Abstract
Cyclodextrins (CDs) are abundantly explored in the field of medicines for the design of various types of drug delivery systems. They are cyclic oligosaccharides carrying α (1,4) glucopyranose units and able to build aqueous soluble inclusion complexes with various small and large drug molecules. These molecules have a unique structural feature and categorized into hydrophobic, hydrophilic and ionic derivatives. Villiers and Schardinger in 1891, first described the chemical nature and types of cyclodextrin. Cramer and co-workers in 1955 illustrated their latent to make water soluble inclusion complexes with various active components. These CDs molecules are used in pharmaceutical field practically and economically to improve the stability, solubility as well as bioavailability of drug molecules. In this review article physical characteristic, chemical nature and applications of different cyclodextrin and their derivatives in different drug delivery systems and toxicological effects are engrossed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction and history
Now days, researches are aimed to improve the safety and efficacy of previously established and newly developed chemical entities. These chemical entities are obtained by some physical/chemical changes in the parent candidate and implementation of better formulation strategies that meet the specific needs [1]. In the past few years, development of therapeutics has been enormously explored and has witnessed a tremendous development and progress in this area. Natural polymers obtained from different origins like microbial (xanthan gum, dextran), plant (pectin, guar gum, psyllium), algal (alginate), animal origin (chitosan, chondroitin) etc. have been massively explored as such and even after varied functionalization [2,3,4,5]. Polymers have a number of active groups, different molecular mass, variability in chemical composition, structure and properties that help in the synthesis of large number of derived polymers. The natural polymers are practically safe, non-toxic, abundantly available and biodegradable in nature. The presence of active groups on molecular chain makes them chemically and bio-chemically active which results in formation of various derivatives. A number of molecular, natural and synthetic macro and micro-carriers have been induced in the pharmaceutical design in order to meet controlled, sustained and immediate release of the drug to the specified targets [6,7,8]. The natural polymers carrying very large number of carboxyl, amino and hydroxyl groups which form non-covalent bonds with biological membrane and also aid in improving solubility of the active compound by forming complexes [9].
For designing drug delivery systems cyclodextrins plays an important role which have been known for over 100 years. The importance of cyclodextrins is increasing day by day because of its ability to modify the physicochemical properties of the guest molecules via the formation of inclusion complexes (Fig. 1a). The well known application of cyclodextrin in various fields is to improve the solubility, bioavailability and stability of the guest molecules [9].
Initially, French scientist Villiers (1891) isolated small quantity of crystalline product from 1000 g of starch. He has given its configuration (C6H10O5)2·3H2O and called this product “cellulosine” since the product didn’t have any reducing properties and seems to be opposing acid hydrolysis [8]. In 1903 and 1904, Schardinger was capable to isolate the crystalline products of dextrins A and B from the bacterial strain of Bacillus macerans which were additionally related through their lack of reducing power [9]. Moreover, in 1948, the X-ray crystallography of γ-cyclodextrin and its capability to make inclusion-complexes with several active molecules were revealed [10,11,12,13]. Till the end of 1960, the different methods of preparation of cyclodextrins at laboratory scale and the existence of different cyclodextrins like α, β, γ andη CD was confirmed. Further, their various physical and chemical properties, structures, mode of preparation, industrial potential and toxicological properties have been studied by many researchers and it was recorded as successful “new” pharmacological excipients [14,15,16].
Chemical structure and properties of cyclodextrins
Origin and nature
The chemical structures of CD look like a hollow truncated cone with a hydrophobic centre (Fig. 1b) allowing the evolution of number of inclusion complexes with suitable guest molecules. CDs are achieved from starch and α-1,4-glucan substrates by transglycosylation (cyclization) reaction in the presence of cyclodextrin glucanotransferase (CGTase) enzyme [17]. CGTase is one of the members of family of amylase enzymes and is used in many reactions like coupling, disproportionation and hydrolytic reactions [18]. There are many cyclodextrins derivatives but α, β and γ-CDs are commonly used in pharmaceutical and food industry. The CDs composed of nine to twenty one glucose units and shows different chemical structure and properties. Moreover, due to variations in sizes and structural features of CDs it could found a novel host molecule in various nano-biotechnological applications and molecular recognition in biotechnology [16, 19].
Nomenclature and shape
Lichtenthaler and Immel in 1994 has introduced a complete nomenclature, such as pre-α-CD later named as cyclo-α(1–4)-glucopentaoside and this nomenclature suggested for the minor derivatives of CDs, cyclic oligosaccharides and other derivatives. Frequently, industrially produced and practically important CDs (α, β and γ) are unambiguous and their symbols and nomenclature need not to be changed [20].
Based on the geometry of the cyclodextrin it have truncate shape in which the wider part is formed with secondary hydroxyl groups (2&3) while the narrower side is formed by the primary hydroxyl group (6). The size and dimension of the cyclodextrin cavity depends upon the number of glucose units. The cavity is creased via the hydrogen [H] atoms and glycosidic oxygen bridges. The lone pair (non bonding) electrons of the glycosidic oxygen bridges are directly towards the inner side of the cavity with high electron density and lending to its Lewis base character. In cyclodextrin, the specific positions of the active functional groups make it comparatively hydrophobic and the external parts are hydrophilic in nature. Moreover, the adjacent glucose units intra-molecularly linked by the 2 and 3-hydroxyl groups and form hydrogen bond ring [21, 22]. The crystal structure study showed that the CDs crystallized in two major kinds of crystal packing i.e. cage and channel structures. These all crystal structures of the cyclodextrins inclusion complexes acquire the ‘round’ shape with glucopyranose units due to the 4C1 chair conformation in the molecule [23].
Classification of cyclodextrins
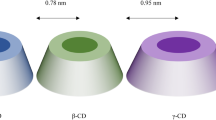
The cyclodextrins are classified into two main categories that are natural and synthetic cyclodextrins. According to the first generation, three main types of natural CDs i.e. α, β and γcyclodextrin as shown (Fig. 2) carrying six, seven and eight α (1,4)-linked glycosyl units [24]. The physical and chemical properties of the cyclodextrins are shown in Table 1. Due to high reactivity of primary (1°) and secondary (2°) hydroxyl groups of cyclodextrins different derivatives of cyclodextrin have been synthesized by various reactions like esterification, amination and etherification. The solubility of the synthesized cyclodextrin derivatives mostly depends upon the degree of substitution that differs from its parent cyclodextrin. Each and every derivative has a specific internal (hydrophobic) cavity volume and these alterations help to improve solubility and stability towards light/oxygen as well as control the chemical activity of the guest molecules [23].
On the other hand, different synthesized cyclodextrin derivatives are obtained by optimization of reaction conditions using regioselective reagents and high-quality separation techniques to obtain good products. The CDs and their derivatives carry a number of hydroxyl groups making it prone to an electrophilic attack and help in the formation of esters and ethers. The esters and ethers have been formed by using different reagents like alkyl halides (CH3X), isocyanates, acyl, epoxides derivatives and by some inorganic acid derivatives such as sulphonic acid chloride. Moreover, they also undergo a nucleophilic attack by some chemical reagents such as azide ions, thiols, halides, amines and thiourea which activate the oxygen atom by an electron-withdrawing group [25].The various synthetic CD derivatives like (2-hydroxypropyl-β-CD (2-HP-β-CD) [26], methyl-β-CD (M-β-CD), randomly methylated-β-CD [21], branched CDs (glucosyl- and maltosyl-β-cyclodextrins), acetylated β- and γ-CDs [27] or sulfated amphiphilic α-, β-, and γ-CDs) [28] respectively, shown in (Fig. 3) (Table 2) had synthesized to enhance the solubility and the biological activity of various active molecules.
