Abstract
Mercury is a frequent, bioaccumulative, extremely toxic pollutant in the environment. Mercury contamination can be accumulated along the food chain and cause a wide range of serious threats to living organisms, and also affect neurological systems and the kidneys. The trace-level detection of heavy and toxic metal ions such as mercury ions is certainly great intense. Chromogenic and fluorogenic recognition of toxic mercury ions has been established to be powerful methods due to their high detection limit, cost-efficiency, simplicity, and applicability in bioimaging. This review will mainly focus on the sensing mechanisms of fluorescent probes that have emerged over the past 5 years, such as PET, ICT, AIE, as well as ring-opening sensing mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorescent chemosensors capable of selectively recognizing heavy and toxic ions are an important target in the field of supramolecular chemistry due to their potential application in bioimaging molecular catalysis, environmental detection, medicine, industrial processes, and human sciences [1,2,3]. Mercury is a toxic environmental pollutant. Due to the extreme toxicity of mercury ions, the World Health Organization (WHO) has determined that the allowable limit of mercury ions in drinking water is 0.5 µg/l [4]. There are many different techniques used to detect the concentration of toxic anions and cations present in water. These methods can be divided into four categories: mechanical, optical, electrochemical, and spectroscopic/spectrometric. Usually, the methods of high-performance liquid chromatography (HPLC), mass spectrometry, atomic emission spectroscopy (AES), atomic absorption spectroscopy (AAS), chromatography, inductively coupled plasma mass spectroscopy (ICP-MS), flow injection analysis, and electrochemical methods are used to analyze the toxic ions [5]. However, these methods suffer either from extensive, time-consuming procedures, or the use of sophisticated instrumentation. The instrumentation methods are not very expedient and versatile for ion detection. The development of chromogenic sensors is increasingly appreciated since naked eye detection can offer qualitative and quantitative information without resorting to any spectroscopic instrumentation. Fluorescence measurement, on the other hand, is usually very sensitive, versatile, and offers a sub-micromolar estimation of guest species [6, 7]. The photo-induced electron transfer, intramolecular charge transfer, ring-opening, and chelation-enhanced fluorescence mechanisms are commonly adopted fluorescence response mechanisms for mercury detection [8, 9]. A few reviews only described the mercury fluorescence sensors from different perspectives, such as molecule types, application in imaging, testing of mercury, and optical and fluorescence recognition [10,11,12]. This review mainly focuses on the fluorescent recognition mechanisms for the detection of mercury ions since 2015. The review is instigated with a brief discussion of the metal’s occurrence, methodologies for detection, sources, applications, toxicity, and the mechanism of the mercury ion sensor. Further, the fluorogenic and chromogenic mercury ion sensors are classified according to their sensing mechanisms (Table 1).
Metal occurrence
Mercury is a naturally occurring element found in rock in the earth’s crust. It is released into the environment from volcanic activity, forest fires, and weathering of rocks. Mercury exists in several forms, such as metallic mercury, inorganic mercury, methyl mercury, and other organic mercury compounds [13]. Elemental mercury is a silver-white metal and is liquid at room temperature. The inorganic mercury compounds are obtained when other elements (S, O, Cl, etc.,) combine with mercury. Methylmercury is the most common organic mercury compound found in the environment. The inorganic mercury is converted to the organic form when the microscopic organisms unite mercury with carbon (Fig. 1).
Sources, applications, and toxic effect of mercury
Sources
Among the metal ions, particular attention has been paid to mercury ions for their extreme toxicity. Mercury contamination occurs through various natural processes such as volcanic eruptions, geothermal springs, geologic deposits, and emissions from the ocean. Human activity is the main cause of mercury releases, particularly coal-fired power stations, burning oil, fossil fuels, raw materials, residential coal burning for heating and cooking, industrial processes, waste incinerators, and as a result of mining for mercury, gold, and other metals. Some of the mercury circulating through today's environment was released years ago. Water, land, and other surfaces can repeatedly re-emit mercury to the atmosphere after its initial release into the environment [14]. The anthropogenic emissions continue to add significantly to the global pool of mercury (Fig. 2).
Applications
Mercury is used in various processes and workplaces. Mercury is used in laboratories for making thermometers, barometers, diffusion pumps, fluorescent lamps, electrical apparatus and instruments, mercury switches, mercury relays, sphygmomanometers, and used as an electrode for making batteries. Mercury is also used as a catalyst in chemical industries. Mercury easily forms alloys with other metals such as gold, silver, and tin, called amalgams. Mercury amalgams were also used in dental fillings. Metal mercury is used as a liquid electrode in the manufacture of chlorine and sodium hydroxide by electrolysis of brine [15]. Mercurous chloride (Hg2Cl2) is used in medicine as a purgative and also used as a standard electrode in electrochemical measurements (Fig. 3).
Major consequences and adverse effects of mercury
Mercury (Hg2+) is well known as one of the most toxic metals, and is widespread in air, water, and soil, generated by many sources such as gold production, coal plants, thermometers, barometers, caustic soda, and mercury lamps. As it can cause strong damage to the central nervous system, the accumulation of mercury in the human body can lead to various cognitive and motor disorders, and Minamata disease. A major absorption source is related to daily diet such as fish. Thermometer manufacturing releases a very small amount of mercury (from 0.1 mg to 10 mg) into the atmosphere, which contaminates the soil. This can cause harmful effects, such as nerve, brain, and kidney damage, lung irritation, eye irritation, skin rashes, vomiting, and diarrhea. Metallic mercury mainly causes health effects including neuromuscular changes, headaches, changes in nerve responses, tremors, insomnia, etc. [16] when inhaled as a vapor where it can be absorbed through the lungs (Fig. 4).
Marine organisms like phytoplankton and zoo-planktons easily absorb the toxic methylmercury compound. The organisms are consumed by small fish, which are consumed by large fish, and the large fish are consumed by human beings [17]. The poisonous chemical enters the body of human beings through the food chain. It causes various disorders such as nervous disorders, muscular coordination, severe headaches, and loss of vision and hearing (Fig. 5).
Methodologies for mercury ion detection
In recent years, various accurate and analytical techniques have been reported for the detection of mercury ions such as gas chromatography-triple quadrupole mass spectrometry (GC–MS/MS), mercury analyzers, electrochemical sensors, cold vapor integrated quartz crystal microbalance (CV-QCM), atomic absorption spectroscopy, atomic fluorescence spectroscopy, reduced graphene oxide field-effect transistor (rGO FET), solid-phase extraction, and fluorescence and colorimetric methods. Different types of nanozymes are used to detect mercury ions due to their simplicity and ease of developing a portable sensor. The processing of hydrocarbon samples is generally very complex, and mercury is found in low concentrations. Therefore, the development of highly sensitive analytical methods is still needed for low-level detection of mercury ions. The colorimetry and fluorescent methods are widely used because these methods have an excellent selectivity compared to other methods [18].
Mechanism of mercury sensing
Fluorescence emission takes place from the electronically excited states of molecules. However, given the high reactivity of the electrons in these states, reactions that usually do not occur in the ground states can take place. From the point of view of chemical sensing, the coordination of metal ions could cause an enhancement of the fluorescence or quenching of the fluorescence intensity. The enhancement of fluorescence emission is called the chelation enhanced fluorescence effect (CHEF). The quenching of the fluorescence is called the chelation enhancement quenching effect (CHEQ). Both effects can be coupled with a red or blue shift of the emission band. Upon analyte binding to chemosensors, it is possible to modulate some of these reactions' emissions, and thus take advantage of the different mechanisms for signal transduction (Fig. 6). Conventional mechanisms such as paramagnetic fluorescence quenching, photo-induced electron transfer (PET), intramolecular charge transfer (ICT), fluorescence resonance energy transfer (FRET), photo-induced charge transfer (PCT), photo-induced excimer formation, intersystem crossing or the heavy atom effect, aggregation-induced emission (AIE) and excited-state intramolecular proton transfer (ESIPT) have been frequently adopted for the construction of probe molecules.
Fluorescent sensors for mercury ions
Aggregation-induced emission (AIE)-based Hg2+ ions detection
The restriction of intramolecular motion, rotation, or vibration (RIM, RIR, or RIV) in the aggregates is the main cause of the AIE phenomenon. The AIE active molecules are weakly emissive in the solution state due to unhindered intramolecular motions but become highly emissive upon aggregation in a suitable environment through activation of RIM, RIR, or RIV mechanisms in the excited state. In view of such unusual fluorescence behaviors, the AIE phenomenon is successfully utilized to design fluorescent probes with proper chelating groups for the detection of metal ions [19]. The aggregation of AIE probes can be tuned by metal ions through electrostatic interaction, coordination interaction, or the influence of polarity and viscosity (Fig. 7).
Highly fluorescent aggregation-induced emission-based 1, 8-naphthalimide-sulfamethizole sensor S1 has been reported for Hg2+ and Ag+ ions. Aggregation-induced emission is caused by the hydrophobic nature of naphthalimide fluorogenic moiety in DMSO: water (1:99 v/v, pH 7.2, HEPES buffer). Upon the addition of various metal ions, Hg2+ ions show an increase in absorbance at 267 and 343 nm with a slight red shift (hypochromic effect). An excimer group was obtained from the intramolecular interaction between the naphthalimide moieties in the completion of S1 with Hg2+ ion [20]. Due to this reason, Sensor S1 emission band at 390 nm was quenched and a new intensity band appeared at 483 nm in the presence of Hg2+ ions. The sensor S1-Hg2+ coordination restricts the free rotations of the S1 and increases the rigidity of the molecular assembly, resulting in enhanced fluorescence intensity at 483 nm. Further, significantly enhancing fluorescence intensity was observed by the increasing addition of Hg2+ ions. Mercury ions induce more aggregation of the S1-Hg2+ complex thereby facilitating the aggregation-induced emission enhancement behavior of the S1. Sensor S1 sensitively detects Hg2+ ions and the calculated detection limit of S1-Hg2+ ions is 14.7 nM (Fig. 8).
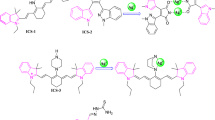
A new fluorescent sensor S2a–b based on sulfonamidospirobifluorenes was synthesized and reported for the selective detection of Hg2+ ions in the DMSO/HEPES buffer mixture [21]. Out of 20 metal ions, sensor S2a–b only shows an excellent selectivity towards Hg2+ ions and showed a selective fluorescence quenching (107-fold). The sulfonamide group coordinates to Hg2+ ions that direct to an aggregation of such complex via the face-to-face stacking of the spirobifluorene cores. At neutral pH, the sulfonamide group coordinates with Hg2+ ions and could promote deprotonation of the –NH group in S2a–b. The detection limits of S2a–b with Hg2+ ions were found to be 10.4 nM and 103.8 nM for the derivatives bearing two and four sulfonamide groups, respectively (Fig. 9).
