Abstract
Bis-benzyl 2-(oxy) benzoate substituted axially silicon phthalocyanine was synthesized by the reaction of silicon phthalocyanine dichloride and benzyl salicylate compounds. Characterization of the compound was done by FT-IR, 1H NMR, 13C NMR, UV-visible and Mass spectrum. Photochemical and photophysical properties of new silicon phthalocyanine (SiPc) was investigated. Biological properties of SiPc was carried out by several different parameters. The highest antioxidant ability of 73.18% was obtained at 100 mg/L concentration while the lowest antioxidant activity of 38.46% was obtained at 6.25 mg/L concentration. The antimicrobial effects of SiPc were investigated against different bacteria and microfungi. The results regarding the antimicrobial activity of this compound 3 showed that E. faecalis (ATCC 29,212) was the most sensitive microorganism to the tested compounds, while C. tropicalis was the most resistant microorganism. In addition, when the antimicrobial photodynamic treatment of SiPc was examined, a better activity was observed against all microorganisms. DNA fragmentation activity and microbial cell viability of compound 3 was investigated. SiPc showed excellent DNA nuclease activity and 99.96% inhibition of cell viability at 100 mg/L. The effect of compound 3 on antibiofilm activity fabricated by S. aureus and P. aureginosa was also measured and a good biofilm inhibition values of 86.51% and 75.24% was achieved at 50 mg/, respectively. In addition, when the antidiabetic effects of the compounds were examined, it showed an antidiabetic effect of 20.14% at 400 mg/L.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phthalocyanine compounds have been recently photosensitizers for photodynamic therapy studies show that (PDT) is promising in the treatment of cancers due to its superior properties. The derivatives of these compounds its properties such as chemical and thermal stability, low dark toxicity, strong absorption in the near-infrared region have forms the researchers focus point [1,2,3]. Phthalocyanines are one of the most researched topics for semiconductors [4], optical data storage [5], liquid crystals [6], sensors [7], photovoltaic devices [8], electrochromic [9], nonlinear optics [10], fuel cell [11] applications. However, phthalocyanine derivatives have poor solubility and can form common aggregates [12–13]. Caused by aggregation in water and organic solvents quenching and inhibiting the production ability of reactive oxygen limits the applications. To eliminate these drawbacks soluble and non-aggregated conditions are established by using sulfonate, quaternary ammonium, hydroxy and carboxylate, phenol, substitutable groups in axial or peripheral positions [14,15,16]. The chemical, photophysical and physicochemical properties of these compounds can be perfected with new substituent groups. The biological properties of phthalocyanines are among the recent researches. Recently, remarkable results have been obtained in the investigation of the biological properties of phthalocyanine compounds. Phthalocyanines, which have interesting properties, are useful for different applications such as biological drug designs and antioxidant material production. They exhibit a reasonable level of microfungi and antibacterial properties, making them preferred for applications [17]. The importance of the use of environmentally friendly products and compounds has increased even more in order to maintain the balance of ecological and environmental conditions. For this reason, it is important to design those that are environmentally friendly and rich in biological properties in the synthesis of new compounds. Recently, phthalocyanine compounds have exhibited reasonable antioxidant and antibacterial properties, as well as the use of photosensitizers and sensors [17–18].
Here, bis-benzyl 2-(oxy) benzoate phthalocyaninato silicon (IV) was obtained by the reaction of benzyl salicylate and SiPcCl2. This compound was characterized by a combination of 1H-NMR, 13C-NMR, UV, LC-MS, FT-IR. Antioxidant, antimicrobial, bacteria and microfungi properties of the compound were investigated in detail in term of biological activity.
Materials and methods
General
SiPcCl2, benzyl salicylate, K2CO3 DMSO (dimethylsulfoxide), toluene DMF (dimethylformamide), chloroform, ethyl acetate, tetrahydrofuran, dichloromethane was purchased from different such as Sigma- Aldrich and Merck. The melting point was measured by a digital electrothermal meter. FT-IR measurements was made by Thermo Scientific FT-IR spectrophotometer. UV-visible measurements were made by Hitachi U-2900 Spectrophotometer. LC- Mass spectrum for mass and Agilent 400 MHz NMR spectrometer for NMR were used for characterization.