Solubility
In terms of solubility, β-cyclodextrin has lower solubility as compared to α and γ-CD [30, 31]. In case of β-CD, presence of C2-OH group on one of the glucopyranoside unit helps to create a new hydrogen bond with the neighboring C3-OH group of the glucopyranose unit. A complete well structured secondary belt became framed by these hydrogen bonds and got an inflexible structure and this intra-molecular hydrogen bond formation is one of the main causes for the low solubility (18.6 mg/mL) of β-CD out of all CDs [26]. CDs are more stable in basic/alkaline solutions. Though, they are undergoing partial hydrolysis and produce glucose units and acyclic maltosaccharides. The stability of the CDs depends upon the acidity and temperature conditions for example the reaction rate constant of the hydrolysis of CDs may change upon the change of the temperature and acidity of the solution [21]. Furthermore, Cyclodextrins are commonly used as building blocks for the development of supramolecular complexes and up to twenty substituents have been connected to β-CD in a regioselective way [16]. For instance, replacement of hydroxyl groups of CDs by sulfate groups confirms its higher water solubility as compared to the parent compound and extensive varieties of biological activities like antiviral, anti-angiogenic, anti-lipemic and anti-inflammatory correspondingly [32]. Amphiphilic cyclodextrins derivatives enhance the connection between CDs (having high external hydrophilicity) with lipophilic biological membranes [33]. These CDs could be used for different pharmaceutical applications owing to their ability to self-organize in aqueous media at various physiological pH as micellar aggregates [34], vesicles [35] and nano-carriers [36] etc. The amphiphilic CDs have been determined by different names like “skirt” CDs [37], “bouquet” CDs [38], “lollipop” CDs [39], and “cup-and ball” CDs depending upon their structure and features [40]. Currently, water-soluble CDs are synthesized utilizing effective cross-linking agents i.e. epichlorohydrin [41], diisocyanates [42], polycarboxylic acids [43] or anhydrides [44] etc. Epichlorohydrin is one of the most accepted cross linking agent used in the chemical, pharmaceutical and food industry for the development of β-CD polymers [41].
Thermal behavior of CDs
The thermal behavior of natural CDs and their derivatives are quite similar, the only differences is found on the basis of total water content, beginning temperature of thermal degradation and the weight loss values at specified given temperature. For example, natural β-CD is the most popular and widely used in pharmaceutical inclusion complex formation and its thermal behavior commonly divided into three phase (a) loss of bound and unbound water at ambient temperature 120 °C, (b) thermal degradation take place by oxidation at 250–300 °C and (c) the final phase is ignition that occur in the presence of air above 300 °C. The melting point affects the shape of the thermodynamic curves [45]. In case of α and γ-CD a small weight loss is reported at 260–270 °C and also attributed to loss of very tightly bound water. According to the order of relative thermal stability, β-CD is the least thermally stable as compared to the α andγ-CD. The chemically synthesized cyclodextrin derivatives such as acetylated, methylated, hydroxy-propylated and sulphobutyl ether are more stable and have higher temperature of decomposition as compared to the parent/natural CDs. The melting point of the synthesized derivative is roughly similar to the parent CDs but sometimes slightly differ depending upon the modification and degree of substitution [46].
Mechanism of inclusion complex formation
The X-ray investigations of CDs revealed that it carries primary and secondary hydroxyl groups with a central hydrophobic cavity, helping to form inclusion complexes with a huge number of guest lipophilic molecules (Eq. 1) [16, 47]. In aqueous solution, cyclodextrins used to form “inclusion complexes” whereas the hydrophobic cavity of CD occupied by water molecules is bounded with “weak forces” and is energetically unfavored [16]. Therefore, water molecules positioned within the hydrophobic central cavity are substituted by hydrophobic guest molecules and lipophilic moieties. Depending upon the size of the central cavity; one or more than one lipophilic guest molecules may be entrapped [22].
The hydroxyl groups present on the outer surface of CDs could be able to form hydrogen bonds with many other molecules like non-cyclic oligosaccharides, polysaccharides and cyclodextrin itself also [48, 49]. The Linear homologs of maltodextrins have the potential to bind fluorescence probes, but the binding constants are very smaller as compared to those of their cyclic counterparts [50]. Furthermore, β- cyclodextrin form two types of complexes i.e. inclusion and non-inclusion complex with dicarboxylic acids and these complexes simultaneously exist in equilibrium state in aqueous solutions [51]. The existence of equilibrium state depends upon either the concentration of the cyclodextrins and guest molecule or the methods applied for the formation of inclusion complex [52]. The cyclodextrin complexes are based upon the fitting of “guest” molecule into the central hydrophobic cavity along with the ratio of host-guest- complex [16]. For example, if the guest molecule is very small, it can easily penetrate in and out from the cyclodextrin cavity with slight or no bonding. On the other hand if the guest molecule is larger than the cyclodextrin in that case the complex is made in a way only some particular functional groups and side chain enter into the cavity. The guest molecules which are less polar as compared to water can easily create complexes with cyclodextrin. The stability of the inclusion complex is directly proportional to the hydrophobic nature of the guest molecule [53]. Furthermore, the inclusion complexes of CDs with organic molecules offer many potential outcomes to build supra molecules such as catenanes, polyrotaxanes, rotaxanes, and tubes [25]. The supramolecular host–guest complexation process is also useful for many applications such as bioimaging, photovoltaics and photocatalysis. For example, the γ-cyclodextrin-modified DPA is recently use to enhance the triplet lifetime of the sensitizer with the acceptor in water [54]. The chemically modified CDs have ability to significantly enhance the binding and chiral properties of the molecules. For example, the CDs and curcubituril have been utilized as wheels of rotaxanes because of their proper round shape and complex formation ability with organic guests’ molecules in aqueous solution. The hydrogen bonding formed between CD and curcubituril plays a major role for rotaxanes formation and these rotaxanes have been using as a supramolecular sensitizer [55].
Chiral/stereo-nature of CDs
Cyclodextrins have ability to form dia-stereoisomeric inclusion complexes by chiral guest molecules. When one enantiomeric form of the guest molecule form higher stable inclusion complex with CDs as compared to the another enationmer then it could be recognized as the enantio-selective recognition. The variation of the stability between both the enatiomeric forms of complex is known as the effectiveness of this chiral discrimination feature. The chemical modifications of the natural CDs are needed to enhance their physico-chemical properties and sometimes grafting is also done to improve covalent anchoring at surfaces. But it is necessary to note down that all these chemical changes are done in such a way that there is no disturbance in the chiral inclusion characteristics of the native CDs. For example, the α-Amino acids are stereotypical guests having central chirality used for the chiral recognition. The different forms of α-Amino acids (R&S) are able to bind with the α-CD with different binding constant value. In this case, the (R)-enantiomers are the better guest for parent CDs having higher binding constant value [56].
Application in drug delivery
Cyclodextrins can increase the aqueous solubility and chemical stability of different active constituents against various chemical reactions like hydrolysis, dehydration, oxidation, and photo decay; therefore improve the shelf life and stability of the drugs. As we know, CDs enhance stability by forming an inclusion complex and prevents the interaction between drugs and vehicles or inhibit drug alteration at the site of absorption. Owing to the molecular shielding effect of CDs and the formation of inclusion complexes with guest molecules, several applications have been evolved for the delivery of drug/guest molecule by different routes of administration.
Oral drug delivery
The oral route is one of the common and easy routes of drug administration. In this, the active moiety must be completely dissolved and then absorbed via the gastrointestinal tract in such a way that it produces a sufficient amount of drug at the site of action and obtained drug effects in a reproducible manner. Currently, more than 40% of the drug entities have poor solubility and permeability and these drawbacks create problems in the formulation of drug delivery system because of their poor biopharmaceutical properties [57]. The FDA and many other regulatory agencies have classified the drug into different four types on the basis of solubility and permeability of the drug characteristics and this type of classification is known as the Biopharmaceutical Classification System (BCS) (Fig. 4) [58, 59]. The formulations strategies based on the fact that the class II (low solubility high permeability) material act like a class I compound, with a resultant increase in oral bioavailability [60, 61]. The drug design methodologies are focused on class II compounds and CDs is one the main polymer that enables the technology for these compounds [62].The CDs inclusion complexes in a formulation may differ widely and are precise to the administration route or physicochemical issues that have to be overcome [48, 63].Right now, CDs and their derivatives are implemented for oral delivery purposes because they have the potential to improve their bioavailability of active constituents by rising the time period of their dissolution and the release rate [64, 65].