Li et al. reported [22] a novel fluorescence sensor S3 for selective and sensitive detection for Hg2+ and CN− ions in DMF-H2O (13:12, v/v). Sensor S3 shows a nonemissive spectrum in a pure organic solvent (DMF) and low concentrated aqueous system. When the water content is increased to 60%, the fluorescence emission band at 650 nm increased significantly, and in 80% water the emission intensity band reached its maximum (120 fold). A slight decrease in fluorescence intensity was observed when adding further water in S3. This is due to the formation of amorphous aggregates. The emission intensity of S3 gradually inclined and reaches the maximum along with a small bathochromic shift (50 nm) upon the addition of Hg2+ ions. Under a UV lamp (360 nm), the S3-Hg2+ complex results in a strong red fluorescence, and the calculated detection limit to be 6.6 nM. The enhancement of fluorescence is due to the aggregation induced by the coordination of thymine units with Hg2+ ions (Fig. 10).
A novel aggregation-induced emission-based sensor S4 designed [23], synthesized, and reported for selective recognition of Hg2+ ions in a mixture of CH3CN: H2O (60%). Sensor S4 was further used to quantitatively measure the bioaccumulation of Hg2+ within a small invertebrate, D. carinata (Fig. 11). When exciting sensor S4 at 350 nm, the emission intensity increased from 0.17 to 1038.6 upon the gradual addition of Hg2+ ions (6100-fold). D. carinata alone shows no fluorescence signals and when incubated in S4 showed blue fluorescence (460–500 nm). In the presence of Hg2+ ions, D. carinata-S4 showed red fluorescence in the red channel in the 570 to 610-nm wavelength range. The fluorescent microscopy studies recognize in vivo dispersion and distribution of Hg2+ in D. carinata.
X. Han and research group [24] designed a gold (I) complex sensor S5 and reported for Hg2+ ion detection in CH3CN-H2O (1∶1, V∶V) solution. Usually, gold (I) complexes show unique optical properties because of their intermolecular gold–gold interactions. Sensor S5 reveals an aggregate-induced emission (AIE) in CH3CN-H2O mixtures and exhibits a high selectivity towards Hg2+ ions. In a pure CH3CN sensor, S5 exhibits almost no emission. When increasing the percentage of water (60%) in sensor S5, CH3CN: H2O, 40:60) results in a fluorescence enhancement band at 572 nm with a hypochromatic shift to 536 nm. The increasing percentage of water endorses the aggregation of sensor S5 and changes its form from a well-dispersed state to an aggregated state, which induces the emission (Fig. 12). The addition of Hg2+ ions into S5 exhibited an effective fluorescence quenching over the other metal ions such as Al3+, Ca2+, Cd2+, Cu2+, Fe2+, K+, Li+, Mn2+, Na+, Ni2+, Pb2+, Ag+, Zn2+, and Fe3+ ions. Exciting S5 at 340 nm exhibited a strong fluorescence emission at 575 nm in the presence of Hg2+ ions.
A novel 8-hydroxyquinoline functionalized pillar[5]arene sensor S6 was synthesized and the sensor selectively recognizes toxic Hg2+ ions based on the AIE fluorescence mechanism in an aqueous solution [25]. Sensor S6 almost shows non-fluorescence in a pure organic system (DMF). On increasing the water content, S6 displays strong fluorescence emission intensity at 410 nm (7.88-fold) due to aggregation of fluorescence. Upon the addition of various metal ions into S6, Hg2+ ions could significantly quench the fluorescence over other ions at 410 nm. Under a UV lamp, the fluorescence color of S6 (20% H2O) changed from blue to colorless upon the addition of Hg2+ ions (Fig. 13). The detection limit of S6 towards Hg2+ ions was calculated to be 0.24 nM. Further, based on the above results, the Hg2+ detection test kit was prepared by using an S6 sensor on a silica gel plate and the test kit could detect Hg2+ ions more conveniently and effectively.
Cyanostilbene derivative fluorescent sensor S7 was developed for selective and sensitive detection of Hg2+ ions in THF/H2O (2:8, v/v) medium [26]. Sensor S7 shows three absorption bands at 245, 291, and 365 nm. Sensor S7 has a longer π-conjugated unit and showed a blue-shift band due to a more twisted conformation caused by the cyano and vinyl groups (Fig. 14). When S7 dispersed in its “solution” state (THF), sensor S7 showed a non-emissive property. Upon the addition of water (80%), THF had a rigorous band at 537 nm and the fluorescence intensity enhanced by 79 times compared to the emission spectrum in pure THF and THF with a small fraction of water. These results correlated well with the fact that more aggregates were formed in lesser solvents. The enhanced fluorescence of S7 (THF-H2O) can be quenched linearly upon interacting with Hg2+ ions. The detection limit of Hg2+ is 37 nM.
Sensor S8 was prepared by condensation of 1,4- dimethylquinolin-1-ium iodide with 4-(1,2,2-triphenylvinyl) benzaldehyde under a refluxed condition in ethanol [27]. Highly fluorescent sensor S8 was further applied for the detection of Hg2+ ions in an aqueous medium. Sensor S8 is based on a tetraphenylethene functionalized quinolinium salts with hexafluorophosphate (PF6−) as the counterion and exhibits non-fluorescence properties in pure DMSO solution. When increasing the water fraction from 0 to 80% into S8, it exhibits a very low-level emission intensity spectrum. Above 80% of water in DMSO, the emission band at 610 nm enhanced sharply, and reaches a maximum (13-fold) when compared to pure DMSO solution. Meanwhile, the colorless sensor S8 solution is converted into strong red emission color under the UV light at 365 nm (Fig. 15). The observed fluorescence changes could be recognized as the formation of molecular aggregates, which suppresses the non-radiative relaxation channels. Upon the addition of I– fluorescence, emission of S8 started quenching. The quenching of fluorescence is due to synergetic electrostatic interaction and drastic collision between aggregates of S8 and I–. Fluorescence “Turn-On” was observed when S8- I– complex interact with Hg2+ ions and the detection limit for Hg2+ is as low as 71.8 nM.
Fluorescence sensor S9 has been prepared by reaction of tetraphenylethene containing ketone with 1, 2-ethanedithiol [28] and reported for detection of Hg2+ ions THF/H2O mixtures. In THF solution state sensor S9 shows a nearly nonemissive property. Upon the addition of water, the aggregates of sensor S9AIEgens were formed and the fluorescence emission intensity of AIEgens increased promptly when a large amount of water was added (> 80%) into sensor S9. Sensor S9 emitted sky blue luminescence due to the breaking of the conjugated system and the changing of the intramolecular charge transfer (ICT) efficiency upon excitation at the aggregation state. Upon the addition of Hg2 + ions, the absorption peak at 316 nm redshifted to 338 nm. When introducing mercury ions into S9, the luminescent color change from sky blue to yellow-green could happen instantly over the other metal ions. The detection limit of S9 towards Hg2+ ions was calculated to be 10 μM (Fig. 16).
Tetraarylethylenes with metal chelating 1,1-bis(2-pyridylethylene) fragments and thiophene/bithiophene substituents-based sensor S10 have been prepared by Gabr and Pigge [29]. Sensor S10 acts as an AIE active fluorescent sensor for Hg2+ ions detection. In a pure CH3CN sensor, S10 revealed a weak fluorescence emission at 512 nm. The addition of H2O into S10 initially shows a slight red-shifted emission band followed by a decrease in emission intensity at 520 nm and the appearance of a new blue-shifted emission band at 404 nm. Further increasing the water fraction in S10 (9:1 H2O: CH3CN), the enhanced emission intensity band was observed at 404 nm. The sensors exhibit red-shifted and enhanced emission in the presence of Hg2+ in an aqueous solution. The limit of detection for Hg2+ was determined to be 48 nM (Fig. 17).
A dual-emission ratiometric fluorescent sensor S11 based on AIE organic fluorescence nanoparticles and Au nanoclusters for detection of Hg2+ ion has been reported by Niu and coworkers [30]. When sensor S11 is in organic medium (THF), the twisted conformation makes it easy to rotate and vibrate, and the enhanced non-radiative transitions decrease its fluorescence emission intensities. Further increasing the concentration of H2O (80%), the intramolecular rotations are restricted to some extent, so its fluorescence intensities are stronger. In 90% of H2O, the face-to-face interactions, nonradiative transitions, and intramolecular rotation are all circumventing, thus its enhanced fluorescence emission intensity (blue shift). With increasing the concentrations of Hg2+ ions, the sensor displays continuous color changing from red to yellow to green and exhibits significant fluorescence quenching (Fig. 18).
A series of novel pyridopyrazine derivatives-based sensor S12a–d were synthesized and developed for selective and sensitive detection for Hg2+ ions in the H2O-CH3CN mixture [31]. Sensor S12a showed highly twisted conformation with no π-π stacking interactions. All the sensors S12a–d showed a weak fluorescence emission in CH3CN solution. In higher water fractions, S12a–b displayed a strong enhancement of emission intensity with a slight red shift in emission maxima. Pyridopyrazine derivatives of sensors S12a–b bearing electron-withdrawing biphenyl rings showed “turn-on”, whereas S12c–d bearing electron-donating biphenyl rings showed “turn-off” fluorescent response towards Hg2+ ions in aqueous media (Fig. 19). The detection limits of probes S12a–d towards Hg2+ were found to be in the submicromolar range. The other competitive metal ions such as Na+, K+, Mg2+, Ca2+, Ba2+, Cr3+, Fe3+, Fe2+, Co2+, Ni2+, Cu2+, Ag+, Zn2+, Cd2+, Al3+ and Pb2+ did not show a fluorescence response with sensors S12a–d.