Bis -benzyl 2-(oxy) benzoate phthalocyaninato silicon(IV) (3)
Benzyl salicylate (benzyl 2-hydroxybenzoate) (1) (55 mg or 47µL, 0.24 mmol), SiPcCl2 (2) (75 mg, 0.12mmol), and K2CO3 (94 mg, 0.68 mmol) were refluxed mixed with dry toluene (10 mL) and for 29 h. After cooling the reaction mixture, the product was purified by extraction. Yield: 25 mg (21%), m.p. > 300 °C. The obtained compound is readily soluble in solvents such as dichloromethane, CHCl3, ethyl acetate, DMSO, THF, DMF. IR (ATR), ν/cm− 1: 3190,3064,3034 (Ar-H),2926,2854,1672(C = O) 1612,1585, 1485, 1463, 1382, 1298,1247,1209, 1155, 1134, 1085, 1031, 952, 912, 844, 752, 694. 1H NMR (400 MHz, DMSO-d6), (δ): 10.48, 7.81, 7.51, 7.47, 7.38, 6.97, 6.96, 6.95, 6.93, 6.90, 5.37, 3.33, 2.47. 13C NMR (400 MHz, DMSO-d6), (δ):169.68, 168.92, 160.51,136.18, 136.10, 134.77, 133.04, 130.52, 129.00, 128.70, 128.51, 123.38, 119.92, 117.87, 113.52, 109.98, 66.97, 66.94, 66.92, 40.56, 40.35, 40.14, 39.93, 39.73, 39.52, 39.31. MS (ESI), (m/z): Calculated for C60H38N8O6Si: 994.3; Found: 995.27 [M + H]+. UV–Vis (1 × 10− 5 M, THF): λmax/nm (log ε): 690 (5.30), 620 (4.62), 360 (4.92).
Antioxidant activity
The free radical scavenging abilities of compound 3 were evaluated using previously published literature with minor changes [19]. The stock solution of compound 3 was diluted in various concentrations as 6.25, 12.5, 25, 50 and 100 mg/L. A 250 µL sample and 1000 µL methanolic DPPH solution were mixed and shaken vigorously. After the solution samples were incubated in a dark place at 25 ºC for 30 min, absorption measurements were made at 517 nm. If the compound behaves like the radical scavenger, the DPPH solution decolorizes and the color is altered from dark purple to pale yellow. As a control, the assay mixture devoid of any compound was employed. The percent inhibition was calculated with the formula shown below, which is commonly used.
Antidiabetic Activity
First, test compound was incubated with alpha amylase enzyme at 37 °C for 10 min at concentrations of 100, 200 and 400 mg/L. Starch was then added to all test tubes and the test tubes were again incubated at 37 °C for 20 min. After 20 min of incubation, 3,5 dinitro salicylic acid (3.5 DNS) was added to each test tube and the test tubes were boiled at 100 °C for 5 min. After the tubes cooled, dilution was made and spectrophotometric measurements were made at 540 nm. The tube without test samples was used as a control and the antidiabetic ability was calculated according to the formula below.
DNA nuclease experiments
The gel electrophoresis technique was used to examine the DNA nuclease ability of compound 3 according to Barut and Demirbaş, assay with minor changes [20]. Supercoiled pBR322 plasmid DNA was incubated at 37 ºC for 90 min with increasing concentrations of them (50, 100 and 200 mg/L). The same step was applied without compound 3. After that, the loading buffer was adjoined and the compound 3’s was loaded on a 1.0% agarose gel and stained with EthBr in a tris-acetic acid-EDTA buffer. Using an electrophoresis apparatus, the compounds were run at 120 V for 1.5 h. Following the electrophoresis process, photographs of the bands were taken under UV illuminator.
Antimicrobial assays
The SiPc was viewed for their in vitro antimicrobial efficacy against Gram (+) species (including E. faecalis (ATCC 29,212), E. hirae (ATCC 10,541), S. aureus (ATCC 25,923)), Gram (-) (including E. coli (ATCC 25,922), L. pneumophila subsp. pneumophila (ATCC 33,152), P. aeruginosa (ATCC 27,853)), as well as fungal strains (C. tropicalis (ATCC 750) and C. albicans) using the standard broth microdilution method. These species were grown at 37 ºC overnight before the application. The initial wells of the microplates were filled with 1024 mg/L of each of the compound 3’s under study, and the remaining wells were filled with diluted versions of the compound. Then, each well received an inoculation of 10 µL of microorganism culture solution. On a shaker, microplates were incubated at 37 ºC for 24 h [21].
Photodynamic antimicrobial therapy activity
This study was carried out in the same methodology as the antimicrobial assays section mentioned above. However, in this procedure, compound 3 was subjected to LED light for 30 min prior to microbial inoculation. The results of the study were evaluated as MIC values.