The naturally obtained α, β, γ-CDs and their derivatives having large central cavity helps in the formation of inclusion complexes with many hydrophobic drugs and make the process of oral administration easy (Table 2). The α, β, γ-cyclodextrin, sulfated-α/ β CD, carboxymethyl-β-CD (CM-β-CD), and 2-HP-β-CD have been used for improving oral hygiene products. The oral hygiene products/oral derived malodor contain volatile sulfur compounds like hydrogen sulphide (H2S) and mehtyl mercaptan (CH3SH), amines and amino acids [66] that bind to CDs residing in the mouth, further it lowers the local gastrointestinal tract irritation and bitter taste of the drugs [67]. Few methods are also available to overcome the bitterness of active constituents like creation of a “host–guest” complexes and the interaction between CDs with the so-called gatekeeper proteins which is localized on the taste bud and paralyzing them [68].
The main purpose of using CDs and their derivatives to overcome undesirable physicochemical properties of the active constituent like low water solubility, dissolution rate, and drug stability, many recent reviews focused on the use of CDs as good excipients for the oral delivery of various drugs [69,70,71]. Therefore, the value of CDs had demonstrated in the preparation process of various formulation that carry different types of drugs (Table 3) such as danazol, peptides,tolbutamide, itraconazole, gliclazide, cilostazol, diclofenac, paclitaxel, saquinavir, repaglinide,vinpocetine, albendazol and many others [72,73,74,75,76,77,78,79,80,81,82,83]. The water-soluble cyclodextrins inclusion complexes of the drugs have enhanced their diffusion through the mucosal barrier and improved their oral bioavailability [68].
Nasal drug delivery
Nasal drug delivery is the most promising approach for the delivery of drugs since the nose has a huge surface area for the drug absorption. Basically, nasal cavity lined with mucous membrane carries a huge number of microvilli and subepithelial layer and is highly vascularized. The venous blood from the nose directly passes into the systemic circulation and also avoids the first-pass/hepatic metabolism of the drugs. Therefore, it offers many advantages like a lower drug dose, quick attainment of therapeutic blood levels and rapid pharmacological action [87]. β-cyclodextrin is least soluble in water so different derivatives of β -CD had prepared to enhance its solubility. In nasal drug delivery, few derivatives of β-CDs had used, such as, dimethyl-β-CD, randomly methylated β-CD, trimethyl-β-CD and hydroxypropyl-β-CD [88]. Cyclodextrins also act as an absorption enhancers and used for the delivery of a large number of compounds like luteinizing hormone-releasing hormone analogues, oligopeptides, buserelin, leuprolide calcitonin, glucagon, insulin, recombinant human granulocyte colony-stimulating reason, polypeptides and proteins [88, 89]. The α- cyclodextrin also act as an enhancer and its complex with leuprolide administered as nasal drops [90]. The liquid formulation of insulin has zero percent internasal bioavailability in rabbits and in men, so its complex with dimethyl-β-CD has been broadly investigated and used as a powder [91]. The morphine hydrochloride and other opioids in solution have higher systemic availability when given via nasal route as compared to other routes like oral solution and rectal suppository. The plasma level concentration of morphine and its metabolites via nasal route was two times higher than those of oral and rectal administrations. Additionally, the nasal delivery of morphine produced a dose-dependent effect i.e. the higher level of analgesic response is produced [92]. Heptakis (2,6-di-O-methyl)-β-CD accelerates the nasal epithelial permeability which considerably improved the rate of nasal absorption and simultaneously increased the entry of the morphine and other opioids into the cerebrospinal fluid. However, 2-hydroxypropyl-g-CD retards the plasma concentration of morphine because it forms the complex that has less permeability through biological membranes [93]. The heptakis (2,6-di-O-methyl)-β-CD used as a solubilizer in estradiol containing nasal spray that is useful in estrogen insufficiency approach. Therefore, suitable use of cyclodextrins and their derivatives in nasal opioids formulation may offer enough analgesia for both acute and chronic pain [94].
Ophthalmic drug delivery
In ophthalmology, the drugs are either delivered either local orsystemic to the eye. Generally, the locally applied drugs have incredibly low (< 5%) ocular bioavailability [95]. In the eye, cornea, conjunctiva, and sclera are the main ocular barriers to drug permeability although the membrane exterior that is hydrophilic mucin film and aqueous tear also act as a barrier. The drug moiety must carry both properties i.e. somewhat hydrophilic can easily permeate via the aqueous peripheral part of the eye but simultaneously the molecule shall be a bit hydrophobic and able to penetrate the ocular barrier [96]. One of the main standards for ophthalmic formulation is that it must be non-irritating to the eye surface because irritation causes impulse blinking and tearing that may cause quick wash-out of the drug. Cyclodextrin and their derivatives were used in ophthalmic preparation because it increases the solubility, stability as well as evading of incompatibilities of drugs such as discomfort and irritation [97].
Cyclodextrin derivatives have many potential advantages in ocular drug delivery such as 2-hydroxypropyl-β-CD and sulfobutyl-β-CD are well tolerated and nonirritating to the eye well so used in the formation of liquid eye drop [98, 99]. Hydrophilic cyclodextrins do not pass through the corneal barriers of the eye but improve the ocular bioavailability of hydrophobic drugs. The water-loving CDs derivatives aid in maintaining the drug in solution as well as increase their presence at the corneal surface [100, 101]. The presence of a small amount of pre-corneal the fluid does not cause any dissociation effect on complex although a few endogenous lipids can replace the drug from the complex and enhance the level of free drug at the corneal surface. For example, the 2-hydroxypropyl-β-CD form inclusion complex with dexamethasone acetate which is used as an ophthalmic solution increases the ocular bioavailability of dexamethasone acetate via preventing its conversion to dexamethasone that shows less corneal permeability as compared to the pure drug [102]. Presently, some studies paying attention to the combination of CDs with other polymers, such as the blend of 2-hydroxypropyl-CD and hydroxy propyl methyl cellulose (HPMC) are able to use for enhancing the surface delivery of carbonic-anhydrase inhibitors [103]. Moreover, cyclodextrin and drug (1:1) ratio used to enhance the chemical stability and water solubility of dipivefrin. Further, the effect of 2-hydroxypropyl-β-CD and sulfobutyl ether β-cyclodextrin (SBE-β-CD) on the stability and aqueous solubility of dipivefrin and pilocarpine was also studied [104].
Dermal drug delivery
In last decade, the dermal drug delivery system has gained more attention since large numbers of drugs have been successfully transferred through this route. Though, the stratum corneum outer most epidermal layer of skinis the main restricted barrier toward the dermal drug transport and prevents release of active compounds by this route. Consequently, different methods have been used to enhance drug absorption like tissue targeting (localized drugdelivery) and enhancement of drug retention in the skin [105]. Cyclodextrin and their derivatives provide a major safety margin to dermal drug delivery like; it improve the solubility, stability [106], enhance transdermal absorption [107], sustain the drug release [108], avoid undesirable side effects [109] and knowing also for local and systemic use [110]. For dermal system, selection of a specific vehicle should be necessary so that cyclodextrins completely deploy their functions for example the hydrophilic cyclodextrins with water carrying ointments like absorptive, hydrophilic, and polyacrylic base enhance the in vitro release of corticosteroids. Conversely, the hydrophilic cyclodextrin with other ointments such as propylene glycol and macrogol base delay the drug release. The water-soluble complex formation can be increased with increase in concentration, diffusibility and solubility of the active constituent in aqueous phase of the ointment and keep a high level of thermodynamic activity [111, 112].
In case of ointments and suppositories the cyclodextrin-drug-complexs in which the drug may be replaced by other ingredients of the ointment based upon the magnitude and stability constant of the complex [113]. Accordingly, the release of the drug from the cyclodextrin complex possibly depends upon the interaction of cyclodextrin complex with the skin components [114, 115]. Cyclodextrins may also alter the barriers of the skin to facilitate the drug absorption and avoid irritation effects on the skin. For example, methylated β-cyclodextrin brings down the barrier function of the skin by removing all the major lipids fromthe skin layer especially (stratum corneum [116]. In 1992, Kawahara et al., prepared diethyl-β-CD and indomethacin complex and studied the in vivo absorption and release rates of indomethacin from gel ointment [117]. Further, addition of small quantity of polyvinyl pyrrolidone (PVP) and HPMC to the dexamethasone–2- hydroxypropyl-β-CD suspension improve the dermal delivery of dexamethasone into the skin [106]. The role of CDs in transdermal drug delivery is given in Table 4.
