suspension of bone adhesive was studied after co-culture with Hela cells for 24 h. As can be seen from Fig. 14, when the concentration of the three bone adhesive added is low (0.1 and 0.5 mg/mL), the survival rate of the cells is high, all of which are above 90%. This may be because the concentration of the added samples is low, which does not have a great influence on the growth of cells. When the concentration of the sample was further increased to 1 mg/mL, the survival rate of the cells decreased to about 84% on average. The survival rate of the cells added with MP-PA-Gel-9-CD-8 bone adhesive was higher than that of the other two bone adhesives. When the concentration of bone adhesive continued to increase to 2 and 4 mg/mL, the survival rate of the cells added with the three bone adhesives showed an increasing trend. When the concentration was 4 mg/mL, the survival rate of the cells added with montmorillonite reached 92%. The cell survival rate of MP-PA-Gel-9-CD-8 and MP-PA-Gel-9-MMT-1.4 bone adhesive was higher than that of MP-Pa-Gel-9-MMT-1.4 bone adhesive. According to ISO-10993-5, a material is considered non-cytotoxic if the percentage of living cells is greater than 70% of the untreated control [35]. Therefore, the magnesium phosphate- based bone adhesive prepared in the experiment has enough biocompatibility.
Conclusion
In this study, magnesium phosphate-based bone adhesive has been prepared by means of solid–liquid blending crosslinking. Phytic acid could chelate calcium ions and magnesium ions in broken bones and calcined dolomite, and phytic acid could also form ionic-based cross-linking with gelatin molecules, thus making the prepared magnesium phosphate-based bone adhesive have good bonding and compression properties. It is found that the mechanical properties of bone adhesive could be improved by adding a certain concentration of gelatin on the basis of phytic acid. Especially, the bonding strength, after adding gelatin, the bonding strength of bone adhesive was significantly improved. However, different amounts of calcined dolomite and gelatin concentration will affect the bonding properties and compressive properties of bone adhesive. When the addition amount of calcined dolomite is 8 wt% and gelatin concentration is 9 wt%, the bone adhesive has better performance and the structure is the densest when observed under scanning electron microscope. FT-IR and XRD also confirmed this.
The prepared bone adhesive was immersed in SBF solution for 14 and 21 days, respectively. There were rod and massive particles deposited on the surface of the bone adhesive, and the deposited particles gradually matured with the extension of soaking time. EDS analysis showed that Ca/P ratio was 1.89, indicating that the deposited particles were bone-like apatite. FT-IR results also confirmed this idea. It indicates that the bone adhesive prepared has the ability of mineralization and osteogenesis.
In vitro biological activity test also showed that the bone adhesive has good biocompatibility and has the potential to be used as medical biological bone adhesive. However, bone adhesive is ultimately intended to be used in vivo, so it is necessary to conduct in vivo biocompatibility experiments to observe whether they have clinical application potential.
Data Availability
No datasets were generated or analysed during the current study.
References
Ekegren, C.L., Edwards, E.R., De Steiger, R., Gabbe, B.J.: Incidence, costs and predictors of non-union, delayed union and mal-union following long bone fracture. Int. J. Environ. Res. Public Health 15(12), 2845 (2018). https://doi.org/10.3390/ijerph15122845
Böker, K.O., Richter, K., Jäckle, K., Taheri, S., Grunwald, I., Borcherding, K., Byern, J.V., Hartwig, A., Wildemann, B., Schilling, A.F., Lehmann, W.: Current state of bone adhesives—necessities and hurdles. Materials 12(23), 3975 (2019). https://doi.org/10.3390/ma12233975
Whitehouse, M.R., Atwal, N.S., Pabbruwe, M., Blom, A.W., Bannister, G.C.: Osteonecrosis with the use of polymethylmethacrylate cement for hip replacement: thermal-induced damage evidenced in vivo by decreased osteocyte viability. Eur. Cells Mater. 27, 50–63 (2014). https://doi.org/10.22203/ecm.v027a05
Farrar, D.F.: Bone adhesives for trauma surgery: a review of challenges and developments. Int. J. Adhes. Adhes. 33, 89–97 (2012). https://doi.org/10.1016/j.ijadhadh.2011.11.009