Schiff base type-two novel fluorogenic sensors S13a–b containing AIE luminogen have been reported for Hg2+ ions in aqueous media [32]. In pure THF solution, sensor S13a showed almost non-fluorescence properties and S13b displayed weak emission intensity. When the water fraction increased from 0 to 50%, both sensors displayed a bathochromic shift. Upon the addition of excessive water (99%), the fluorescence intensity of S13a–b increased dramatically due to its aggregated nature. Upon increasing the concentration of water, sensors S13a–b could aggregate to form nanoparticles and be dispersed in water because they possessed many hydrophobic aromatic rings. Due to the two strong electron-donating alkoxy groups and longer conjugation lengths, the quantum yield of S13b was higher than that of S13a because of the stronger internal charge transfer effect. Once encountered the Hg2+ ions, the maximum fluorescence emission intensity of S13a–b gradually decreased significantly (Fig. 20).
Cephalexin molecular assembly-based fluorescence sensor S14 prepared by Pradeep Kumar Singh et al. and reported for Hg2+ ion sensor [33]. An emission study shows that cephalexin has very feeble fluorescence properties while after laser treatment, a strong blue-colored fluorescence due to self-assembly fluorescence. The fluorescent assembly is shown to detect very low concentrations of Hg2+ ions in an aqueous solution due to the presence of a negative sulfur molecule binding site. Upon UV irradiation, the weak interaction between α-cyclodextrin and cis-azobenzene may drive some of the α-Cyclodextrin to slide onto the alkyl chain, and thus the self-organization of azo complexes with α-cyclodextrin could form different self-assembled aggregates. Sensor S14 was applied for Hg2+ ions detection in an aqueous medium due to its affinity toward negative sulphur molecular binding site in sensor and shows fluorescence quenching (Fig. 21).
Rod-coil cyanostilbene amphiphile sensor S15 was prepared by the Knoevenagel condensation method and reported for Hg2+ ions detection in an aqueous medium [34]. Applying two trifluoromethyl groups on the rod segment of S15 is predictable to increase its hydrophobicity, which shows a strong tendency to form supramolecular assemblies with prominent aggregation-induced emission behaviors. Under UV light irradiation (365 nm), a typical orange emission signal can be directly visualized for S15a towards Hg2+, which is distinct from green emission signals for other metal ion species. The sensor system S15 shows an excellent selectivity toward Hg2+ ions over other metal ions. The detection limit for the sensor S15 towards Hg2+ ions is determined to be 0.11 μM (Fig. 22).
Aggregation-induced emission (AIE) active linear conjugated Schiff base and containing α-cyanostilbene unit sensor S16 was reported for Hg2+ ions detection [35]. In THF solvent, sensor S16 was well dispersed and displayed structured absorption spectra and almost non-fluorescence emission. In the aggregation state, the twisted conformation with larger torsion angles between the benzene rings avoiding strong π-π stacking interactions as well as their excimer formation When increasing the percentage of water the absorbance gradually decreased and the maximal absorption wavelength blue-shifted slightly from 390 to 375 nm and an absorption peak appeared at 302 nm. These spectral changes are due to aggregating in the sensor phase by gradually increasing the water fraction. Up to 30% of the water sensor exhibits weak fluorescence intensity and the fluorescence intensity was gradually enhanced and reached its maximum at 40% of water fraction. When increasing the percentage of water fraction, the fluorescence emission intensity gradually decreases. Once triggered Hg2+ ions into S16 significantly the fluorescence turn-on behavior was observed and the calculated detection limit was to be 3.4 nM (Fig. 23).
An anthracene-based fluorescent sensor S17 exhibited novel AIE characteristics in H2O-THF mixtures at high water content and reported for Hg2+ ions [36]. In THF solution, S17 exhibits a weak fluorescence emission at 498 nm (Φ = 0.002). It was observed that aggregation switched on in mixed aqueous media (THF/H2O) by varying the volume of water percentages gradually. Increasing the volume of H2O fractions up to 70% in the binary solvent mixture (THF/H2O) at 518 nm shows enhanced fluorescence and simultaneously the colorless non-fluorescent solution changed to the strong fluorescent green color solution. A strong fluorescence quenching of S17 was observed in the presence of Hg2+ ions via a complex interplay through the ground state complexation between S17 and Hg2+ ions and external heavy atom induced perturbation by Hg2+ ions to the excited states of the S17 (Fig. 24).
Intramolecular charge transfer (ICT) based Hg2+ ions detection
Intramolecular charge transfer (ICT)-based molecules require both electron-donating and electron-accepting groups conjugated into one molecule that gives rise to a ‘push–pull’ π-electron system in the excited state. ICT mechanisms have been widely used for cation sensing. Intramolecular charge transfer (ICT) involves an excited molecule and a neighboring molecule; one serves as an acceptor and the other as a donor molecule, involving charge redistribution in the excited molecule which produces a very large excited-state dipole moment. Upon excitation of the fluorophore, redistribution of electron density occurs so that a substantial dipole is created, resulting in intramolecular charge transfer from the donor to the acceptor. The electron donor (D) group interacts with an analyte, reducing the ICT process due to decreased electron-donating capacity, which leads to a blue shift in the absorption spectrum. In contrast, when analytes bind with the electron acceptor (A) group, an apparent red shift is observed in the absorption spectrum due to the increased ICT process [37]. Most of the fluorescent molecules are derived from the ICT mechanism by changing either the π-conjugation degree, electron-donating, or electron-withdrawing ability to interact with the target analyte (Fig. 25).
Thioacetal modified pyrene-based fluorescent sensor S18 was reported for selective and sensitive recognition of Hg2+ ions in the aqueous medium over the other ions [38]. The emission spectra of S18 are recorded in ethanol/PBS (2:1, v/v) solution and exhibits a strong fluorescence emission intensity centered at 457 nm (Φ = 0.33). The fluorescence emission of S18 (red shift) is due to the intramolecular charge transfer (ICT) mechanism in polar solvents. Upon the addition of Hg2+ ions into S18, the fluorescence intensity at 457 nm increased gradually (100-fold). The limit of detection of S18 towards Hg2+ ions is determined to be 1.49 nM. The 1H NMR titrations are confirmed the thioacetal moiety in S18 can be converted to an aldehyde group upon the addition of Hg2+ ions (Fig. 26).
A novel acenaphtoquinoxaline-based fluorescent sensor S19 was successfully synthesized and act as a selective fluorescent sensor for Hg2+ ions in acetonitrile [39]. In the presence of various metal ions such as Ba2+, Ca2+, Cd2+, Cs+, Co2+, Cr3+, Fe3+, Fe2+, Hg2+, K+, Li+, Mg2+, Mn2+, Na+, Ni2+, Sr2+, Hg2+ and Zn2+ into S19, an enhanced fluorescence emission intensity (red shift) is observed at 520 nm only in the presence of Hg2+ ions over other ions. The detection limit was as low as 42 ppb. The binding stoichiometry between S19 and Hg2+ ions was found to be 1:1. The sensing mechanism of Hg2+ ions towards S19 through the intramolecular charge transfer was investigated by DFT calculations. From the results, the sensor S19 can be considered as a highly selective and reliable chemosensor for Hg2+ ion detection (Fig. 27).
A novel fluorescent sensor S20, which contained a conjugated dicyanomethylene-benzopyran structure as fluorophore and dithia-dioxa-monoaza crown ether moiety as the receptor was developed and reported for Hg2+ ions detection [40]. The sensor S20 showed an excellent selectivity and sensitivity towards Hg2+ ions with no significant interference from other competitive metal ions and anions. Sensor S20 showed strong fluorescence emission intensity at 645 nm. When an Hg2+ ion was added, a strong fluorescence quenching was observed. The quenching of fluorescence is due to the complexation of Hg2+ with dithia-dioxa-monoaza crown and weakened electron-donating ability of the recognition site, which resulted in the blocking of the intermolecular charge transfer (ICT) process. The fluorescence sensor S20 was successfully used in real aqueous samples and fluorescent imaging for Hg2+ in living cells and zebrafish larvae with low cytotoxicity (Fig. 28).
A novel bithiophene-based fluorescent sensor S21 was designed and developed for mercury ions detection in an aqueous medium [41]. A sequence of solutions with different ratios between ethanol and water with increasing water content (0 ~ 100%) were selected for test and finally found 100% aqueous solution is suitable for sensing action. Sensor S21 showed a maximal absorption band at 334 nm, attributed to the absorption of bithiophene moiety. Once trigged with Hg2+ ions into S21, the appearance of a new absorbance band at 370 nm with a large red shift (36 nm). Enhanced fluorescence emission (Φ = 0.460) at 470 nm was observed in the presence of Hg2+ ions and an instant fluorescence color change from colorless to blue was also noted. The distinctive fluorescent enhancement signal could be recognized to the formation of new species via Hg2+ ions promoted dethioacetalization on S21, which induced the intramolecular charge transfer (ICT) process from bithiophene moiety to aldehyde group turned on. The detection limit of S21 towards Hg2+ was calculated to be 19 nM (Fig. 29).
A simple and novel coumarin-based fluorescence sensor S22 (7-(propargylamino)-4-methyl-2H-chromene-2-one) was designed and reported by Duan et. al., [42] for the selective and sensitive detection of Hg2+ ions in an aqueous solution. When excited at 350 nm, sensor S22 displayed the greatest emission peak at 450 nm. This is due to the electrons diverted from 7-amino-4-methylcoumarin to alkynyl being blocked and the ICT process was rancid. Upon the addition of Hg2+ ions into sensor S22, the alkynyl group would be converted to keto, a comparatively strong electron-withdrawing part, which resulted in enhancement of the ICT process, and quenched the fluorescence. The fluorescent color of S22 solutions changed from blue-green to blue under the UV (365 nm) light. Further, the sensor S22 was successfully applied for the Hg2+ determinations in water samples with satisfying recovery and on agar gels (Fig. 30).
The intramolecular charge transfer based on a novel phenothiazine fluorescent sensor S23 was designed and synthesized by simple methods [43]. Absorption spectrum sensor S23 displayed an absorption band at 310 nm and a clear red-shifted band was observed at 390 nm upon the addition of Hg2+ ions. The color of sensor S23 changed drastically from colorless to yellow (deprotection reaction) after adding the Hg2+ ions. When excited at 390 nm, there is an obvious fluorescence emission band at 610 nm (Φ = 0.115) exhibited upon interaction with Hg2+ ions into sensor S23. The fluorescence sensor S23 detects Hg2+ ions based on a deprotection reaction. In this sensor, 10-ethylphenothiazine acted as an electron donor, and 2- (demethylation) -methine was acting as a weak electron donor to form an electron donor. The donor system prevents the intra-molecular charge transfer process (ICT). In the presence of Hg2+ ions, an electron-deficient aldehyde group was formed and the aldehyde group was used as the electron acceptor 10-ethylphenothiazine (Fig. 31). The detection limit was calculated to be 0.212 nM.