McCormack and Gregoriadis (1994) for the first time studied the role of CDs in development of liposomes. They studied the CD inclusion effects on entrapment and loading into liposomes. The complexation process useful in increasing drug solubility, stability and it provide better in vivo controlled release rate [118]. In 2006, Maestrellia et al., developed ketoprofen CD complexes, ketoprofen is BCS-II drug that stand for low water solubility and used as a pain reliever for the cure of osteoarthritis. It can cause gastrointestinal irritation when administered via oral route, thus, transdermal application of this is an appealing substitute. The ketoprofen 2-HP-β-CD complex incorporated in liposome simulating the skin behavior, increase permeability across artificial membranes and have showed an extended release effect. The liposome and the concentration of complex in aqueous phase both are responsible for the encapsulation efficiency of ketoprofen–2-HP-β-CD in liposomes [119]. The Prostaglandin E1 is used in the treatment peripheral vascular disorders but they are chemically unstable and poorly permeable to the skin. But cyclodextrin and their derivatives help in the formation of complexes with the prostaglandin E1 and improve its chemical stability as well as permeability [120].
Rectal drug delivery
Many reports have indicates that CDs and their derivative have also been applied to optimize the rectal delivery of drugs. The rectal absorption of drugs from suppository bases has stabilizing effects on drugs. In general, the rectal fluid is viscous and low in volume as contrast to gastrointestinal fluid. However, number of obstacles occurred during the administration of drug through rectal route, for instances, the number of drugs are inadequately absorbed via rectal mucosa and drug metabolism in the rectum [142]. CDs and their derivatives make the drugs insoluble in oleaginous suppository base hence the lesser interaction of the complexes with the vehicles which enhance the release of poorly water-soluble drugs. These complexes not only improve drug dissolution at an interface but it also slow down the reverse diffusion of the drug into the vehicles [143]. The combinations of α-CD and xanthan gum (polysaccharide) exhibit good stability that inhibit the conversion of morphine and facilitated the transport of drug through the rectal mucosa [144]. Though, the methylated-β-CD complexes has higher stability as compared with parent β-CD, the methylated-β-CDconsiderably increase the rectal absorption of lipophilic drugs for example carmofur [145], flurbiprofen [146], and biphenyly lacetic acid [147] in oleaginous suppository. Moreover, 2-Hydroxypropyl-β-CD is also used for enhancing the rectal absorption of anti inflammatory ethyl 4-biphenylyl acetate (prodrug of biphenyly lacetic acid) from oleaginous bases [148] and increase the release rate of the drug and lowering the affinity of the drugs to the oleaginous suppository base [149]. A few hydrophilic cyclodextrins improved the rectal absorption of opioids from the hollow type oleaginous suppository bases by enhancing the mucosal membrane permeability to drug instead of release rate of drug from the vehicle. In conclusion, a large number of reports have demonstrated that the effects of CDs in rectal drug delivery depend upon the viscosity enhancing agent (polysaccharides), type of vehicle (hydrophilic or oleaginous) and the physicochemical properties of the complexes [150].
Sublingual drug delivery
Oral mucosal route is one of the effective and an alternative pathway for systemic administration of several active pharmaceutical agents. The drug delivery via sublingual system offers a number of advantages over both internal and injectable delivery. The drugs given via sublingual route avoid the fate of enteric ally administered drugs like gastric pH, enzymatic degradation and first-pass metabolism [151]. In the sublingual formulations the CDs-drug-complexes enhances the bioavailability of hydrophobic drugs, for instances 2-HP-β-CD has been used to enhance the bioavailability of 17β-oestradiol [152], androstenediol [153], clomipramine [154] and danazol [155]. The increased in bioavailability can be achieved by cyclodextrins due to the increased in aqueous solubility moreover, they act as conventional penetration enhancers.
Some other applications of cyclodextrins
Cyclodextrins in cosmetics
The ultraviolet (UV) rays are very harmful for the skin and cause skin damaging and photoaging (freckling and wrinkling). Principally, the UV rays are of three types according to their wavelength such as UV-A (400–320 nm), UV-B (320–280 nm) and UV-C (280–200 nm). The UV-C rays are engrossed by the ozone layer but UV-A and UV-B are not absorbed by the ozone layer and reach to the surface of the earth and causing skin damage. Even a small time exposure of the skin to these UV rays cause sunburn and other damage [156]. Therefore, the protection of skin from UV rays has been intensively focused in cosmetic industry. The cyclodextrins inclusion complexes formation increasing day by day not only in the field of pharmaceutical, food and aroma but also in the field of fragrance, flavor, personal care products and cosmetic industries. Hence, the inclusion complexes are used for masking the obnoxious taste and smell of the compounds, physic-chemical stability of the volatile oils and improve the efficiency of odorant substances [157]. For example, Ferulic acid is natural UV absorbers obtained from flavonoids but due to their low solubility it has not been used in cosmetic products. But its inclusion complex formation with various cyclodextrin derivatives is useful for the improvement of solubility and stability [156].
Cyclodextrins self-aggregates
The natural CD and their derivatives have ability to interact with themselves and play a unique role in the preparation of CD complexes aggregates. The self aggregation formation of cyclodextrin molecules have tendency to lower the interaction of cyclodextrin with drug molecules. The self aggregate formation of cyclodextrins is increased with increase in CD concentration, temperature and mechanical forces. During the formation of self aggregation the addition of chaotropic agents for example sodium chloride and urea may cause depression of the process of self-association [158]. The Formation of CDs aggregates in aqueous solutions via non-inclusion complexation process/micellar formation may act as drug reservoirs which have tendency to improve the aqueous solubility of hydrophobic drugs. The self-aggregate complexes are identified by various microscopic methods and the diameters of naturally CD aggregates have been reported in the range of 200–300 nm [159].
Drug release from cyclodextrin complexes
In aqueous media the CDs complexes carry drug molecules in dynamic equilibrium state (bound/unbound) i.e. the CDs/drug complexes are continuously being formed and dissembled. The CDs/drug complexes association and dissociation value rate constants are reported in the range of 107 to 108 M−1 s−1 and 105 s−1. Moreover, the value of association and dissociation constant values for poorly water-soluble drugs and lipophilic drug molecules are within the range 102 to 103 M−1 s−1. Additionally, the competitive displacement of the drug and efficiency of drug protein binding will improve the release of drug from the CDs complexes [160]. CDs also serve as immediate and delayed drug release carriers. For example, the micro-extrusion 3D printed (printelets) formulation of HP-β-CD-carbamazepine inclusion complexes for the fine dose adjustment and immediate release of drug from the complexes. However, number of studies also revealed that CDs control the drug release from the polymeric matrix systems [161].
Toxicological consideration
The safety and toxicity profiles of all the naturally obtained cyclodextrins and their synthesized derivatives depend upon the route of administration [162]. Likewise starch, the CDs and their derivatives can be partial or complete hydrolyzed by the enzyme (amylases) present in the digestive tract after oral administration [163]. The hydrolysis of CDs also depends upon the size of internal cavity, for example, γ-CD having a large cavity and can be quickly hydrolyzed as compared to α-CD and β-CD which exhibits a small internal cavity. CDs also form complexes with other compounds like biliary salts that are present in the digestive tract in a reversible manner [164]. All the toxicity studies shows that orally delivered cyclodextrins are virtually non-toxic because of lack of absorption and hydrolysis from the gastrointestinal tract [93]. Though, the parenteral delivery of CDs causes harmful effects on the excretory organ because the removals of CDs from systemic circulation are carried out by kidney. CDs and their derivatives concentrated in the proximal convoluted tubule after glomerular filtration that causes toxic effect on the body [165]. Moreover, some of the CDs for exampleγ-CD, 2-HP-β-CD, sulphated β-CD, sulphobutyl ether β-CD, and maltosyl β-CD seems to be safer when administered parenterally. However, some of the parent compound like α, β-CD and the methylated β-CDs have shown toxicological effects and are not appropriate for parenteral administration [25]. The intravenous and subcutaneous administration of β-CD in high dose cause nephrotoxicity and reduce body weight [166]. By intravenous route, β-CDs may impair the blood components by forming inclusion complexes with some of their components and cause hemolysis. However, some derivatives of CDs for example 2-HP-β-CD and SBE β-CD (Captisol) are well tolerated by animal (rats, mice, and dogs) particularly when given by oral route. Furthermore, the intravenous administration has neither lethal nor carcinogenic effects on the kidneys and other organs [167].