Test of microbial cell viability
The talent of SiPc to inhibit bacterial cell viability was tested using the strain of E. coli (ATCC 25,922). E. coli was inoculated into Nutrient Broth (NB) and grown at 37.0 ºC for one day in a shaker. After one day of incubation, E. coli was separated by centrifugation at 5500 rpm for 6 min. The residue of the NB medium was then rinsed off the bacterial pellet with a sterile 0.9% saline solution. After the purification step, E. coli strain was slinged in 10 mL of saline. The microbial cell viability experiment was conducted using this microbial suspension (2.8 × 109 CFU/mL). E. coli was treated at 0, 25, 50 and 100 mg/L concentrations of compound 3 at 37 ºC for 90 min. The mixes were then diluted in a variety of ratios, inoculated on NB agar, and left to incubate for one day at 37 ºC. At the end, the colonies were be counted. The following formula was applied to calculate the microbial cell viability [22].
Antibiofilm ability
P. aeruginosa and S. aureus were applied as model microorganisms to assign the effect of compound 3 on biofilm inhibition. Microorganisms were added to well plates containing test compound 3 additions of 0, 12.5, 25 and 50 mg/L. The plates were then incubated at 37 ºC for three days. The wells of the plate were carefully drained after incubation and washed twice with distilled water. The biofilms were stained with crystal violet (CV) and then incubated for 60 min. Then, CV was suspended and the plates were slowly rinsed with distilled water. Ethanol was suffixed to plates and allowed for 15 min for recovery of absorbed CV. Using a spectrophotometer, the biofilm inhibition was measured at 595 nm. Calculation of biofilm inhibition was done with the following formula [23].
Results and discussion
Bis-benzyl 2-(oxy) benzoate phthalocyaninato silicon(IV) compound was get by the reaction of SiPcCl2 and benzyl salicylate compounds. The general synthesis route of the original compound is given in scheme 1. Characterization of the compound was done by 1H NMR, 13C NMR, FT-IR, UV-visible and Mass spectrum. The IR spectroscopy of the compound gives the vibrations at 3066 cm− 1 (Ar-H), the vibrations of the CH2 groups at 2926 cm− 1 and 2854 cm− 1. It gives C = O vibrations at 1672 cm− 1. gives C = C vibrations at 1612 cm− 1 and 1585 cm− 1. It shows the Si-O-C vibration at 1085 cm− 1. These spectral IR vibration data support the expected structure. Analysis of compound 3 by ESI mass spectrometry revealed the expected ion peak of 995.27 [M + H]+. This supports the structure at the summit as expected. Aromatic protons characteristic in the 1H NMR spectrum give peaks in the range of 7.81–6.90 ppm. At the same time, the aliphatic CH2 protons characteristic for the compound support the structure with a peak at 5.37 ppm. In addition, the peaks of the 13C NMR spectrum are in the range of 169.68 to 109.98 ppm in the aromatic region, while aliphatic peaks are observed at 66.94 ppm and 66.92 ppm. The peaks seen from these spectra support the structure.
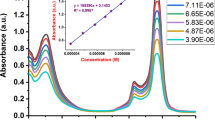
Compound 3 shows the Q and B bands specific to phthalocyanines at 690 and 360 nm in THF solvent. The peak called shoulder in phthalocyanines is observed at 620 nm. The peaks mentioned for phthalocyanine compounds are related to electronic structure. Q and B bands, which are important electronic transitions in the characterization of the phthalocyanine structure, contain information about the structure. It serves information about both the phthalocyanine ring structure and whether metal is bonded. For this reason, the absorption data of electronic transitions in phthalocyanine chemistry are closely related to the structure [24–25]. The electronic absorption of SiPc in the space of certain concentrations is given in Fig. 1. Figure 1 shows that the compound does not aggregation in the measured concentration range and obeys Lambert-Beer’s law. Non-aggregation behavior of the compound is also an important parameter for applications such as drugs or antioxidants. It contributes positively to the applicability of the compound.
The solubility and non-agglomeration of phthalocyanine compounds in diverse solvents is an essential parameter for photodynamic therapy. Demonstrating this with spectral data is shown in Fig. 2. It is necessary for different applications that the compound be soluble in commonly used solvents such as dichloromethane, CHCl3, DMF, THF, DMSO.
The compound 3 appears to have some redshift from the fluorescence spectrum in THF, DMF and DMSO solvents. The spectra in the aforementioned THF, DMF, DMSO solvents are 6, 9 and 11 nm and the stokes absorption shift values were determined. Stokes shift values are shown in Table 1. Diagrams showing the fluorescent emission, excitation, absorption spectra of the compound in THF, DMF and DMSO solvents are shown in Figs. 3, 4 and 5. The fluorescence quantum yield (ΦF) of compound 3 in DMF and DMSO was found 0.18 and 0.15, respectively. The quantum yield of this phthalocyanine complex is intimate to the reported value of silicon phthalocyanine for DMF and DMSO solvents [26]. It is reported that the fluorescence quantum yield varies depending on the substituent group [27].