A novel oligothiophene-based fluorescence sensor S24 acts as a colorimetric and ratiometric fluorescent sensor for Hg2+ ions based on intramolecular charge transfer (ICT) mechanism [44] in EtOH/H2O (1:1, v/v) solution. Sensor S24 exhibited a maximal absorption band at 360 nm (oligothiophene moiety). Upon the addition of Hg2+ ions into S24, the band at 360 nm gradually decreased and a new red-shift absorption band is formed at 400 nm along with the solution color change from colorless to pale yellow. In emission spectrum, sensor S24 showed a blue emission band centered at 448 nm and the fluorescence enhancement intensity at 552 nm (turn-on) and a significant decrease of fluorescence intensity at 448 nm in the presence of Hg2+ ions. Under a UV lamp at 365 nm, the sensor S24 solution color changed from blue to bright green. The large red shift (104 nm) and the fluorescent enhancement signals in S24 are due to electron-rich dithioacetal moiety, which could be removed by Hg2+ ions to release the electron-deficient aldehyde group, and produced a strong push–pull electronic system, leading to the ICT process from oligothiophene moiety to aldehyde group being turned on (Fig. 32).
Photo-induced electron transfer (PET)-based Hg2+ ions detection
The photo-induced electron transfer mechanism is a deactivation process involving an internal redox reaction between the excited state of the fluorophore and another species able to donate or accept an electron. A fundamental point explaining this process is to consider that in the excited state the properties of the species are quite different compared with those of the ground state. In particular, due to its higher energy content, an excited state is both a stronger reducing and oxidant than the corresponding ground state. Generally, in fluorescent metal sensors, PET takes place from a lone pair of the coordinating atoms (e.g., N, O, S, P) to the HOMO of the excited fluorophore. The presence of a coordinated metal ion lowers the energy of the lone pair involved in the coordination preventing the PET, thus causing the switch-ON of the fluorescence. PET strongly depends on the solvent polarity, which affects the oxidation potential of the lone pairs of the coordinating moiety. Higher solvent polarities make the electron transfer easier; as a consequence, the PET-mediated quenching effect of the fluorescence occurs more quickly in high-polar environments [45]. The PET-type fluorescent response does not cause any spectroscopic shifts in the emission band upon the complexation of metal ions (Fig. 33).
A novel 7-nitrobenzo-2-oxa-1, 3-diazolyl-based fluorescence sensor S25 containing a piperazine derivative was synthesized and developed for Hg2+ ions detection in a 100% aqueous medium [46]. In UV–Vis spectrum sensor S25 shows a strong band at 495 nm due to the ICT process from the anilino group to the strong electron-withdrawing nitro group of sensor S25. Upon the gradual addition of Hg2+ ions, the ICT band at 495 nm shows a gradual increase in the absorption intensity with a blue shift. In the emission spectrum, sensor S25 showed a very weak emission (Φ = 0.011) band centered about 543 nm when excited at 495 nm. This is due to an efficient PET process from the piperazine nitrogen atom to the photo-excited sensor S25 fluorophore. The sensor S25 displays a significant fluorescence enhancement (Φ = 0.14) toward Hg2+ ions through blocking of the photo-induced electron transfer process, which selectively senses Hg2+ ions as low as 19.2 nM (Fig. 34).
Borondipyrromethane (BODIPY)-based fluorescence sensor S26 has been designed and developed for selective and sensitive detection of mercury ions [47]. The free sensor S26 induces a weak emission band centered at 510 nm when excited at 496 nm (Ф = 0.048). The weak emission is probably due to the efficient fluorescence quenching induced by the photo-induced electron transfer mechanism (PET) from the electron-donating amine moiety to the BODIPY framework. A strong new emission band at 514 nm with an enhancement in fluorescence intensity has appeared in the presence of Hg2+ ions into sensor S26. The fluorescence turn-on is due to the blocking of PET using Hg2+ ions coordination through the ether-O, pyridine-N, and amine-N of the sensor S26 (Fig. 35).
Calix[6]arene-based fluorescence sensor S27 has been synthesized by using p-tert-butylcalix[6]arene is functionalized with 5-methylfurfural and reported for a Hg2+ ions detection [48]. The sensor S27 shows an absorption band at 303 nm. Upon the addition of Hg2+ ions into sensor S27, a new band near 326 nm with a bathochromic shift in an aqueous medium. The sensor S27 exhibited a very weak emission band at 345 nm and this band is due to the photoinduced electron transfer (PET) mechanism. When adding Hg2+ ions into S27, significant fluorescence enhancement with bathochromic shifts was observed near 425 and 545 nm. The other ions such as K+, Be2+, Mg2+, Ca2+, Sr2+, Co2+, Cu2+, Zn2+, Cd2+, Hg2+, Pb2+, Al3+, Sb3+, Eu3+, Gd3+, Th4+, W4+ and U4+ are not interference with this sensor action. The binding constant of sensor S27 with Hg2+ ions is 3.382 × 106 M−1 with a 1:1 binding mode (Fig. 36).
A novel fluorescein-based fluorescence sensor S28 was reported for highly selective and sensitivity towards Hg2+ ions in an aqueous medium [49]. The spectrum studies of sensor S28 were studied in an aqueous solution (20 mM HEPES buffer, pH = 7.4). Sensor S28 exhibited a weak fluorescence emission band of fluorescein at 529 nm when excited at 470 nm. Various metal ions such as Mn2+, Fe2+, Fe3+,Co2+, Cr3+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+, Pb2+, Ag+, Mg2+, Ca2+, K+, Na+, La3+, Eu3+, and Er3+were added to sensor S28, and only Hg2+ ions show a significant enhancement of fluorescence with the emission maximum (green color emission) at 539 nm (51-fold). The detection limit of Hg2+ was measured to be 22.06 ppb. The green fluorescence of the cells is similar to that of S28-Hg2+ in solution, which indicates that sensor S28-Hg2+ is membrane permeable (Fig. 37).
A novel pyridyl-based fluorescence sensor S29 has been prepared by using a mixture of 2,6-diaminopyridine and di-2-pyridyl ketone in ethanol [50]. The sensing ability of sensor S29 in the presence of various cations such as Na+, Ag+, Ca2+, Ba2+, Cu2+, Cr3+, Fe2+, Fe3+, Co2+, Hg2+, Cd2+, Zn2+, Ni2+ and Pb2+ were studied in methanol/water solution (4:1, v/v) pH = 7.51. Sensor S29 alone displayed two main absorption bands at 243 and 310 nm, and small absorption bands at 268 and 338 nm, which can be attributed to π–π* and n-π* transitions from the imine group. Upon the addition of Hg2+ ions into sensor S29, a bathochromic shift at 310 nm and an increase in the absorbance at 338 nm was observed due to the donor and acceptor system in the sensor S29, resulting in enhanced intramolecular charge transfer (ICT). The Hg2+ ions bind with sensor S29, leading to the disruption of PET, and affects decay processes of the excited states in the systems with unbound lone pair of an electron in the vicinity of the fluorophore. Enhanced emission intensity was prominent in the presence of Hg2+ ions with sensor S29 since a non-radioactive decay of the excited state was inhibited (Fig. 38).
The naphthalimide-based fluorescence solid sensor S30 has been developed by inserting N-(2-hydroxyethyl)-4-(4-(1Hbenzo[d] imidazol-2-yl) methyl) piperazine-1-yl)-1,8-naphthalimide to a photocrosslinked membrane reaction through the acid chloride groups [51]. The sensor S30 was reported for selective and sensitive detection of mercury ions over other interference metal ions. Upon the addition of Hg2+ ions into S30, the effective fluorescence intensities were significantly enhanced caused by Hg2+ ions interaction and the formation of a sensor S30-Hg complex. The resulting fluorescence sensor S30 undergoes fluorescence enhancement upon binding Hg2+ ions which aggravates a photo-induced electron transfer (PET) inhibition process from the piperazine to the naphthalimide moiety.
The limit of detection was calculated to be 0.73 nM (Fig. 39).
Another naphthalimide-based fluorescence sensor, S31, has been synthesized and reported for Hg2+ ions in aqueous solution selectively and sensitively [52]. The emission properties of the sensor S31 were evaluated in an aqueous solution (20 mM HEPES buffer, pH = 7.4). Sensor S31 exhibited an emission band at 550 nm (4-amino-1, 8-naphthalimide) with a weak green color emission. The addition of Hg2+ ions to sensor S31, the iminodiacetic acid, and picolinic acid as a metal chelating group caused a remarkable fluorescence enhancement (25-fold) at 550 nm. This fluorescence change is due to the energy level of the iminodiacetic acid and picolinic acid moiety being lower than that of the HOMO of the excited 4-amino-1, 8-naphthalimide, the electron transfer is not energetically favored, so the fluorescence is “switched on”. The binding constant of sensor S31 with Hg2+ ions was measured to be 1.46 × 108 M−1 with 1:1 binding mode (Fig. 40).
A novel Hg2+ ion sensor S32 based on 7-nitrobenzo-2-oxa-1,3-diazolyl fluorophore connected with thiophene ionophore was prepared and reported by Kraithong and coworkers [53]. Fluorescence sensor S32 showed a high selectivity toward Hg2+ ions in aqueous acetonitrile solutions and the color change of sensor S32 changed from orange to purple. Sensor S32 exhibited weak fluorescence at 587 nm (λex = 520 nm), which could be attributed to the presence of the thiophene group, which led to fluorescent quenching via photoinduced electron transfer (PET) from a sulfur atom of the thiophene moiety. When increasing the concentration of Hg2+ ions into S32, the fluorescence enhancement (50-fold) was observed at 587 nm. This fluorescence turn “OFF–ON” of sensor S32 was caused by the interaction between a sulfur atom of the thiophene ionophore and Hg2+ ions, which led to inhibition of the PET process upon binding of Hg2+ ions (Fig. 41).