In cosmetology, surfactants are used as a solubilizer but they have several drawbacks for example cloudiness of the formulation, cutaneous irritation and foaming property. The hydroxypropyl-α-CD has been used in place of surfactants due to its good solublizing fragrance and retention at the skin surface. Presently, the hydroxypropyl-α-CD carrying cosmetics sustain scent formulation used for a longer period of time. The HP-α-CD has been studied for their topical irritation, antigenicity and mutagenicity proven that it is a safe materials and used in cosmetics and perfumes preparation [158].
Conclusion
This review focused on the chemical nature and physicochemical properties of the cyclodextrin and their derivatives. It includes the pharmaceuticals applications of natural and chemically synthesized CDs in the development of different drug delivery system. The CDs have a number of applications in pharmaceutical industry and drug delivery because of their ability to form complexes with a number of drug molecules. The CDs are used to improve the solubility, safety, stability and bioavailability of the drugs. The CDs have ability to interact with poorly water soluble drugs and resulting in the formation of non covalent dynamic inclusion complexes. Moreover, it also used in the formation of self aggregates and act as a permeation enhancer in the cosmetic preparations. Certainly, the multifunctional characteristics and bio-adaptability of CDs make them a good carrier for the drug delivery system.
References
Dutta, R.C.: Drug carriers in pharmaceutical design: promises and progress. Curr. Pharm. Des. 13, 761–769 (2007)
Ngwuluka, N.C., Ochekpe, N.A., Aruoma, O.I.: Naturapolyceutics: the science of utilizing natural polymers for drug delivery. Polymers. 6(5), 1312–1332 (2014)
Bhatia, M., Ahuja, M.: Psyllium arabinoxylan: carboxymethylation, characterization and evaluation for nanoparticulate drug delivery. Int. J. Biol. Macromol. 72, 495–501 (2015)
Bhatia, M., Ahuja, M., Mehta, H.: Thiol derivatization of xanthan gum and its evaluation as a mucoadhesive polymer. Carbohydr. Polym. 131, 119–124 (2015)
Ahuja, M., Bhatia, M., Saini, K.: Sodium alginate–arabinoxylan composite microbeads: preparation and characterization. J. Pharm. Invest. 46(7), 645–653 (2016)
Siemoneit, U., Schmitt, C., Alvarez-Lorenzo, C., Luzardo, A., Otero-Espinar, F., Concheiro, A., Blanco-Méndez, J.: Acrylic/cyclodextrin hydrogels with enhanced drug loading and sustained release capability. Int. J. Pharm. 312, 66–74 (2006)
Liao, R., Lv, P., Wang, Q., Zheng, J., Feng, B., Yang, B.: Cyclodextrin-based biological stimuli-responsive carriers for smart and precision medicine. Biomater. Sci. 5, 1736–1745 (2017)
Cui, X., Wang, N., Wang, H., Li, G., Tao, Q.: pH sensitive supramolecular vesicles from cyclodextrin graft copolymer and benzimidazole ended block copolymer as dual drug carriers. Int. J. Polym. Mater. Polym. Biomater. 68, 733–740 (2019)
Barse, B., Kaul, N., Banerjee, N., Kaul, C.L., Banerjee, U.: Cyclodextrins: emerging applications. Chim. Oggi 21, 48–54 (2003)
Hedges, A.R.: Industrial applications of cyclodextrins. Chem. Rev. 98, 2035–2044 (1998)
Lu, X., Chen, Y.: Chiral separation of amino acids derivatized with fluoresceine-5-isothiocyanate by capillary electrophoresis and laser-induced fluorescence detection using mixed selectors of β-cyclodextrin and sodium taurocholate. J. Chromatogr. A 955, 133–140 (2002)
Baudin, C., Pean, C., Perly, B., Gosselin, P.: Inclusion of organic pollutants in cyclodextrins and derivatives. Int. J. Environ. Anal. Chem. 77, 233–242 (2000)
Larson, S.B., Day, J.S., McPherson, A.: X-ray crystallographic analyses of pig pancreatic α-amylase with limit dextrin, oligosaccharide, and α-cyclodextrin. Biochemistry 49, 3101–3115 (2010)
Higuchi, T.: A phase solubility technique. Adv. Anal. Chem. Instrum. 4, 117–211 (1965)
Cramer, F., Saenger, W., Spatz, H.C.: Inclusion compounds. XIX. 1a the formation of inclusion compounds of α-cyclodextrin in aqueous solutions. Thermodynamics and kinetics. J. Am. Chem. Soc. 89, 14–20 (1967)
Szejtli, J.: Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 98, 1743–1754 (1998)
Qi, Q., Zimmermann, W.: Cyclodextrin glucanotransferase: from gene to applications. Appl. Microbiol. Biotechnol. 66, 475–485 (2005)
Qi, Q., Mokhtar, M.N., Zimmermann, W.: Effect of ethanol on the synthesis of large-ring cyclodextrins by cyclodextrin glucanotransferases. J. Incl. Phenom. Macrocycl. Chem. 57, 95–99 (2007)
Li, Z., Wang, M., Wang, F., Gu, Z., Du, G., Wu, J., Chen, J.: γ-Cyclodextrin: a review on enzymatic production and applications. Appl. Microbiol. Biotechnol. 77, 245 (2007)
Lichtenthaler, F.W., Immel, S.: Cyclodextrins, cyclomannins, and cyclogalactins with five and six (1→ 4)-linked sugar units: a comparative assessment of their conformations and hydrophobicity potential profiles1. Tetrahedron Asymmetry 5, 2045–2060 (1994)
Saokham, P., Muankaew, C., Jansook, P., Loftsson, T.: Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 23, 1161 (2018)
Bai, L., Xu, X.M., He, J., Pan, S.Z.: Inclusion complexation, encapsulation interaction and inclusion number in cyclodextrin chemistry. Coord. Chem. Rev. 253, 1276–1284 (2009)
Szejtli, J.: Past, present and futute of cyclodextrin research. Pure Appl. Chem. 76, 1825–1845 (2007)
Li, S., Purdy, W.C.: Cyclodextrins and their applications in analytical chemistry. Chem. Rev. 92, 1457–1470 (1992)
Del Valle, E.M.: Cyclodextrins and their uses: a review. Process Biochem. 39, 1033–1046 (2004)
Loftsson, T., Fridriksdottir, H.: The effect of water-soluble polymers on the aqueous solubility and complexing abilities of β-cyclodextrin. Int. J. Pharm. 163, 115–121 (1998)
Szente, L., Szejtli, J.: Highly soluble cyclodextrin derivatives: chemistry, properties, and trends in development. Adv. Drug Deliv. Rev. 36, 17–28 (1996)
Dubes, A., Degobert, G., Fessi, H., Parrot-Lopez, H.: Synthesis and characterisation of sulfated amphiphilic α-, β-and γ-cyclodextrins: application to the complexation of acyclovir. Carbohydr. Res. 338, 2185–2193 (2003)
Yamamoto, M., Yoshida, A., Hirayama, F., Uekama, K.: Some physicochemical properties of branched β-cyclodextrins and their inclusion characteristics. Int. J. Pharm. 49, 163–171 (1989)
Sakuraba, H., Natori, K., Tanaka, Y.: Asymmetric oxidation of alkyl aryl sulfides in crystalline cyclodextrin complexes. J. Org. Chem. 56, 4124–4129 (1991)
Pitha, J., Milecki, J., Fales, H., Pannell, L., Uekama, K.: Hydroxypropyl-β-cyclodextrin: preparation and characterization; effects on solubility of drugs. Int. J. Pharm. 29, 73–82 (1986)