As is generally known, the emission spectra give a mirror similar of the absorption spectra. Therefore, the highly symmetrical view of the emission and absorption spectra is an indication that the emission match to like absorption transitions [28]. It is known that diamagnetic zinc phthalocyanine compounds are effective as photosensitizers for photodynamic therapy. In addition to its biological properties, this compound also has photosensitizer potential.
Antioxidant activity
One of the most commonly used methods for the valuation of antioxidant activity is the DPPH radical capture test. The DPPH test is one of the feasible methods because of its inexpensive, usefulness and impact. This method is based on the reduction of DPPH with a compound made, synthesized or naturally obtained. Due to this reduction, a color change occurred from purple to yellow. This color change was measured at 517 nm with the aid of a spectrophotometer device. The antioxidant effects of compound 3 examined at different concentrations in our study. It was monitored that tested compound showed good antioxidant activity at different concentrations. The results obtained as a result of the study are presented in Fig. 6. The antioxidant activity of compound 3 was 49.48%, 59.66%, and 65.28% at 12.5, 25, and 50 mg/L, respectively. The DPPH scavenging abilities of compound 3 was found to increase from 38.46 to 73.18% when concentration rises from 6.25 mg/L to 100 mg/L. Celik et al. showed the various biological properties of new axisymmetric and unsymmetrical compound 3 with anti-inflammatory groups and also investigated the antioxidant activities of these compounds [29]. Among the four different Silicon phthalocyanine derivatives, the highest antioxidant ability was obtained as 73.48%. Colak et al. they appear to have examined the antioxidant effects of other phthalocyanine derivatives and reported their results [30]. . The antioxidant ability of sulfobetaine substituted compounds was 48.72% and they found that other compounds didn’t show any scavenging effect at 200 mg/L. Günsel et al. reported that, Among the newly synthesized metallophthalocyanines, the highest DPPH scavenging activity was 40.82% by the CoPc (4) at 100 mg/mL [31]. The order of DPPH radical scavenging activities was 91.95% > 90.33% > 89.93% > 17.52% > 9.63%. Barut and Demirtaş showed the antioxidant ability properties of metal-free, copper and nickel Pc. They found that metallophthalocyanines had higher antioxidant properties than metal-free analogues and also, CuPc displayed the highest DPPH radical inhibition ability with IC50 values of 1.11 ± 0.02 mM [20]. Compared with the studies, it has been seen that newly synthesized silicon phthalocyanine can be strong applicants due to their superior antioxidant abilities. With this rationale, silicon phthalocyanines have the potential to be another synthetic antioxidants for human, animal or in vivo enforcements after anymore research.
Antidiabetic activity
In the case of diabetes, some drug formulations have been approved for controlled insulin delivery. Standard preclinical and clinical approaches to assessing the toxicity and antidiabetic properties of new drugs are required to support the market introduction of a wide variety of diabetes drugs. So we evaluated the antidiabetic properties of compound 3 at different concentrations. Antidiabetic percentage of compound 3 was 12.66%, 16.33% and 20.14% at 100, 200 and 400 mg/L, respectively (Fig. 7). Ozturkmen et al. investigated the antidiabetic activity of some phthalocyanines and it was found that the compounds had the potential to treat Diabetes mellitus(DM). While the highest inhibition percentages of DFP-Mn were 72.96 ± 4.95%, respectively at 100 µM on AChE. In the presence of DFP-Mn at 100, the maximum inhibition percentages of DFP-Mn were as 52.18 ± 2.22% against tyrosinase at 100 µM [32]. Değirmencioğlu et al., informed that they studied the α-amylase and tyrosinase inhibition properties of newly substituted phthalocyanines. It was determined that among the test phthalocyanines PbPc compund was the most effective compound against tyrosinase (IC50 value 6.7 ± 0.6 µM) and α-amylase (IC50 value 7.3 ± 0.3 µM) in antidiabetic study [33]. It was determined that phthalocyanines were most active against α-amylase and tyrosinase. Based on these results, it can be said that compound 3 has the back demand to treat DM. However, further studies are needed to confirm the antidiabetic effect with other tests.
DNA cleavage studies with agarose gel electrophoresis
In this study, gel electrophoresis was enforced to determine the DNA degradation action of compound 3. The view of the DNA fragmentation activity of SiPc is presented in Fig. 8. According to the results, compound 3 caused single strand break at 50 and 100 mg/L additions and also DNA completely fragmented at 200 mg/L concentration. In a study by Barut et al., the DNA fragmentation ability of overcoiled pBR322 plasmid DNA in the asset of SiPc compounds is analyzed [34]. It is reported that the compounds did not show significant degradation activities in the study. Farajzadeh et al. investigated the anticancer and biological properties of new axially substituted SiPcs [35]. They investigated the DNA cleavage of SiPc compounds using agarose gel electrophoresis and used pBR322 plasmid DNA for DNA cleavage studies. As a result of the study, it was found that all quaternized SiPc compound exhibited acceptable DNA fragmentation activities. As reviewed in the literature, the data obtained for silicon Pcs showed that these Pcs can be significant sources for drug contrive and studies after upward research.