Thiocarbohydrazide-based Schiff base fluorescence sensor S33 act as a selective colorimetric and fluorescent sensor for Hg2+ ions over other interference ions such as Al3+, Ag+, Ba2+, Ca2+, Cd2+, Co2+, Cu2+, Cr3+, Fe3+, K+, Mg2+, Mn2+, Na+, Ni2+, Pb2+, Hg2+, Zn2+, Th4+, and Bi3+ ions [54]. Sensor S33 consists of an electron donor triphenylamine center and electron acceptor thiourea unit. The interaction of S33 with Hg2+ ions in CH3CN: H2O (6:4, v/v) results in a color change from colorless to yellow. In absorption studies, a new peak appeared at 386 nm (red shift) upon interaction with Hg2+ ions into S33. The sensor S33 shows an emission band at 485 nm upon excitation at 375 nm and the band was found to be quenched completely when interacting with Hg2+ ions. These changes may be due to combined chelation enhanced quenching (CHEQ) and a strong photo-induced electron transfer (PET) mechanism. The limit of detection for Hg2+ ions was found to be 1.26 nM (Fig. 42).
Pyrenylthioureayl alanine-based fluorescence sensor S34 was prepared by a simple condensation reaction of 3-amino pyrene with isothiocyanyl alanine in CH3CN: DCM (1:3) solvent at 50 °C and reported for the detection of the Hg2+ ions [55]. Upon the addition of various metal ions such as Mg2+, Mn2+, Fe3+, Co2+, Ni2+, Zn2+, Ag+, Cu+, Cd2+, Pb2+, Hg2+, and Cu2+ ions to the sensor S34, a slight blue shift of pyrenyl absorption bands along with a new band at 359 nm was observed only in the presence of Hg2+ ions. The enhancement of emission intensity was found in the presence of S34-Hg2+ ions upon excitation at 342 nm. The detection limit is found to be 93 nM and a 1:2 metal–ligand complexation possibly via the coordination with the S atom of the thiourea unit (Fig. 43).
Mercaptosuccinic acid capped CdTe/ZnS core/shell quantum dots S35 have been synthesized and reported for selective detection of Hg2+ ions in an aqueous medium [56]. Quantum dots S35 having a shell material with a wider band gap than that of the core material causes the improvement of the confinement of electrons and holes in the low band gap core. Upon the addition of Hg2+ ions to sensor S35 (λex = 400 nm), a significant fluorescence quenching was observed and this intensity decreases due to the photoinduced electron transfer (PET) between sensor S35 and Hg2+ ions. The detection limit of sensor S35 with Hg2+ ions was calculated to be 1 pM, which is remarkably less than the tolerance limit of mercury. The real-time analysis was further carried out with drinking water and tap water solutions and the sensor S35 show remarkably good quenching in these solutions (Fig. 44).
A novel fluorescent sensor S36 based on a metal–organic framework/DNA hybrid system was developed and reported for Hg2+ ions detection in an aqueous medium [57]. The fluorescence intensity decreased rapidly along with the UiO-66-NH2 concentration ranging from 0–0.15 µg µl−1. In the presence of Hg2+ ions, UiO-66-NH2 showed a fluorescence enhancement band at 518 nm in Tris–HCl buffer (pH 7.4) medium. The detection limit was calculated to be 17.6 nM. The other metal ions such as Ca2+, Cd2+, Co2+, Cu2+, Fe2+, Fe3+, Mg2+, Mn2+, Ni2+, and Pb2+ only showed a slight response and indicated that the sensor possesses an excellent selective signal towards Hg2+ with respect to other metal ions (Fig. 45).
The novel fluorescent sensor S37 was constructed by polyaniline nanoclips (PANCs) embedded with FAM-ssDNA and reported for sensitive and selective detection of Hg2+ ions [58]. The FAM-ssDNA has an absorption band at 495 nm (n-π* electronic transition). When polyaniline nanoclip concentration is increased, the absorption band became saturated due to the intermolecular interaction. Upon the addition of Hg2+ ions, it leads to retaining the absorption band due to the dissociation of FAM-ssDNA. FAM-ssDNA has shown an emission peak with high emission intensity and in the presence of polyaniline nanoclips the fluorescence intensity effectively turns off due to photo-induced electron transfer (PET). Upon the addition of Hg2+ ions into the sensor S37, Hg-induced nucleobases of FAM-ssDNA and interacted with Hg2+ via hydrogen bonds. The LOD was calculated to be 4 nM (Fig. 46).
Bhatti and coworkers reported a water-soluble p-sulphonatocalix[4]arene appended with rhodamine fluorescence sensor S38 for selective detection of Hg2+ ions over the other metal ions such as Pb2+, Cu2+, Zn2+, Cr3+, Ni2+, Co2+, Al3+, Cd2+, and Fe2+ ions [59]. Upon the incremental addition of Hg2+ ions into sensor S38, a distinct increase in fluorescence intensity was observed. This effect can be explained based on the electron transfer process, whereas sensor S38 is functionalized with N(CH2CH2NH2)3 structure, which possesses nonbonding electrons and PET process overcome due to intermolecular oxidation process. Upon addition of Hg2+ ions, the intramolecular PET fluorescence quenching effect derived from the electron pairs of N donor atoms was fully blocked and relieved by reducing the electronic density of lone pairs through metal–donor binding interaction and consequently increases the sensor emission. The detection limit of sensor S38-Hg2+ was found to be 3.55 × 10–13 mol L−1 and the stoichiometry of complex to be 1:1 (Fig. 47).
Ring-opening mechanism-based Hg2+ ion detection
The fluorescence sensor S39 based on rhodamine immobilized electrospun chitosan nanofibrous material has been developed and reported for mercury ion detection [60]. In the presence of various competing ions such as Ni2+, Pd2+, Zn2+, Mg2+, Hg2+, Pb2+, Fe2+, Cu2+, Co2+, Cr2+, and Cd2+ ions, only the addition of Hg2+ ions shows an enhanced fluorescence intensity. This enhanced fluorescence is due to the opening of the spirolactam ring of the rhodamine unit followed by cyclization. Sensor S39 has a high surface area of nanofibers and provides a high number of functional groups. A divergent change in fluorescence emission from colorless to red-pink under 366-nm UV light was obtained for the sensor S39 in the presence of Hg2+ ion addition (Fig. 48).
A novel fluorescence sensor S40 based on 1,2,3-triazole and its rhodamine B derivative has been developed for the Hg2+ ions sensor [61]. The sensing behavior of sensor S40 towards various metal ions such as Pb2+, Mn2+, K+, Na+, Ag+, Ca2+, Cd2+, Co2+, Cu2+, Fe3+, Zn2+, Ni2+, Hg2+, Li+ and Mg2+ was studied in DMF/H2O (v/v = 1:1, Tris–HCl, pH = 7.4) solutions. Sensor S40 alone displayed a non-emissive spectrum, which indicated the rhodamine moiety in ring-closed spirolactam form. Upon the addition of the Hg2+ ion, an enhanced fluorescence emission band (4500-fold) at 584 nm (λex = 563 nm) was observed. Simultaneously, the color of sensor S40 solution changed from colorless to pink in the presence of Hg2+ ions. The enhanced fluorescence change is due to ring-opening from the spirolactam (S40) to a ring-opened amide. The fluorescence imaging of Hg2+ ions in HeLa cells was successfully applied in sensor S40 and demonstrates a good membrane-permeable reagent for biological imaging applications (Fig. 49).
Rhodamine-bearing cage-like silsesequioxanes-based fluorescence sensor S41 has been developed for Hg2+ ions in 10% aqueous ethanol solutions [62]. With the addition of increasing concentration of Hg2+ ions, a new absorption band significantly appeared at 555 nm at the same time. The colorless sensor S41 solution changed to pink color. When excited at 520 nm, the solution of sensor S41 showed a weak fluorescence signal (turn off). Upon the addition of Hg2+ ions, the intensity of the fluorescence emission was rapidly enhanced, and “turn on” fluorescence intensity was observed at 580 nm corresponding to the spirolactam ring opened-form of rhodamine fluorophores and the detection limit was found to be 0.63 ppb (Fig. 50).
Rhodamine 6G-based fluorescence sensor S42 has been synthesized by using rhodamine hydrazide, anhydrous triethylamine, and a solution of triphosgene in dichloromethane and applied for selective detection of Hg2+ ions in ethanol/water (1/1 v/v) solutions [63]. The fluorescence spectra of sensor S42 exhibited very weak fluorescence intensity at 561 nm, which is attributed to the colorless and non-fluorescent spirocyclic form of rhodamine. Among the addition of various metal ions, only the Hg2+ ions alone showed a high fluorescence enhancement band at 561 nm and the color of the sensor S42 solution changed from colorless to pink. Sensor S42 has an excellent specificity for Hg2+ ions and a very low detection limit of 1.3 ppb. Fluorescence bio imaging shows that sensor S42 has good cell membrane permeability and can be applied to monitor intracellular Hg2+ ions in living cells, animal tissues, and plant tissues (Fig. 51).
Three novel rhodamine fluorescence sensors S43a-c act as a selective colorimetric and fluorescence sensor for Hg2+ ions in Tris—HCl/C2H5OH (v: v = 3: 7, 10 mM, pH = 7.2) [64]. Colorless sensors S43a-c are turned to pink color once they interact with Hg2+ ions. Upon the incremental addition of Hg2+ ions into sensors, a strong fluorescence enhancement was observed at 576 nm (20-fold) and also noted that the colorless sensors solutions changed to orange-red under UV irradiation. This fluorescence enhancement is due to a ring-opening of the spirolactam unit after coordination with sensors S43a-c and the paramagnetic effect of the Hg2+ ions. The binding constants between sensors S43a-c and Hg2+ are 5.0 × 106 M−1, 1.8 × 106 M−1, 1.5 × 106 M−1, respectively. The other cations and anions such as Cd2+, Co2+, Cr3+, Cu2+, Mn2+, Ni2+, Zn2+, Pb2+, Fe3+, Ag+, Ba2+, Bi3+, Sr+, Na+, K+, Ca2+, Mg2+, H2PO4−, HPO42−, PO43−, SO42−, C2O42−, CO32−, ClO−, NO3−, NO2−, SCN−, Ac−, F−, Cl−, Br−, I−, CN−, HSO4− ions did not show any changes in naked-eye and fluorescence emission peaks. The limits of detections (LOD) were calculated to be 18, 16, and 56 nM, respectively (Fig. 52).