Zhang, H., Bre, L., Zhao, T., Newland, B., Da Costa, M., Wang, W.: A biomimetic hyperbranched poly(amino ester)-based nanocomposite as a tunable bone adhesive for sternal closure. J. Mater. Chem. B 2(26), 4067–4071 (2014). https://doi.org/10.1039/c4tb00155a
Azarpira, M.R., Vazani, K., Ayatollahi, M., Azarpira, N., Kaviani, M.: Comparison of healing intra-articular fracture of distal femur using a kirschner wire and autologous fibrin glue in an animal model. J. Pediatr. Orthop. B 26(5), 454–457 (2017). https://doi.org/10.1097/BPB.0000000000000444
Mestres, G., Ginebra, M.P.: Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 7(4), 1853–1861 (2011). https://doi.org/10.1016/j.actbio.2010.12.008
Yu, Y., Xu, C., Dai, H.: Preparation and characterization of a degradable magnesium phosphate bone cement. Regener. Biomater. 3(4), 231–237 (2016). https://doi.org/10.1093/rb/rbw024
Shi, Y., Yu, L., Gong, C., Li, W., Zhao, Y., Guo, W.: A bioactive magnesium phosphate cement incorporating chondroitin sulfate for bone regeneration. Biomed. Mater. 16(3), 035034 (2021). https://doi.org/10.1088/1748-605X/abf5c4
Fiume, E., Tulyaganov, D., Ubertalli, G., Verne, E., Baino, F.: Dolomite-foamed bioactive silicate scaffolds for bone tissue repair. Materials (Basel) 13(3), 628 (2020). https://doi.org/10.3390/ma13030628
Cui, Z.K., Kim, S., Baljon, J.J., et al.: Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 10, 3523 (2019). https://doi.org/10.1038/s41467-019-11511-3
Ruiz-Hitzky, E., Darder, M., Wicklein, B., Castro-Smirnov, F.A., Aranda, P.: Clay-based biohybrid materials for biomedical and pharmaceutical applications. Clays Clay Miner. 67, 44–58 (2019). https://doi.org/10.1007/s42860-019-0005-0
Nita, L.E., Chiriac, A.P., Ghilan, A., Rusu, A.G., Tudorachi, N., Timpu, D.: Alginate enriched with phytic acid for hydrogels preparation. Int. J. Biol. Macromol. 181, 561–571 (2021). https://doi.org/10.1016/j.ijbiomac.2021.03.164
Xiong, P., Jia, Z., Zhou, W., Yan, J., Wang, P., Yuan, W., Li, Y., Cheng, Y., Guan, Z., Zheng, Y.: Osteogenic and ph stimuli-responsive self-healing coating on biomedical mg-1ca alloy. Acta Biomater. 92, 336–350 (2019). https://doi.org/10.1016/j.actbio.2019.05.027
Meininger, S., Blum, C., Schamel, M., Barralet, J.E., Ignatius, A., Gbureck, U.: Phytic acid as alternative setting retarder enhanced biological performance of dicalcium phosphate cement in vitro. Sci. Rep. 7, 558 (2017). https://doi.org/10.1038/s41598-017-00731-6
Santoro, M., Tatara, A.M., Mikos, A.G.: Gelatin carriers for drug and cell delivery in tissue engineering. J. Control Release 190, 210–218 (2014). https://doi.org/10.1016/j.jconrel.2014.04.014
Ma, A.B.: Research progress on the interface bonding properties of magnesium-based bone adhesive and bone tissue. Adhesion 43, 13–15 (2020)
Clearfield, H.M., Mcnamara, D.K., Davis, G.D.: Adhesion and adhesives, pp. 7–8. Elsevier, Amsterdam (1965)
Lei, K., Zhu, Q., Wang, X., Xiao, H., Zheng, Z.: In vitro and in vivo characterization of a foam-like polyurethane bone adhesive for promoting bone tissue growth. ACS Biomater. Sci. Eng. 5(10), 5489–5497 (2019). https://doi.org/10.1021/acsbiomaterials.9b00918
Jin, H., Liu, X., Lin, Y.M.: Preparation, structure and properties of gelatin composite film modified by phytic acid. Chem. Ind. Eng. Pro. 40, 3847–3853 (2020). https://doi.org/10.16085/j.issn.1000-6613.2020-1525
Tashi, Z., Zare, M., Parvin, N.: Application of phytic-acid as an in-situ crosslinking agent in electrospun gelatin-based scaffolds for skin tissue engineering. Mater. Lett. 264, 127275 (2020). https://doi.org/10.1016/j.matlet.2019.127275
Cai, Q., He, Y., Huang, Z.L., Bai, Z.H., Li, X.H.: The coordination of gelatin with calcium and magnesium ions. J. Honghe Univ. 3, 10–12 (2005). https://doi.org/10.13963/j.cnki.hhuxb.2005.06.005
Cao, X., Lu, H., Liu, J., Lu, W., Guo, L., Ma, M., Zhang, B., Guo, Y.: 3d plotting in the preparation of newberyite, struvite, and brushite porous scaffolds: using magnesium oxide as a starting material. J. Mater. Sci. Mater. Med. 30, 88 (2019). https://doi.org/10.1007/s10856-019-6290-2
Han, L., Li, J., Fei, X., Wang, M., Liu, S., Zhang, X., Xue, Q.: Stabilization and strengthening of chromium(vi)-contaminated soil via magnesium ascorbyl phosphate (map) and phytase addition. J. Hazard. Mater. 448, 130860 (2023). https://doi.org/10.1016/j.jhazmat.2023.130860
Wang, D., Xu, F., Lin, M., Hu, J.: Phytic acid/graphene oxide nanocomposites modified electrode for electrochemical sensing of dopamine. Mater. Sci. Eng. C-Mater. 71, 1086–1089 (2017). https://doi.org/10.1016/j.msec.2016.11.023