In vitro antimicrobial activity and photodynamic antimicrobial therapy
Accordingly, antimicrobial activities of compound 3 were determined by Gram (+) bacteria, Gram (-) bacteria and yeast were investigated. The antimicrobial properties of compound 3 were examined by microdilution method and the data are given in Table 2. E. faecalis was accepted as the most susceptible microorganism. MICs of compound 3 were obtained as 128 mg/L for S. aureus, 64 mg/L for E. coli, 128 mg/L for P. aeruginosa, 128 mg/L for L. pneumophila, 64 mg/L for E. hirae, 32 mg/L for E. feacalis, 128 mg/L for C. albicans, and 256 mg/L for C. tropicalis. Masilela et al. investigated the photophysicochemical and antimicrobial behavior of zinc and silicon phthalocyanines [36]. The highest antimicrobial activity was obtained opposite B. subtilis check against to S. aureaus, both in the dark and under light illumination. SiPc exhibited no significant reduce in microbial viability, however, under the same irradiation conditions, SiPc + Ch and SiPcCh showed efficiently inactivate both B. subtilis and S. aureus. B. subtilis was inactivated to the detection limit with 5 W/cm2 light (9 J/cm2) after 5 min irradiation when 0.2 mg/mL SiPcCh was utilized. For complete inactivation of E. coli required concentration and light dose was higher (0.4 mg/mL SiPcCh or 24 µg mL equivalent of SiPc, 63 J/cm2) [37]. Compared with the studies in the literature, these study findings are expected to conduce to the growth of antimicrobial drugs after upward research is completed.
Antimicrobial photodynamic activities of synthesized compound 3 was investigated using Gram (+), Gram (-) bacteria and fungi. Photodynamic antimicrobial talents of the compound 3 was investigated using the microdilution method and are listed in Table 3. compound 3 exhibited important photodynamic antimicrobial action opposite Gram-negative, Gram-positive bacteria and yeasts. E. faecalis was also accepted as the most susceptible microorganism. MICs of compound 3 was 64 mg/L opposite S. aureus, 32 mg/L against E. coli, 32 mg/L opposite P. aeruginosa, 64 mg/L against L. pneumophila, 32 mg/L against E. hirae, 8 mg/L opposite E. feacalis, 32 mg/L opposite C. albicans and 64 mg/L opposite C. tropicalis. Zhao et al. investigated the effect of various silicon (IV) phthalocyanines on photodynamic antimicrobial activities [38]. The new compound 3 containing tri-arginine oligopeptides exhibited an extremely superior in vitro photodynamic action not only opposite Gram (+)/(-) bacteria but also opposite fungi, clearly superior to its mono-arginine-containing analogues. In addition, compound 3 showed a higher photodynamic inactivation than methylene blue (MB), a known antimicrobial photosensitizer, opposite S. aureus, E. coli and C. albicans cells. Bıyıklıoğlu et al. examined the antimicrobial and antimicrobial photodynamic abilities of SiPcs on Gram (+) and Gram (-) bacteria. Antimicrobial abilities of the test compounds were determined by microdilution process. The MIC values of Es-SubPc and Es-SiPc were found as 256 mg/L against E. coli and 128 mg/L against S. aureus. After light irradiation, the growth of E. coli and S. aureus were effectively decreased [39]. The results showed that aPDT had promising antibacterial effects on Gram-positive and Gram-negative bacteria in the presence of synthesized SiPc. The results show that compound 3 has a very good capacity for photodynamic antimicrobial therapy and is a promising photosensitizer, subject to further research.
Microbial cell viability
Microbial cell viability of compound 3 opposite bacterial strain of E. coli was tested. The results obtained are shown in Fig. 9. It was defined that compound 3 cased very powerful E. coli growth inhibition. As understand in Fig. 9, SiPc inhibited E. coli growth by 99.96% at 100 mg/L, 99.89% at 50 mg/L and 99.46% at 25 mg/L, respectively. The results show that compound 3 exhibits a strong inhibitory effect. Therefore, upward research is needed to determine the antimicrobial impact of compound 3 using in-vivo animal models. Chemicals that reduce cell proliferation are known to be an important area of drug find and cell biology research, and too much research is being done in this area. Masilela et al. showed the photophysicochemical and antimicrobial behavior of zinc and silicon phthalocyanines and they found that the most effective bacterial inhibition was achieved with silicon phthalocyanines [36]. Celik et al. viewed microbial cell viability of a series of silicon phthalocyanines [29]. The effect of microbial cell viability of the SiPcs was performed using E. coli. SiPc1, SiPc2, SiPc3, and SiPc4 were decreased the E. coli growth as 89.73%, 95.29%, 95.04, and 94.25%, respectively at 125 mg/L. On the other hand, E. coli growth was also inhibited as 95.84% for SiPc1, 99.05% for SiPc2, 98.77% for SiPc3, and 98.41% for SiPc4 at 250 mg/L. They found that all of SiPcs the compounds exhibited 100% cell viability inhibition at 500 mg/L. According to the data get from the results of our study, synthesized compound 3 can be used as alternate materials in the cure of many diseases, including resistant bacteria, after further research.