Xu and coworkers reported a simple fluorescence sensor S44 for Hg2+ ion detection [65]. The sensor S44 was prepared by reaction of rhodamine B hydrazide with 2-amino-4-ferrocenylthiazole and applied for molecular recognition studies with analytes. A significant absorbance band was noticed at 560 nm upon the addition of Hg2+ ions. No significant emission response was observed after the addition of other metal ions such as Mn2+, Co2+, Ni2+, Hg2+, Fe2+, Na+, Sr2+, Cu2+, K+, Ba2+, Ca2+, Mg2+, Zn2+, Al3+, Fe3+, Cd2+, and Cs+ ions. Sensor S44 shows a weak fluorescence emission band when exciting the sensor. Upon the addition of Hg2+ ions to sensor S44, a new fluorescence enhanced band positioned at 621 nm in addition to color change from colorless to pink was observed by the naked eye. This enhancement is mainly due to the spirolactam ring of rhodamine opening when Hg2+ ions coordinate with sensor S44. The detection limit was calculated to be as low as 0.53 μM with a 1:1 binding coordination ratio (Fig. 53).
Rhodamine 6G bearing [5]Helicene dye-based hybrid fluorescent sensor S45 was synthesized successfully [66] and reported for the detection of Hg2+ ions in HEPES buffer/methanol solution (1:1 v/v, 5 mM, pH 7.2). The color of sensor S45 solution was yellow and exhibited absorption peaks at 310 and 373 nm. Upon the addition of Hg2+ ions, the absorption band at 535 nm increased significantly and the yellow color S45 solution turned to pink. These changes are mainly due to the mercury-promoted spirolactam ring-opening behavior. Weak fluorescence intensity of sensor S45 was observed at 560 nm when exciting the solution at 373 nm. Upon the incremental addition of Hg2+ ions, a notable fluorescence enhancement (Φ = 0.53) was observed via the FRET process and displayed a non-fluorescent color to orange fluorescence. The FTIR studies revealed that Hg2+ bonded with the oxygen atoms of imine of [5]helicene-like imide and sulfur atom of rhodamine thioamide of sensor S45 (Fig. 54).
Rhodamine-based fluorescence sensor S46 was developed and synthesized for Hg2+ ions detection in THF/H2O (9:1, v/v) solution [67]. Sensor S46 displayed selective sensing of Hg2+ ions and showed a color change from colorless to pink color in solution state and red to pink color in solid-state. The sensor S46 displayed a very weak single fluorescence emission band at 400 nm. Upon addition of Hg2+ ions, sensor S46 exhibited a prominent fluorescence enhancement with a red shift band observed at 570 nm (16 fold). The addition of Hg2+ ions leads the spirocycle unit open via coordination, resulting in color change and the generation of a strong fluorescence. The association constant of the S46-Hg2+ complex was calculated to be 8.25 × 109 M−1, and the detection limit for Hg2+ ions was found to be 27 ppb (Fig. 55).
A novel rhodamine-6G-based fluorescence sensor S47 [68] acts as a highly selective fluorescence sensor for Hg2+ ions in HEPES buffer (10 mM, pH-7.4)/CH3CN (40:60, V/V). The colorless sensor S47 shows a dramatic yellow color and displayed a new absorption peak that appeared at 533 nm in the presence of Hg2+ ions. The other cations are not in interference with the color change as well as in spectral changes. When increasing the Hg2+ ion concentration, the fluorescence emission spectra of sensor S47 changed significantly, where the emission peak appeared at 560 nm. The enhancement may be due to the structure of sensor S47 transformation from spirolactam to the ring-opened amide form with adding Hg2+ ions. The practical application of sensor S47 towards Hg2+ ions was investigated in test strips and shows an obvious color change in strips (Fig. 56).
A simple sensor S48 based on p-tert-butylcalix[4]arene thiospirolactam rhodamine b acts as a fluorescence sensor for Hg2+ ions [69]. The sensing studies towards sensor S48 with metal ions such as Ag2+, Ba2+, Ca2+, Co2+, Cd2+, Cu2+, Fe2+, Fe3+, K+, Mn2+, Mo2+, Na+, Ni2+, Sn2+, Sr2+, Zn2+ and Hg2+ were investigated. Except for Hg2+, the other ions did not interfere in the molecular recognition studies, and this confirms the sensor S48 sense Hg2+ ions selectively and sensitively in ethanol–water (v/v 8/2, Tris–HCl 20 mM pH = 7.0). The colorless solution of sensor S48 changed to pink color in the presence of Hg2+ ions. When the concentration of Hg2+ ion increases, there is an appearance of a new peak at 562 nm with a slight red shift. The appearance of the new peak may be due to the thiospirolactam ring opening of rhodamine caused by the Hg2+ ion. Sensor S48 shows an emission maximum at 430 nm (0.079) due to the spirolactam structure of rhodamine moiety. In the presence of Hg2+ ions, the enhanced emission fluorescence intensity was observed at 582 nm (0.082) and this peak is responsible for spirolactam ring opening. Sensor S48 is successfully penetrated the HeLa cells and gives fluorescence quantification of Hg2+ ions under biological conditions (Fig. 57).
A novel rhodamine-based fluorescence sensor S49 was reported [70] for Hg2+ ions detection in CH3CN/HEPES buffer (1/99, v/v, pH = 7.05). Upon addition of various interference metal ions into sensor S49, only Hg2+ exhibited a new absorption band centered at 561 nm along with a clear color change from colorless to red. Further, the emission studies of sensor S49 towards Hg2+ ions were studied in an aqueous solution. Upon the addition of Hg2+ ions, a remarkable enhancement of the fluorescence intensity was noticed at 578 nm (170-fold) with an emerging brilliant orange fluorescence. These results indicated that Hg2+ ions induced the ring-opening of spirolactam in sensor S49 and sensor S49 might be a highly selective and sensitive colorimetric and fluorescent sensor for Hg2+. The detection limit of sensor S49 towards Hg2+ ions was calculated to be 0.14 μM (Fig. 58).
A novel non-sulfur rhodamine derivative with an ethylene moiety-based sensor S50 selectively and sensitively sense Hg2+ ions in acetonitrile [71]. The free sensor S50 is colorless in CH3CN, showing almost no absorption at approximately 450–650 nm. Upon the addition of tested metal ions, only Hg2+ leads to a color change to pink and exhibited a strong absorption band at 557 nm (Fig. 57). When exciting the sensor S50 at 525 nm, no significant fluorescence emission was detected between 535 and 650 nm. These interpretations indicate that the sensor S50 exists as a nonfluorescent spirocyclic form. The Hg2+ ion displays strong fluorescence enhancement (640-fold) with the maximum emission at 584 nm (Φ = 0.25). The fluorescence enhancement of S49-Hg2+ is due to the coordination of Hg2+ to the ethylene moiety and oxygen atom of the carbonyl group could induce the breaking of the C–N bond in the spirocyclic ring of sensor S50 (Fig. 59). The detection limit was determined to be 0.2 μM.
Conclusions
Mercury is one of the most toxic and heavy metal elements. Mercury contamination is extensive and occurs through various processes, e.g., volcanic emissions, mining, solid waste incineration, and in the combustion of fossil fuels. It is to be frightened that mercury-containing chemicals have been linked with a number of human health problems, including myocardial infarction, Minamata disease, damage to the brain, kidneys, and some kinds of autism, and damage to the sensory parts of the central nervous system, immune system, and endocrine system. The most common method used for the detection process of toxic and heavy Hg2+ ions is the chromogenic and fluorogenic reaction. The new development of fluorescence sensors that can monitor Hg2+ ions is very important. Fluorescent sensing is becoming a tool for molecular recognition due to its potential application in biological and environmental. Fluorescent sensors have their advantages in high sensitivity, selectivity, simplicity, low detection limit, and application to bioimaging.
This review is mainly focused on the recognition of toxic mercury ions in an aqueous medium without compromising the selectivity and sensitivity based on their sensing mechanism. However, the methods have some limitations, like low complex stability in complex matrix compounds. Conventional signaling mechanisms for the design of fluorescent chemosensors including PET, ICT, MLCT, and FRET have been widely investigated and successfully applied in a versatile range of fields during the last few years. For the past 5 years, some new mechanisms for designing fluorescence sensors have materialized to meet diverse design and application requirements. This review covers the general design principles for fluorescent sensors based on different photophysical mechanisms and recapitulates the recent advances in new mechanisms such as ring-opening and AIE for the past 5 years. On reading this tutorial review, it may seem to young researchers that all the great problems in chemosensors research have already been solved. The reported fluorescence sensor probes will help readers regarding which material should be chosen for the best design of interesting fluorescence probes with fascinating applications.
Data availability
All data underlying the results are available as part of the article and no additional source data are required.