Uekama, K., Hirayama, F., Irie, T.: Cyclodextrin drug carrier systems. Chem. Rev. 98, 2045–2076 (1998)
Memisoglu, B., Erem, A., Bochot, L., Trichard, D., Duchene, Hıncal, A.A.: Amphiphilic cyclodextrins and microencapsulation. In: Microencapsulation, 2nd Edn. 270 Madison Avenue New York (2005)
Auzely-Velty, R., Djedaini-Pilard, F., Desert, S., Perly, B., Zemb, T.: Micellization of hydrophobically modified cyclodextrins. 1. Micellar structure. Langmuir 16, 3727–3734 (2000)
Sukegawa, T., Furuike, T., Niikura, K., Yamagishi, A., Monde, K., Nishimura, S.I.: Erythrocyte-like liposomes prepared by means of amphiphilic cyclodextrin sulfates. Chem. Commun. 5, 430–431 (2002)
Chen, X., Qiu, Y.K., Owh, C., Loh, X.J., Wu, Y.L.: Supramolecular cyclodextrin nanocarriers for chemo-and gene therapy towards the effective treatment of drug resistant cancers. Nanoscale. 8, 18876–18881 (2016)
Zhang, P., Chang-Chun, L., Coleman, A.W., Parrot-Lopez, H., Galons, H.: Formation of amphiphilic cyclodextrins via hydrophobic esterification at the secondary hydroxyl face. Tetrahedron Lett. 32, 2769–2770 (1991)
Canceill, J., Jullien, L., Lacombe, L., Lehn, J.M.: Channel-type molecular structures. Part 2. Synthesis of bouquet-shaped molecules based on a β-cyclodextrin core. Helv. Chim. Acta 75, 791–812 (1992)
Bellanger, N., Perly, B.: NMR investigations of the conformation of new cyclodextrin-based amphiphilic transporters for hydrophobic drugs: molecular lollipops. J. Mol. Struct. 273, 215–226 (1992)
Koehler, J.E.H., Saenger, W., Van Gunsteren, W.F.: Conformational differences between α-cyclodextrin in aqueous solution and in crystalline form: a molecular dynamics study. J. Mol. Biol. 203, 241–250 (1988)
Renard, E., Deratani, A., Volet, G., Sebille, B.: Preparation and characterization of water soluble high molecular weight β-cyclodextrin-epichlorohydrin polymers. Eur. Polym. J. 33, 49–57 (1997)
Cadars, S., Foray, M.F., Gadelle, A., Gerbaud, G., Bardet, M.: High-resolution solid-state 13C NMR study of per (3, 6-anhydro)-α-cyclodextrin based polymers and of their chromium complexes. Carbohydr. Polym. 61, 88–94 (2005)
Zhao, D., Zhao, L., Zhu, C.S., Huang, W.Q., Hu, J.L.: Water-insoluble β-cyclodextrin polymer crosslinked by citric acid: synthesis and adsorption properties toward phenol and methylene blue. J. Incl. Phenom. Macrocycl. Chem. 63, 195–201 (2009)
Girek, T., Kozlowski, C.A., Koziol, J.J., Walkowiak, W., Korus, I.: Polymerisation of β-cyclodextrin with succinic anhydride. Synthesis, characterisation, and ion flotation of transition metals. Carbohydr. Polym. 59, 211–215 (2005)
De Brauer, C., Merlin, M.P., Germain, P., Guerandel, T.: Thermal behaviour of anhydrous α-, β-and γ-cyclodextrin at low temperature. J. Incl. Phenom. Macrocycl. Chem. 37(1–4), 75–82 (2000)
Giordano, F., Novak, C., Moyano, J.R.: Thermal analysis of cyclodextrins and their inclusion compounds. Thermochim. Acta 380, 123–151 (2001)
Ritter, H., Tabatabai, M.: Cyclodextrin in polymer synthesis: a green way to polymers. Prog. Polym. Sci. 9, 1713–1720 (2002)
Loftsson, T., Brewster, M.E.: Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 85, 1017–1025 (1996)
Tomasik, P.: Complexes of starch with inorganic guests. Adv. Carbohydr. Chem. Biochem. 53, 263–343 (1996)
Aoyama, Y., Nagai, Y., Otsuki, J.I., Kobayashi, K., Toi, H.: Selective binding of sugar to β-cyclodextrin: a prototype for sugar–sugar interactions in water. Angew. Chem. Int. Ed. Engl. 31, 745–747 (1992)
Gabelica, V., Galic, N., De Pauw, E.: On the specificity of cyclodextrin complexes detected by electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 13, 946–953 (2002)
Loftsson, T., Magnusdottir, A., Masson, M., Sigurjonsdottir, J.F.: Self-association and cyclodextrin solubilization of drugs. J. Pharm. Sci. 91, 2307–2316 (2002)
Mura, P.: Analytical techniques for characterization of cyclodextrin complexes in aqueous solution: a review. J. Pharm. Biomed. Anal. 101, 238–250 (2014)
Lai, H., Zhao, T., Deng, Y., Fan, C., Wu, W., Yang, C.: Assembly-enhanced triplet-triplet annihilation upconversion in the aggregation formed by Schiff-base Pt (II) complex grafting-permethyl-β-CD and 9, 10-diphenylanthracence dimer. Chin. Chem. Lett. 30, 1979–1983 (2019)
Liu, R., Zhang, Y., Wu, W., Liang, W., Huang, Q., Yu, X., Xu, W., Zhou, D., Selvapalam, N., Yang, C.: Temperature-driven braking of γ-cyclodextrin-curcubit [6] uril-cowheeled [4] rotaxanes. Chin. Chem. Lett. 30, 577–581 (2019)
Xu, J., Wang, Q., Xuan, C., Xia, Q., Lin, X., Fu, Y.: Chiral recognition of tryptophan enantiomers based on β-cyclodextrin-platinum nanoparticles/graphene nanohybrids modified electrode. Electroanalysis 28(4), 868–873 (2016)
Prentis, R.A., Lis, Y., Walker, S.R.: Pharmaceutical innovation by the seven UK-owned pharmaceutical companies (1964–1985). Br. J. Clin. Pharmacol. 25, 387–396 (1988)
Dressman, J.B., Amidon, G.L., Reppas, C., Shah, V.P.: Dissolution testing as a prognostic tool for oral drug absorption: immediate release dosage forms. Pharm. Res. 15, 11–22 (1998)
Ahr, G., Voith, B., Kuhlmann, J.: Guidances related to bioavailability and bioequivalence: European industry perspective. Eur. J. Drug Metab. Pharmacokinet. 25, 25–27 (2000)
Davis, M.E., Brewster, M.E.: Cyclodextrin-based pharmaceutics: past, present and future. Nat. Rev. Drug Discov. 3, 1023 (2004)
Dressman, J., Butler, J., Hempenstall, J., Reppas, C.: The BCS: where do we go from here? Pharm. Tech. 25, 68–76 (2001)
Strickley, R.G.: Solubilizing excipients in oral and injectable formulations. Pharm. Res. 21, 201–230 (2004)
Kurkov, S.V., Loftsson, T.: Cyclodextrins. Int. J. Pharm. 453, 167–180 (2013)
Adeoye, O., Cabral-Marques, H.: Cyclodextrin nanosystems in oral drug delivery: a mini review. Int. J. Pharm. 531, 521–531 (2017)
Loftsson, T., Matthiasson, K., Masson, M.: The effects of organic salts on the cyclodextrin solubilization of drugs. Int. J. Pharm. 262, 101–107 (2003)
Lantz, A.W., Rodriguez, M.A., Wetterer, S.M., Armstrong, D.W.: Estimation of association constants between oral malodor components and various native and derivatized cyclodextrins. Anal. Chim. Acta 557, 184–190 (2006)
Szejtli, J., Szente, L.: Elimination of bitter, disgusting tastes of drugs and foods by cyclodextrins. Eur. J. Pharm. Biopharm. 61, 115–125 (2005)
Vyas, A., Saraf, S., Saraf, S.: Cyclodextrin based novel drug delivery systems. J. Incl. Phenom. Macrocycl. Chem. 62, 23–42 (2008)