Antibiofilm activity
As the National Institutes of Health explains, microbial biofilm-forming microorganisms are responsible for most common infections. The biofilms are the most extensive form of life in which they adhere to each other and to other wet surfaces in a well-organized bacterial group. It is known that microorganisms have biofilms with many unique properties that give resistance to antimicrobial agents. Prevention of biofilms is an exciting and important area of research for protection opposite bacteria and bacterial infections. In this study, the biofilm inhibition abilities of compound 3, which are known to form biofilms, opposite S. aureus and P. aeruginosa at diverse concentrations were tested. Results regarding the biofilm inhibition ability of P. aeruginosa and S. aureus are given in Figs. 10 and 11. Antibiofilm ability of compound 3 against both test microorganisms was investigated at 12.5 mg/L, 25 mg/L and 50 mg/L. The biofilm inhibition ability of SiPc was found to be concentration dependent. Antibiofilm activities of compound 3 at 25 mg/L and 50 mg/L were 69.82% and 75.24% for P. aeruginosa and 99.89% and 99.96% for S. aureus, respectively. Taskin et al. showed the biofilm inhibition ability of SiPcs in a study they carried out [40]. From the results of this report, they reported that the bacterial biofilm revealed complete penetration into its biomass after 48 h. Celik et al. They stated that they investigated the effect of silicon phthalocyanines with anti-inflammatory groups on biofilm inhibition [29]. It was informed that the biofilm inhibition was found as 33.69%, 26.44%, 45.39%, and 41.82% for SiPc(1–4), respectively at concentration of 125 mg/L. When concentration of SiPcs rised up 250 mg/L to 500 mg/L, the biofilm inhibition percentages rised up 43.12–65.58% for SiPc1, from 39.18 to 64.39% for SiPc2, and from 52.73 to 73.25% for SiPc4. Compound SiPc3 showed excellent biofilm inhition as 82.14% at 500 mg/L. It can be finalized that the synthesized compound 3 can be used as a strong option in medical and environmental applications to reduce the effect of infection with biofilm inhibition and increase the performance of wastewater treatment.
Conclusion
As a result, silicon phthalocyanine was synthesized as original in this study. The compound was characterized by 1H NMR, 13C NMR, FTIR, UV-vis, LC-MS spectroscopies. The absorption of the compound in diverse solvents and the absorption in the same solvents reveal that the compound can be used for different applications. In addition, various in vitro biological activities of newly synthesized silicon phthalocyanine was evaluated. Compound 3 exhibited antioxidant and antidiabetic activities at all tested concentrations. Compound 3 also showed more significant antimicrobial abilities especially against bacteria. Compound 3 displayed powerful antibiofilm abilities opposite S. aureus and P. aeruginosa. It can be clearly seen that compound 3 showed high biofilm inhibition activity against both test microorganisms at all doses tested. Silicon phthalocyanines had 99.96% bacterial reduction against E. coli. The photodynamic antimicrobial therapy (PDAT) activity of the investigated compounds was also tested. Compound 3 were found to have a more effective activity on Gram (+) bacteria than Gram (-) bacteria. In addition, it was observed that while single chain was obtained at 50 and 100 mg concentrations, and also compound 3 completely degraded DNA at 200 mg/L concentration. As a result, the data obtained in this study can be an important preliminary resource for alternative drug designs and new studies.