References
Liu, S., Wang, Y.M., Han, J.: Fluorescent chemosensors for copper(II) ion: structure, mechanism and application. J. Photochem. Photobiol. C 32, 78–103 (2017). https://doi.org/10.1016/j.jphotochemrev.2017.06.002
Kwon, N., Hu, Y., Yoon, J.: Fluorescent chemosensors for various analytes including reactive oxygen species, biothiol, metal ions, and toxic gases. ACS Omega 3, 13731–13751 (2018). https://doi.org/10.1021/acsomega.8b01717
Wu, D., Sedgwick, A., Gunnlaugsson, T., Akkaya, E., Yoon, J., James, T.D.: Fluorescent chemosensors: the past, present and future. Chem. Soc. Rev. 46, 7105–7123 (2017). https://doi.org/10.1039/C7CS00240H
Bernhoft, R.A.: Mercury toxicity and treatment: a review of the literature. J. Environ. Public. Health. 2012, 460508 (2012). https://doi.org/10.1155/2012/460508
Saleh, T.A., Fadillah, G., Ciptawati, E., Khaled, M.: Analytical methods for mercury speciation, detection, and measurement in water, oil, and gas. Trends Anal. Chem. 132, 116016 (2020). https://doi.org/10.1016/j.trac.2020.116016
Berhanu, A.L., Gaurav, M.I., Malik, A., Aulakh, J., Kumar, V., Kim, K.-H.: A review of the applications of Schiff bases as optical chemical sensors. Trends Anal. Chem. 116, 74–91 (2019). https://doi.org/10.1016/j.trac.2019.04.025
Udhayakumari, D., Naha, S., Velmathi, S.: Colorimetric and fluorescent chemosensors for Cu2+. A comprehensive review from the years 2013–15. Anal. Methods. 9, 552–578 (2017). https://doi.org/10.1039/C6AY02416E
Udhayakumari, D., Velmathi, S.: Azo linked polycyclic aromatic hydrocarbons-based dual chemosensor for Cu2+ and Hg2+ ions. Ind. Eng. Chem. Res. 54, 3541–3547 (2015). https://doi.org/10.1021/acs.iecr.5b00775
Zu, Y.R., Li, H., Shi, B.B., Qu, W.J., Zhang, Y.M., Lin, Q., Yao, H., Wei, T.B.: A reversible fluorescent chemosensor for the rapid detection of mercury ions (ii) in water with high sensitivity and selectivity. RSC Adv. 4, 61320–61323 (2014). https://doi.org/10.1039/C4RA09961C
Samanta, T., Shunmugam, R.: Colorimetric and fluorometric probes for the optical detection of environmental Hg(II) and As(III) ions. Mater. Adv. 2, 64–95 (2021). https://doi.org/10.1039/D0MA00521E
Liu, C., Chen, X., Zong, B., Mao, S.: Recent advances in sensitive and rapid mercury determination with graphene-based sensors. J. Mater. Chem. A. 7, 6616–6630 (2019). https://doi.org/10.1039/C9TA01009B
Chen, G., Guo, Z., Zeng, G., Tang, L.: Fluorescent and colorimetric sensors for environmental mercury detection. Analyst. 140, 5400–5443 (2015). https://doi.org/10.1039/C5AN00389J
Peakall, D.B., Lovett, R.J.: Mercury: its occurrence and effects in the ecosystem. Bioscience 22, 20–25 (1972). https://doi.org/10.2307/1296180
Bishop, K., Shanley, J.B., Riscassi, A., Wit, H.A., Eklöf, K., Meng, B., Mitchell, C., Osterwalder, S., Schuster, P.F., Webster, J., Zhu, W.: Recent advances in understanding and measurement of mercury in the environment: Terrestrial Hg cycling. Sci. Total Environ. 721, 137647 (2020). https://doi.org/10.1016/j.scitotenv.2020.137647
Yeoh, T.S., Lee, A.S., Lee, H.S.: Absorption of mercuric sulphide following oral administration in mice. Toxicology 41, 107–111 (1986). https://doi.org/10.1016/0300-483X(86)90108-3
Mao, L., Liu, X., Wang, B., Lin, C., Xin, M., Zhang, B.T., Wu, T., He, M., Ouyang, W.: Occurrence and risk assessment of total mercury and methylmercury in surface seawater and sediments from the Jiaozhou Bay, Yellow Sea. Sci. Total Environ. 714, 136539 (2020). https://doi.org/10.1016/j.scitotenv.2020.136539
Ackerman, J.T., Kraus, T.E.C., Fleck, J.A., Krabbenhoft, D.P., Horwath, W.R., Bachand, S.M., Herzog, M.P., Hartman, C.A., Bachand, P.A.M.: Experimental dosing of wetlands with coagulants removes mercury from surface water and decreases mercury bioaccumulation in fish. Environ. Sci. Technol. 49, 6304–6311 (2015). https://doi.org/10.1021/acs.est.5b00655
Escudero, L.B., Olsina, R.A., Wuilloud, R.G.: Polymer-supported ionic liquid solid phase extraction for trace inorganic and organic mercury determination in water samples by flow injection-cold vapor atomic absorption spectrometry. Talanta 116, 133–140 (2013). https://doi.org/10.1016/j.talanta.2013.05.001
Wang, Z., Si, S., Luo, Z., Qin, T., Xu, Z., Liu, B.: An AIE-based fluorescent probe for detection of picric acid in water 50, 103–105 (2021). https://doi.org/10.1246/cl.200618
Bahta, M., Ahmed, N.: An AIEE active 1, 8-naphthalimide- sulfamethizole probe for ratiometric fluorescent detection of Hg2+ ions in aqueous media. J. Photochem. Photobiol. A. 391, 112354 (2020). https://doi.org/10.1016/j.jphotochem.2020.112354
Silpcharu, K., Sukwattanasinitta, M., Rashatasakhon, P.: Novel sulfonamidospirobifluorenes as fluorescent sensors for mercury(II) ion and glutathione. RSC Adv. 9, 11451–11458 (2019). https://doi.org/10.1039/C9RA00004F
Li, Y., Zhou, H., Chen, W., Sun, G., Sun, L., Su, J.: A simple AIE-based chemosensor for highly sensitive and selective detection of Hg2+ and CN-. Tetrahedron 72, 5620–5625 (2016). https://doi.org/10.1016/j.tet.2016.07.054
He, T., Ou, W., Tang, B.Z., Qin, J., Tang, Y.: In vivo visualization of the process of Hg2+ bioaccumulation in water flea Daphnia carinata by a novel aggregation-induced emission fluorogen. Chem. Asian J. 14, 796–801 (2019). https://doi.org/10.1002/asia.201801538
Han, X., Lü, X., Chen, Z., Yu, G., Yin, J., Liu, S.: A Fluorescent probe for Hg2+ based on Gold(I) complex with an aggregation-induced emission feature. Chin. J. Chem. 33, 1064–1068 (2015). https://doi.org/10.1002/cjoc.201500324
Ma, X.Q., Wang, Y., Wei, T.B., Qi, L.H., Jiang, X.M., Ding, J.D., Zhu, W.B., Yao, H., Zhang, Y.M., Lin, Q.: A novel AIE chemosensor based on quinoline functionalized Pillar[5]arene for highly selective and sensitive sequential detection of toxic Hg2+ and CN−. Dyes Pigm. 164, 279–286 (2019). https://doi.org/10.1016/j.dyepig.2019.01.049
Wang, A., Yang, Y., Yu, F., Xue, L., Hu, B., Fan, W., Dong, Y.: A highly selective and sensitive fluorescent probe for quantitative detection of Hg2+ based on aggregation-induced emission features. Talanta 132, 864–870 (2015). https://doi.org/10.1016/j.talanta.2014.10.048
Zhang, R.X., Li, P.F., Zhang, W.J., Li, N., Zhao, N.: A highly sensitive fluorescent sensor with aggregation-induced emission characteristics for the detection of iodide and mercury ions in aqueous solution. J. Mater. Chem. C 4, 10479–10485 (2016). https://doi.org/10.1039/C6TC03696A
Ruan, Z., Shan, Y., Gong, Y., Wang, C., Ye, F., Qiu, Y., Liang, Z., Li, Z.: Novel AIE-active ratiometric fluorescent probes for mercury(II) based on the Hg2+-promoted deprotection of thioketal, and good mechanochromic properties. J. Mater. Chem. C. 6, 773–780 (2018). https://doi.org/10.1039/C7TC04712F
Gabr, M.T., Pigge, F.C.: A turn-on AIE active fluorescent sensor for Hg2+ by combination of 1,1-bis(2-pyridyl)ethylene and thiophene/bithiophene fragments. Mater. Chem. Front. 1, 1654–1661 (2017). https://doi.org/10.1039/C7QM00085E
Niu, C., Liu, Q., Shang, Z., Zhao, L.: Ouyang J Dual-emission fluorescent sensor based on AIE organic nanoparticles and au nanoclusters for the detection of mercury and melamine. Nanoscale 7, 8457–8465 (2015). https://doi.org/10.1039/C5NR00554J
Gupta, S., Milton, M.D.: Synthesis of novel AIEE active pyridopyrazines and their applications as chromogenic and fluorogenic probes for Hg2+ detection in aqueous media. N. J. Chem. 42, 2838–2849 (2018). https://doi.org/10.1039/C7NJ04573E
Wang, K., Li, J., Ji, S., Li, L., Qiu, Z., Pan, C., Zhang, J., Huo, Y.: Fluorescence probes based on AIE luminogen: application for sensing Hg2+ in aqueous media and cellular imaging. N. J. Chem. 42, 13836–13846 (2018). https://doi.org/10.1039/C8NJ02245C
Singh, P.K., Prabhune, A., Ogale, S.: Pulsed laser-driven molecular self-assembly of cephalexin: aggregation-induced fluorescence and its utility as a mercury ion sensor. Photochem Photobiol. 91, 1340–1347 (2015). https://doi.org/10.1111/php.12526
Wang, X., Gao, Z., Zhu, J., Gao, Z., Wang, F.: Aggregation-induced emission of cyanostilbene amphiphile as a novel platform for FRET-Based ratiometric sensing of mercury ion in water. Polym. Chem. 7, 5217–5220 (2016). https://doi.org/10.1039/C6PY01109H
Fang, W., Zhang, G., Chen, J., Kong, L., Yang, L., Bi, H., Yang, J.: An AIE active probe for specific sensing of Hg2+ based on linearconjugated bis-Schiff base. Sens. Actuators B 229, 338–346 (2016). https://doi.org/10.1016/j.snb.2016.01.130
Shyamal, M., Maity, S., Maity, A., Maity, R., Roy, S., Misra: A Aggregation induced emission based “turn-off” fluorescent chemosensor for selective and swift sensing of mercury (II) ions in water. Sens. Actuators B 263, 347–359 (2018). https://doi.org/10.1016/j.snb.2018.02.130
Wang, J., Qian, X., Cui, J.: Detecting Hg2+ ions with an ICT fluorescent sensor molecule: remarkable emission spectra shift and unique selectivity. J. Org. Chem. 71, 4308–4311 (2006). https://doi.org/10.1021/jo052642g
Gao, Y., Ma, T., Ou, Z., Cai, W., Yang, G., Li, Y., Xu, M., Li, Q.: Highly sensitive and selective turn-on fluorescent chemosensors for Hg2+ based on thioacetal modified pyrene. Talanta 178, 663–669 (2018). https://doi.org/10.1016/j.talanta.2017.09.089