Erment, M., Kahrman, F.: Ability of near infrared spectroscopy and chemometrics to measure the phytic acid content in maize flour. Spectrosc. Lett. 18, 520–527 (2021). https://doi.org/10.1080/00387010.2021.1950189
Meininger, S., Blum, C., Schamel, M., Barralet, J.E., Ignatius, A., Gbureck, U.: Phytic acid as alternative setting retarder enhanced biological performance of dicalcium phosphate cement in vitro. Sci. Rep. 7(1), 558 (2017). https://doi.org/10.1038/s41598-017-00731-6
Duconseille, A., Andueza, D., Picard, F., Sante-Lhoutellier, V., Astruc, T.: Molecular changes in gelatin aging observed by nir and fluorescence spectroscopy. Food Hydrocolloids 61, 496–503 (2016). https://doi.org/10.1016/j.foodhyd.2016.06.007
Christel, T., Christ, S., Barralet, J.E., Groll, J., Gbureck, U., Ginebra, M.P.: Chelate bonding mechanism in a novel magnesium phosphate bone cement. J. Am. Ceram. Soc. 98(3), 694–697 (2015). https://doi.org/10.1111/jace.13491
Ravichandran, R., Seitz, V., Reddy Venugopal, J., Sridhar, R., Sundarrajan, S., Mukherjee, S., Wintermantel, E., Ramakrishna, S.: Mimicking native extracellular matrix with phytic acid-crosslinked protein nanofibers for cardiac tissue engineering. Macromol. Biosci. 13(3), 366–375 (2013). https://doi.org/10.1002/mabi.201200391
Jafari, J., Emami, S.H., Samadikuchaksaraei, A., Bahar, M.A., Gorjipour, F.: Electrospun chitosan-gelatin nanofiberous scaffold: fabrication and in vitro evaluation. Biomed. Mater. Eng. 21(2), 99 (2011). https://doi.org/10.3233/BME-2011-0660
Wang, S., Xiao, Z., Ma, X., Zhao, Z., Guo, D., Chen, Y., Zhai, S., An, Q., Yang, D.: Hard template-induced internal solidification synthesis of cu nps-supported glutaraldehyde-crosslinked polyethyleneimine-modified calcium alginate beads with enhanced catalytic activity. Appl. Catal. A-Gen. 568, 105–113 (2018). https://doi.org/10.1016/j.apcata.2018.10.001
Shi, Y.B., Yu, L., Gong, C.T., Li, W., Zhao, Y.C., Guo, W.C.: A bioactive magnesium phosphate cement incorporating chondroitin sulfate for bone regeneration. Biomed. Mater. 16(3), 035034 (2021). https://doi.org/10.1088/1748-605X/abf5c4
Huang, L., Zhou, B., Wu, H.Y., Zheng, L., Zhao, J.M.: Effect of apatite formation of biphasic calcium phosphate ceramic (BCP) on osteoblastogenesis using simulated body fluid (SBF) with or without bovine serum albumin (BSA). Mater. Sci. Eng. C 70(2), 955–961 (2017). https://doi.org/10.1016/j.msec.2016.05.115
Balcioglu, S., Gurses, C., Ozcan, I., Yildiz, A., Koytepe, S., Parlakpinar, H., Vardi, N., Ates, B.: Photocrosslinkable gelatin/collagen based bioinspired polyurethane-acrylate bone adhesives with biocompatibility and biodegradability. Int. J. Biol. Macromol. 192, 1344–1356 (2021). https://doi.org/10.1016/j.ijbiomac.2021.09.043
Acknowledgements
The authors wish to acknowledge the financial support from the National Natural Science Foundation of China (41672033), the research grants from Engineering Research Center of Non-metallic Minerals of Zhejiang Province (ZD2023K01; ZD2020K07), and the projects from Qing Yang Institute for Industrial Minerals (KYY-HX-20220336; KYY-HX-20170557).
Funding
This study was funded by the financial support from the National Natural Science Foundation of China (22072136; 41672033), the research grants from Engineering Research Center of Non-metallic Minerals of Zhejiang Province (ZD2023K01; ZD2020K07), and the projects from Qing Yang Institute for Industrial Minerals (KYY-HX-20220336 KYY-HX-20170557).
Author information
Authors and Affiliations
Contributions
CHZ conceived the project and reviewed the draft; XJQ conducted the experiments, collected and analyzed the data, and wrote the original draft; MNH, XLC, JHL, TR, AK, GYW, and DH edited the paper.
Corresponding author
Ethics declarations
Conflict of interest
The author has no financial conflicts to disclose or personal relationship that could have appeared to influence the work reported in this paper.
Ethical approval
No human tissues were involved in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, MN., Qu, XJ., Chen, XL. et al. Fabrication of a novel bone adhesive (crosslinked phytic acid-gelatin coordinated with magnesium phosphate and calcined dolomite, and montmorillonite) for enhancing adhesion strength and biocompatibility. J Incl Phenom Macrocycl Chem 104, 317–334 (2024). https://doi.org/10.1007/s10847-024-01234-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-024-01234-4