References
Guowei, L., et al.: Fluorinated/non-fluorinated triphenylamine axially substituted silicon phthalocyanine: Synthesis and photophysical properties. Inorg. Chem. Comm. 141, 109490 (2022)
Yaşa Atmaca, G., et al.: Comparison of sonodynamic, photodynamic and sonophotodynamic therapy activity of fluorinated pyridine substituted silicon phthalocyanines on PC3 prostate cancer cell line. Photodiagnosis Photodyn Ther. 42, 103339 (2023)
Mendes, L.: Metallated phthalocyanines and their hydrophilic erivatives for multi-targeted oncological photodynamic therapy. J. Photochem. Photobiol. 234, 112500 (2022)
Azzouzi, S., et al.: Novel iron (III) phthalocyanine derivative functionalized semiconductor based transducers for the detection of citrate. Org. Electron. 34, 200–207 (2016)
Aziz, T.: A flexible nickel phthalocyanine resistive random access memory with multi-level data storage capability. J. Mater. Sci. Technol. 86, 151–157 (2021)
Choi, S.-H., et al.: Electro-optical characteristics of polymer-dispersed liquid crystal containing copper (II) phthalocyanine as a function of UV irradiation time. J. Mol. Liq. 363, 119821 (2022)
Ağırtaş, M.S., et al.: Synthesis and Sensor properties of Silicon Phthalocyanine axially substituted with Bis-(Prop-2-Ynyloxy) groups and polymeric phthalocyanines bearing PEG substituent by click Chemistry. Polycycl. Aromat. Compd. 43(4), 3278–3290 (2023). https://doi.org/10.1080/10406638.2022.2067195
Szostak, J., et al.: Photovoltaic properties of cadmium selenide–titanyl phthalocyanine planar heterojunction devices. Chem. Phys. 456, 57–60 (2015)
Şen, P., et al.: Synthesis and electrochemical, electrochromic and electrical properties of novel s-triazine bridged trinuclear zn(II), Cu(II) and Lu(III) and a tris double-decker Lu(III) phthalocyanines. Synth. Met. 161, 1245–1254 (2011)
Gounden, D., et al.: Recent advances in phthalocyanines for chemical sensor, non-linear optics (NLO) and energy storage applications. Coord. Chem. Rev. 420, 213359 (2020)
Wang, S., et al.: Graphene carbon black as catalyst support: The influences of iron phthalocyanine loading and carbon black additive amount on the power generation performance of direct glucose fuel cell. Fuel. 315, 123227 (2022)
Liu, G.: Fluorinated/non-fluorinated triphenylamine axially substituted silicon phthalocyanine: Synthesis and photophysical properties. Inorg. Chem. Commun. 141, 109490 (2022)
Güngördü Solğun, D., et al.: Synthesis of novel tetra (4-tritylphenoxy) substituted metallophthalocyanines and investigation of their aggregation, photovoltaic, solar cell properties. Inorg. Nano-Metal Chem. 48(10), 508–514 (2018)
Ağırtaş, M.S.: Highly soluble phthalocyanines with hexadeca tert-butyl substituents. Dyes Pigm. 79, 247–251 (2008)
Berezin, D.B., et al.: Aggregation of water soluble octaanionic phthalocyanines and their photoinactivation antimicrobial effect in vitro. Mendeleev Commun. 30, 621–623 (2020)
Kırbaç, E., Erdoğmuş, A.: New non-peripherally substituted zinc phthalocyanines; synthesis, and comparative photophysicochemical properties. J. Mol. Struct. 1202, 127392 (2020)
Kobayashi, H., et al.: The chemical basis of cytotoxicity of siliconphthalocyanine-based near infrared photoimmunotherapy (NIR-PIT) and its implications for treatment monitoring. Curr. Opin. Chem. Biol. 74, 102289 (2023)
Erdağ Maden, Y., et al.: Electrochemical and spectroelectrochemical characterizations of phthalocyanines bearing peripherally tetra-4-carboxyethylenephenoxy anchoring groups and usage as photosensitizers of dye-sensitized solar cell. J. Electroanal. Chem. 929, 117104 (2023)
Ağırtaş, M.S., et al.: Novel metal (II) phthalocyanines with 3,4,5-trimethoxybenzyloxy-substituents: Synthesis, characterization, aggregation behaviour and antioxidant activity. Dyes Pigm. 96, 152–157 (2013)
Barut, B., Demirbaş, U.: Synthesis, anti-cholinesterease, α-glucosidase inhibitory, antioxidant and DNA nuclease properties of non-peripheral triclosan substituted metal-free, copper(II), and nickel(II) phthalocyanines. J. Organomet. Chem. 923, 121423 (2020)
Arslan, H., et al.: Antimicrobial and antioxidant activity of phenolic extracts from walnut (Juglans regia L.) green husk by using pressure-driven membrane process. J. Food Sci. Technol. 73–83 (2023)
Farajzadeh, N., et al.: Biological properties of hexadeca-substituted metal phthalocyanines bearing different functional groups. J. Inorg. Biochem. 111888 (2022)