Darroudi, M., Ziarani, G.M., Ghasemi, J.B., Badiei, A.: Acenaphtoquinoxaline as a selective fluorescent sensor for Hg (II) detection: experimental and theoretical studies. Heliyon. 6, e04986 (2020). https://doi.org/10.1016/j.heliyon.2020.e04986
Lv, H., Yuan, G., Zhang, G., Ren, Z., He, H., Sun, Q., Zhang, X., Wang, S.: A novel benzopyran-based colorimetric and near-infrared fluorescent sensor for Hg2+ and its imaging in living cell and zebrafish. Dyes Pigm. 172, 107658 (2020). https://doi.org/10.1016/j.dyepig.2019.107658
Li, C., Niu, Q., Wang, J., Wei, T., Li, T., Chen, J., Qin, X., Yang, Q.: Bithiophene-based fluorescent sensor for highly sensitive and ultrarapid detection of Hg2+ in water, seafood, urine and live cells. Spectrochim. Acta A. 233, 118208 (2020). https://doi.org/10.1016/j.saa.2020.118208
Duan, X., Gu, B., Zhou, Q., Hu, X., Huang, L., Su, W., Li, H.: A simple fluorescent probe for detecting mercury(II) ion in aqueous solution and on agar gels. J. Iran. Chem. Soc. 14, 1207–1214 (2017). https://doi.org/10.1007/s13738-017-1071-7
Sun, Y., Wang, L., Zhou, J., Qin, D., Duan, H.: A new phenothiazine-based fluorescence sensor for imaging Hg2+ in living cells, 34:e5945. Appl. Organomet. Chem. (2020). https://doi.org/10.1002/aoc.5945
Yin, P., Niu, Q., Yang, Q., Lan, L., Li, T.: A new “naked-eye” colorimetric and ratiometric fluorescent sensor for imaging Hg2+ in living cells. Tetrahedron 75, 130687 (2019). https://doi.org/10.1016/j.tet.2019.130687
Lee, J.J., Kim, Y.S., Nam, E., Lee, S.Y., Lim, M.H., Him, C.: A PET-based fluorometric chemosensor for the determination of mercury(ii) and pH, and hydrolysis reaction-based colorimetric detection of hydrogen sulfide. Dalton Trans. 45, 5700–5712 (2016). https://doi.org/10.1039/C6DT00147E
Wang, J.H., Liu, Y.M., Dong, Z.M., Chao, J.B., Wang, H., Wang, Y., Shuang, S.: New colorimetric and fluorometric chemosensor for selective Hg2+ sensing in a near-perfect aqueous solution and bio-imaging. J. Hazard. Mater. 382, 121056 (2020). https://doi.org/10.1016/j.jhazmat.2019.121056
Maity, A., Sil, A., Nad, S., Patra, S.K.: A highly selective, sensitive and reusable BODIPY based ‘OFF/ON’ fluorescence chemosensor for the detection of Hg2+ ions. Sens. Actuators B 255, 299–308 (2018). https://doi.org/10.1016/j.snb.2017.08.016
Mohan, B., Sharma, H.K.: Synthesis of calix[6]arene and transduction of its fur fural derivative as sensor for Hg(II) ions. Inorganica Chim. Acta. 486, 63–68 (2019). https://doi.org/10.1016/j.ica.2018.10.022
Liu, D., Wang, Y., Wang, R., Wang, B., Chang, H., Chen, J., Yang, G., He, H.: Fluorescein-based fluorescent sensor with high selectivity for mercury and its imaging in living cells. Inorg. Chem. Commun. 89, 46–50 (2018). https://doi.org/10.1016/j.inoche.2018.01.016
Ngororabanga, J.M.V., Moyo, C.B., Tshentu, Z.R.: A novel multidentate pyridyl ligand: a turn-on fluorescent chemosensor for Hg2+ and its potential application in real sample analysis. Spectrochim. Acta A. 242, 118651 (2020). https://doi.org/10.1016/j.saa.2020.118651
Fern´andez-Alonso, S., Corrales, T., Pablos, J.L., Catalina, F.: A Switchable fluorescence solid sensor for Hg2+ detection in aqueous media based on a photo crosslinked membrane functionalized with (benzimidazolyl)methyl-piperazine derivative of 1,8-naphthalimide. Sens. Actuators B 270, 256–262 (2018). https://doi.org/10.1016/j.snb.2018.05.030
Liu, D., Zhu, H., Shi, J., Deng, X., Zhang, T., Zhao, Y., Qi, P., Yang, G., He, H.: 1, 8-Naphthalimide-based fluorescent sensor with high selectivity and sensitivity for Hg2+ in aqueous solution and living cells. Anal. Methods. 11, 3150–3154 (2019). https://doi.org/10.1039/C9AY00711C
Kraithong, S., Panchan, W., Charoenpanich, A., Sirirak, J., Sahasithiwat, S., Swanglap, P., Promarak, V., Thamyongkit, P., Wanichacheva, N.: A method to detect Hg2+ in vegetable via a “Turn–ON” Hg2+–fluorescent sensor with a nanomolar sensitivity. J. Photochem. Photobiol. A 389, 112224 (2020). https://doi.org/10.1016/j.jphotochem.2019.112224
Bhaskar, R., Sarveswari, S.: Thiocarbohydrazide based Schiff base as a selective colorimetric and fluorescent chemosensor for Hg2+ with “Turn-Off” fluorescence responses. Chem. Select. 5, 4040–4057 (2020). https://doi.org/10.1002/slct.202000652
Bag, S.S., De, S.: Pyrenylthioureayl alanine as a switch-on fluorescent sensor for Hg(II) ions. Anal. Chem. 3, 11758–11764 (2018). https://doi.org/10.1002/slct.201802249
Saikia, D., Dutta, P., Sarma, N.S., Adhikary, N.C.: CdTe/ZnS core/shell quantum dot-based ultrasensitive PET sensor for selective detection of Hg (II) in aqueous media. Sens. Actuators B 230, 149–156 (2016). https://doi.org/10.1016/j.snb.2016.02.035
Wu, L.L., Wang, Z., Zhao, S.N., Meng, X., Song, X.Z., Feng, J., Song, S.Y., Zhang, H.J.: A metal–organic framework/DNA hybrid system as a novel fluorescent biosensor for mercury(II) ion detection. Chem. Eur. J. 22, 477–480 (2016). https://doi.org/10.1002/chem.201503335
Marieeswaran, M., Panneerselvam, P.: Fluorescent polyaniline nanoclips (PANCs): a highly sensitive and selective chemical sensor for the detection of Hg (II) ions in aqueous media. Anal Chem. 5, 4481–4487 (2020). https://doi.org/10.1002/slct.202000545
Bhatti, A.A., Oguz, M., Yilmaz, M.: New water soluble p-sulphonatocalix[4]arene chemosensor appended with rhodamine for selective detection of Hg2+ ion. J. Mol. Structure. 1203, 127436 (2020). https://doi.org/10.1016/j.molstruc.2019.127436
Horzum, N., Mete, D., Karakus, E., Ucuncu, M., Emrullahoglu, M., Demir, M.M.: Rhodamine-immobilised electrospun chitosan nanofibrous material as a fluorescence turn-on Hg2+ sensor. Chem. Select. 5, 896–900 (2016). https://doi.org/10.1002/slct.201600027
He, W., Liu, R., Liao, Y., Ding, G., Li, J., Liu, W., Wu, L., Feng, H., Shi, Z., He, M.: A new 1,2,3-triazole and its rhodamine B derivatives as a fluorescence probe for mercury ions. Anal. Biochem. 598, 113690 (2020). https://doi.org/10.1016/j.ab.2020.113690
Kunthom, R., Piyanuch, P., Wanichacheva, N., Ervithayasuporn, V.: Cage-like silsesequioxanes bearing rhodamines as highly sensitive and selective fluorescence Hg2+ sensors. J. Photochem. Photobiol. A. 356, 248–255 (2018). https://doi.org/10.1016/j.jphotochem.2017.12.033
Yang, Y., Shen, R., Wang, Y.-Z., Qiu, F.-Z., Feng, Y., Tang, X.-L., Bai, D., Zhang, G.-L., Liu, W.-S.: A selective turn-on fluorescent sensor for Hg (II) in living cells and tissues. Sens. Actuators B. 255:3479–3487 (2018). https://doi.org/10.1016/j.snb.2017.09.180
Wang, Q., Jina, L., Wang, W., Hu, T., Chen, C.: Rhodamine derivatives as selective “naked-eye” colorimetric and fluorescence off-on sensor for Hg2+ in aqueous solution and its applications in bioimaging. J. Lumin. 209, 411–419 (2019). https://doi.org/10.1016/j.jlumin.2019.02.024
Xu, X., Zheng, B., Deng, H., Zhang, X., Shuai, Q.: Synthesis and sensing behavior of a new multichannel sensor based on thiazolyl ferrocene-rhodamine for Hg2+ detection. Microchem. J. 158, 105257 (2020). https://doi.org/10.1016/j.microc.2020.105257
Petdum, A., Faichu, N., Sirirak, J., Khammultri, P., Promarak, V., Panchan, W., Sooksimuang, T., Charoenpanich, A., Wanichacheva, N.: [5]Helicene-rhodamine 6 G hybrid-based sensor for ultrasensitive Hg2+ detection and its biological applications. J. Photochem. Photobiol. A. 394, 112473 (2020). https://doi.org/10.1016/j.jphotochem.2020.112473
Tsai, H.-J., Wan, C.-F., Su, Y., Wu, A.-T.: A selective colorimetric fluorescent chemosensor for Hg2+ in aqueous medium and in the solid state. J. Lumin. 194, 279–283 (2018). https://doi.org/10.1016/j.jlumin.2017.10.023
Bai, C.-B., Qiao, R., Liao, J.-X., Xiong, W.-Z., Zhang, J., Chen, S.-S., Yang, S.: A highly selective and reversible fluorescence “OFF-ON-OFF” chemosensor for Hg2+ based on rhodamine-6G dyes derivative and its application as a molecular logic gate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 202, 252–259 (2018). https://doi.org/10.1016/j.saa.2018.05.050
Anandababu, A., Anandan, S., Ashokkumar, M.: A simple discriminating p-tert-butylcalix[4]arene thiospirolactam rhodamine b based colorimetric and fluorescence sensor for mercury ion and live cell imaging applications. Chem. Select. 3, 4413–4420 (2018). https://doi.org/10.1002/slct.201800044
Hong, M., Chen, Y., Zhang, Y., Xu, D.: A novel rhodamine-based Hg2+ sensor with a simple structure and fine performance. Analyst. 144, 7351–7358 (2019). https://doi.org/10.1039/C9AN01608B
Gao, T., Lee, K.M., Kim, S.H., Heo, J., Yang, S.I.: A Mercuric ion selective fluorescent sensor based on rhodamine B with an ethylene unit. Bull. Korean Chem. Soc. 38, 292–295 (2017). https://doi.org/10.1002/bkcs.11078
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
The author confirms the sole responsibility for the manuscript preparation.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Consent for publication
Not Applicable.
Ethical approval
Not Applicable.
Consent to participate
Not Applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Udhayakumari, D. Review on fluorescent sensors-based environmentally related toxic mercury ion detection. J Incl Phenom Macrocycl Chem 102, 451–476 (2022). https://doi.org/10.1007/s10847-022-01138-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-022-01138-1