Soares, A.F., Carvalho, R.D.A., Veiga, F.: Oral administration of peptides and proteins: nanoparticles and cyclodextrins as biocompatible delivery systems. Nanomedicine (2007). https://doi.org/10.2217/17435889.2.2.183
Stella, V.J., He, Q.: Cyclodextrins. Toxicol Pathol. 36, 30–42 (2008)
Shimpi, S., Chauhan, B., Shimpi, P.: Cyclodextrins: application in different routes of drug administration. Acta Pharm. 55, 139–156 (2005)
Liversidge, G.G., Cundy, K.C.: Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 125, 91–97 (1995)
Haeberlin, B., Gengenbacher, T., Meinzer, A., Fricker, G.: Cyclodextrins—useful excipients for oral peptide administration? Int. J. Pharm. 137, 103–110 (1996)
Veiga, F., Teixeira-Dias, J.J.C., Kedzierewicz, F., Sousa, A., Maincent, P.: Inclusion complexation of tolbutamide with β-cyclodextrin and hydroxypropyl-β-cyclodextrin. Int. J. Pharm. 129, 63–71 (1996)
Glasmacher, A., Hahn, C., Molitor, E., Marklein, G., Sauerbruch, T., Schmidt-Wolf, I.G.H.: Itraconazole trough concentrations in antifungal prophylaxis with six different dosing regimens using hydroxypropyl-β-cyclodextrin oral solution or coated-pellet capsules. Mycoses 42, 591–600 (1999)
Ozkan, Y., Atay, T., Dikmen, N., Isimer, A., Aboul-Enein, H.Y.: Improvement of water solubility and in vitro dissolution rate of gliclazide by complexation with β-cyclodextrin. Pharm. Acta Helv. 74, 365–370 (2000)
Patel, S.G., Rajput, S.J.: Enhancement of oral bioavailability of cilostazol by forming its inclusion complexes. AAPS PharmSciTech. 10, 660–669 (2009)
Miro, A., Rondinone, A., Nappi, A., Ungaro, F., Quaglia, F., La Rotonda, M.I.: Modulation of release rate and barrier transport of Diclofenac incorporated in hydrophilic matrices: role of cyclodextrins and implications in oral drug delivery. Eur. J. Pharm. Biopharm. 72, 76–82 (2009)
Agueros, M., Zabaleta, V., Espuelas, S., Campanero, M.A., Irache, J.M.: Increased oral bioavailability of paclitaxel by its encapsulation through complex formation with cyclodextrins in poly (anhydride) nanoparticles. J. Control. Release 145, 2–8 (2010)
Pathak, S.M., Musmade, P., Dengle, S., Karthik, A., Bhat, K., Udupa, N.: Enhanced oral absorption of saquinavir with methyl-beta-cyclodextrin—preparation and in vitro and in vivo evaluation. Eur. J. Pharm. Sci. 41, 440–451 (2010)
Liu, M., Cao, W., Sun, Y., He, Z.: Preparation, characterization and in vivo evaluation of formulation of repaglinide with hydroxypropyl-β-cyclodextrin. Int. J. Pharm. 477, 159–166 (2014)
Lin, C., Chen, F., Ye, T., Zhang, L., Zhang, W., Liu, D., Pan, W.: A novel oral delivery system consisting in “drug-in cyclodextrin-in nanostructured lipid carriers” for poorly water-soluble drug: vinpocetine. Int. J. Pharm. 465, 90–96 (2014)
Chattah, A.K., Pfund, L.Y., Zoppi, A., Longhi, M.R., Garnero, C.: Toward novel antiparasitic formulations: complexes of albendazole desmotropes and β-cyclodextrin. Carbohydr. Polym. 164, 379–385 (2017)
Celebioglu, A., Uyar, T.: Fast dissolving oral drug delivery system based on electrospun nanofibrous webs of cyclodextrin/ibuprofen inclusion complex nanofibers. Mol. Pharm. 16, 4387–4398 (2019)
Celebioglu, A., Uyar, T.: Metronidazole/Hydroxypropyl-β-Cyclodextrin inclusion complex nanofibrous webs as fast-dissolving oral drug delivery system. Int. J. Pharm. 572, 118828 (2019)
Celebioglu, A., Uyar, T.: Development of ferulic acid/cyclodextrin inclusion complex nanofibers for fast-dissolving drug delivery system. Int. J. Pharm. 584, 119395 (2020)
Turker, S., Onur, E., Ozer, Y.: Nasal route and drug delivery systems. Pharm. World Sci. 26, 137–142 (2004)
Marttin, E., Verhoef, J.C., Merkus, F.W.H.M.: Efficacy, safety and mechanism of cyclodextrins as absorption enhancers in nasal delivery of peptide and protein drugs. J. Drug Target. 6, 17–36 (1998)
Merkus, F.W.H.M., Verhoef, J.C., Marttin, E., Romeijn, S.G., Van der Kuy, P.H.M., Hermens, W.A.J.J., Schipper, N.G.M.: Cyclodextrins in nasal drug delivery. Adv. Drug Deliv. Rev. 36, 41–57 (1999)
Adjei, A., Sundberg, D., Miller, J., Chun, A.: Bioavailability of leuprolide acetate following nasal and inhalation delivery to rats and healthy humans. Pharm. Res. 9, 244–249 (1992)
Merkus, F.W., Schipper, N.G., Verhoef, J.C.: The influence of absorption enhancers on intranasal insulin absorption in normal and diabetic subjects. J. Controlled Release 41, 69–75 (1996)
Kondo, T., Nishimura, K., Irie, T., Uekama, K.: Cyclodextrin derivatives that modify nasal absorption of morphine and its entry into cerebrospinal fluid in the rat. Pharm. Pharmacol. Commun. 1, 163–166 (1995)
Irie, T., Uekama, K.: Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 86, 147–162 (1997)
Schwarz, D.H., Engelke, A., Wenz, G.: Solubilizing steroidal drugs by β-cyclodextrin derivatives. Int. J. Pharm. 531, 559–567 (2017)
Le Bourlais, C., Acar, L., Zia, H., Sado, P.A., Needham, T., Leverge, R.: Ophthalmic drug delivery systems-recent advances. Progr. Retinal Eye Res. 17, 33–58 (1998)
Loftsson, T., Stefansson, E.: Effect of cyclodextrins on topical drug delivery to the eye. Drug Dev. Ind. Pharm. 23, 473–481 (1996)
Loftssona, T., Jarvinen, T.: Cyclodextrins in ophthalmic drug delivery. Adv. Drug Deliv. Rev. 36, 59–79 (1999)
Jarvinen, K., Jarvinen, T., Thompson, D.O., Stella, V.J.: The effect of a modified β-cyclodextrin, SBE4-β-CD, on the aqueous stability and ocular absorption of pilocarpine. Curr. Eye Res. 13, 897–905 (1994)
Jarvinen, K., Jarvinen, T., Urtti, A.: Ocular absorption following topical delivery. Adv. Drug Deliv. Rev. 16, 3–19 (1995)
Kristinsson, J.K., Fridriksdottir, H., Thorisdottir, S., Sigurdardottir, A.M., Stefansson, E., Loftsson, T.: Dexamethasone-cyclodextrin-polymer co-complexes in aqueous eye drops. Aqueous humor pharmacokinetics in humans. Investig. Ophthalmol. Vis. Sci. 37, 1199–1203 (1996)
Jarho, P., Urtti, A., Jarvinen, K., Pate, D.W., Jarvinen, T.: Hydroxypropyl-β-cyclodextrin increases aqueous solubility and stability of anandamide. Life Sci. 58, 181–185 (1996)
Utsuki, T., Imamura, K., Hirayama, F., Uekama, K.: Stoichiometry-dependent changes of solubility and photoreactivity of an antiulcer agent, 2′-carboxymethoxy-4, 4′-bis (3-methyl-2-butenyloxy) chalcone, in cyclodextrin inclusion complexes. Eur. J. Pharm. Sci. 1, 81–87 (1993)
Fridriksdottir, H., Loftsson, T., Stefansson, E.: Formulation and testing of methazolamide cyclodextrin eye drop solutions. J. Control. Release 44, 95–99 (1997)
Jarho, P., Jarvinen, K., Urtti, A., Stella, V.J., Jarvinen, T.: Modified β-cyclodextrin (sbe7-β-cyd) with viscous vehicle improves the ocular delivery and tolerability of pilocarpine prodrug in rabbits. J. Pharm. Pharmacol. 48, 263–269 (1996)