M’barek, I., et al.: Nanocellulose synthesis from Tamarix aphylla and preparation of hybrid nanocellulose composites membranes with investigation of antioxidant and antibacterial effects. Sep. Purif. Technol. 120815 (2022)
Ağırtaş, M.S.: Fluorescence properties in different solvents and synthesis of axially substituted silicon phthalocyanine bearing bis-4-tritylphenoxy units. Heterocycl. Comm. 26(1), 130–136 (2020). https://doi.org/10.1515/hc-2020-0113
Günsel, A., et al.: Novel potential metabolic enzymes inhibitor, photosensitizer and antibacterial agents based on water-soluble phthalocyanine bearing imidazole derivative. J. Mol. Struct. 1237, 130402 (2021)
Kayir, N., Gorduk, S.: Synthesis, characterization, and investigation photophysicochemical properties of axially 2-hydroxymethyl-1,4-benzodioxan di-substituted Silicon(IV) phthalocyanine. J. Organomet. Chem. 990, 122661 (2023)
Yasa Atmaca, G., et al.: Novel axially carborane-cage substituted silicon phthalocyanine photosensitizer; synthesis, characterization and photophysicochemical properties. Spectrochim Acta Mol. Biomol. Spectrosc. 137, 244–249 (2015)
Lakowicz, J.R.: Principles of Fluorescence Spectroscopy, 3rd edn. Center for Fluorescence Spectroscopy, University of Maryland School of Medicine, Baltimore (2006)
Celik, G., et al.: Novel axially symmetric and unsymmetric silicon (IV) phthalocyanines having anti-inflammatory groups-: Synthesis, characterization and their biological properties. Dalton Trans. 7517–7529 (2022)
Colak, S., et al.: Ynthesis, characterization, solution and antioxidant properties of novel tetrakis{4-[N-((3-dimethylamino)propyl)amide]phenoxy}nickel (II) phthalocyanine and its water soluble derivatives. J. Organomet. Chem., 83–89 (2016)
Günsel, A., et al.: Novel biologically active metallophthalocyanines as promising. J. Mol. Struct. 127127 (2020)
Öztürmen, B., et al.: Synthesis, characterization, and α-glucosidase, cholinesterases, and tyrosinase inhibitory effects of axial substituted silicon and peripheral tetra-substituted copper (II), manganese (III) phthalocyanines. Appl. Organomet. Chem. 36(8) (2022)
Değirmencioğlu, I., et al.: The synthesis of novel piperazine-benzodioxole substituted phthalocyanines and investigation of their α-amylase and tyrosinase inhibition properties. J. Organomet. Chem. 122012 (2021)
Barut, B., et al.: Non-aggregated axially disubstituted silicon phthalocyanines: Synthesis, DNA cleavage and in vitro cytotoxic/phototoxic anticancer activities against SH-SY5Y cell line. Dyes Pigm. 107794 (2020)
Farajzadeh, N., et al.: Anticancer and biological properties of new axially disubstituted silicon phthalocyanines. Dalton Trans. 19 (2022) (2022)
Masilela, N., et al.: Axial coordination of zinc and silicon phthalocyanines to silver and gold nanoparticles: An investigation of their photophysicochemical and antimicrobial behavior. J. Porphyr. Phthalocyanines 17, (2013)
Strokov, K., Galstyan, A.: Chitosan-Silicon Phthalocyanine Conjugate as Effective Photo-Functional Hydrogel for Tracking and Killing of Bacteria. Eur. J. Org. Chem. 7327332 (2020)
Zhao, Y., et al.: A novel silicon(IV) phthalocyanine-oligopeptide conjugate as a highly efficient photosensitizer for photodynamic antimicrobial therapy. Dyes Pigm. 107834 (2020)
Biyiklioglu, Z., et al.: Synthesis and antimicrobial photodynamic activities of axially {4-[(1E)-3-oxo-3-(2-thienyl)prop-1-en-1-yl]phenoxy} groups substituted silicon phthalocyanine, subphthalocyanine on Gram-positive and Gram-negative bacteria. Dyes Pigm. 149–158 (2019)
Taşkın, G., et al.: Axially paraben substituted silicon(IV) phthalocyanines towards dental pathogen Streptococcus mutans: Synthesis, photophysical, photochemical and in vitro properties. J. Photochem. Photobiol A 31–40 (2015)
Author information
Authors and Affiliations
Contributions
M. S. A. wrote the main manuscript text. D. G. S. prepared the synthesis and Figs. 1, 2, 3, 4 and 5. S. Ö. prepared text and biological properties. G. T. prepared related biological shapes. All authors reviewed the article.
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest statements.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Solğun, D.G., Özdemir, S., Ağırtaş, M.S. et al. Synthesis and biological properties of benzyl 2-(oxy)benzoate-substituted silicon phthalocyanine. J Incl Phenom Macrocycl Chem 104, 137–148 (2024). https://doi.org/10.1007/s10847-024-01226-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-024-01226-4