Matsuda, H., Arima, H.: Cyclodextrins in transdermal and rectal delivery. Adv. Drug Deliv. Rev. 36, 81–99 (1999)
Sigurdoardottir, A.M., Loftsson, T.: The effect of polyvinyl pyrrolidone on cyclodextrin complexation of hydrocortisone and its diffusion through hairless mouse skin. Int. J. Pharm. 126, 73–78 (1995)
Glomot, F., Benkerrour, L., Duchene, D., Poelman, M.C.: Improvement in availability and stability of a dermocorticoid by inclusion in β-cyclodextrin. Int. J. Pharm. 46, 49–55 (1988)
Tomono, K., Gotoh, H., Okamura, M., Horioka, M., Ueda, H., Nagai, T.: Effect of β-cyclodextrins on sustained release of nitroglycerin from ointment bases. InChem. Abstr. 115, 22–28 (1991)
Tiwari, G., Tiwari, R., Rai, A.K.: Cyclodextrins in delivery systems: applications. J. Pharm Bioall. Sci. 2, 72 (2010)
Hoshino, T., Ishida, K., Irie, T., Uekama, K., Ono, T.: An attempt to reduce the photosensitizing potential of chlorpromazine with the simultaneous use of β-and dimethyl-β-cyclodextrins in guinea pigs. Arch. Dermatol. Res. 281, 60–65 (1989)
Uekama, K., Arimori, K., Sakai, A., Masaki, K., Irie, T., Otagiri, M.: Improvement in percutaneous absorption of prednisolone by β-and γ-cyclodextrin complexations. Chem. Pharm. Bull. 35, 2910–2913 (1987)
Uekama, K., Masaki, K., Arimori, K., Irie, T., Hirayama, F.: Effects of beta-and dimethyl beta-cyclodextrins on release and percutaneous absorption behaviors of prednisolone from some ointment bases. Yakugaku Zasshi 107, 449 (1987)
Orienti, I., Zecchi, V., Bertasi, V., Fini, A.: Release of ketoprofen from dermal bases in presence of cyclodextrins: effect of the affinity constant determined in semisolid vehicles. Arch. Pharm. 324, 943–947 (1991)
Vollmer, U., Muller, B.W., Peeters, J., Mesens, J., Wilffert, B., Peters, T.: A study of the percutaneous absorption-enhancing effects of cyclodextrin derivatives in rats. J. Pharm. Pharmacol. 46, 19–22 (1994)
Bentley, M.V.L., Vianna, R.F., Wilson, S., Collett, J.H.: Characterization of the influence of some cyclodextrins on the stratum corneum from the hairless mouse. J. Pharm. Pharmacol. 49, 397–402 (1997)
Legendre, J.Y., Rault, I., Petit, A., Luijten, W., Demuynck, I., Horvath, S., Cuine, A.: Effects of β-cyclodextrins on skin: implications for the transdermal delivery of piribedil and a novel cognition enhancing-drug, S-9977. Eur. J. Pharm. Sci. 3, 311–322 (1995)
Kawahara, K., Ueda, H., Tomono, K., Nagai, T.S.T.P.: Effect of diethyl β-cyclodextrin on the release and absorption behaviour of indomethacin from ointment bases. STP Pharma Sci. 2, 506–513 (1992)
McCormack, B., Gregoriadis, G.: Drugs-in-cyclodextrins-in liposomes: a novel concept in drug delivery. Int. J. Pharm. 112, 249–258 (1994)
Maestrelli, F., Gonzalez-Rodriguez, M.L., Rabasco, A.M., Mura, P.: Effect of preparation technique on the properties of liposomes encapsulating ketoprofen–cyclodextrin complexes aimed for transdermal delivery. Int. J. Pharm. 312, 53–60 (2006)
Adachi, H., Irie, T., Harayama, F., Uekame, K.: Stabilization of prostaglandin E1 in fatty alcohol propylene glycol ointment by acidic cyclodextrin derivative, O-carboxymethyl-O-ethyl-β-cyclodextrin. Chem. Pharm. Bull. 40, 1586–1591 (1992)
Tenjarla, S., Puranajoti, P., Kasina, R., Mandal, T.: Preparation characterization evaluation of miconazole-cyclodextrin complexes for improved oral topical delivery. J. Pharm. Sci. 87, 425–429 (1998)
Lopez, R.F., Collett, J.H., Bentley, M.V.L.: Influence of cyclodextrin complexation on the in vitro permeation and skin metabolism of dexamethasone. Int. J. Pharm. 200, 127–132 (2000)
Lin, S.Z., Wouessidjewe, D., Poelman, M.C., Duchene, D.: In vivo evaluation of indomethacin/cyclodextrin complexes gastrointestinal tolerance and dermal anti-inflammatory activity. Int. J. Pharm. 106, 63–67 (1994)
Celebi, N., Kislal, O., Tarimci, N.: The effect of β-cyclodextrin and penetration additives on the release of naproxen from ointment bases. Pharmazie. 48, 914–917 (1993)
Abdel Rahman, A.A., Khidr, S.H., Ahmed, S.M., Aboutaleb, A.E.: Evaluation of chloramphenicol-β-cyclodextrin inclusion complex. Eur. J. Pharm. Biopharm. 37, 34–37 (1991)
Loftsson, T., Frioriksdottir, H., Ingvarsdottir, G., Jonsdottir, B., Siguroardottir, A.M.: The influence of 2-hydroxypropyl-β-cyclodextrin on diffusion rates and transdermal delivery of hydrocortisone. Drug Dev. Ind. Pharm. 20, 1699–1708 (1994)
Udupa, N., Bhat, L.: Evaluation of FEW ciprofloxacin (CIP) and norfloxacin (NOR) formulations. Drug Dev. Ind. Pharm. 18, 2197–2205 (1992)
Szeman, J., Ueda, H., Szejtli, J., Fenyvesi, E., Watanabe, Y., Machida, Y., Nagai, T.: Enhanced percutaneous absorption of homogenized tolnaftate/beta-cyclodextrin polymer ground mixture. Drug Des. Deliv. 1, 325–332 (1987)
Amidouche, D., Montassier, P., Poelman, M.C., Duchene, D.: Evaluation by laser doppler velocimetry of the attenuation of tretinoin induced skin irritation by β-cyclodextrin complexation. Int. J. Pharm. 111, 111–116 (1994)
Loftsson, T., Sigurdardottir, A.M.: The effect of polyvinylpyrrolidone and hydroxypropyl methylcellulose on HPβCD complexation of hydrocortisone and its permeability through hairless mouse skin. Eur. J. Pharm. Sci. 2, 297–301 (1994)
Iervolino, M., Cappello, B., Raghavan, S.L., Hadgraft, J.: Penetration enhancement of ibuprofen from supersaturated solutions through human skin. Int. J. Pharm. 212, 131–141 (2001)
Lee, B.J., Cui, J.H., Parrott, K.A., Ayres, J.W., Sack, R.L.: Percutaneous absorption and model membrane variations of melatonin in aqueous-based propylene glycol and 2-hydroxypropyl-β-cyclodextrin vehicles. Arch. Pharmacal Res. 21, 503–507 (1998)
Doliwa, A., Santoyo, S., Ygartua, P.: Transdermal iontophoresis and skin retention of piroxicam from gels containing piroxicam: hydroxypropyl-β-cyclodextrin complexes. Drug Dev. Ind. Pharm. 27, 751–758 (2001)
Thorsteinn Loftsson, B.J.O., Bodora, N.: The effects of cyclodextrins on transdermal delivery of drugs. Eur. J. Pharm. Biopharm. 37, 1 (1991)
Moraes, C.M., Abrami, P., De Araujo, D.R., Braga, A.F., Issa, M.G., Ferraz, H.G., Fraceto, L.F.: Characterization of lidocaine: hydroxypropyl-β-cyclodextrin inclusion complex. J. Incl. Phenom. Macrocycl. Chem. 57, 313–316 (2007)
Okamoto, H., Komatsu, H., Hashida, M., Sezaki, H.: Effects of β-cyclodextrin and di-O-methyl-β-cyclodextrin on the percutaneous absorption of butylparaben, indomethacin and sulfanilic acid. Int. J. Pharm. 30, 35–45 (1986)
Chang, S.L., Banga, A.K.: Transdermal iontophoretic delivery of hydrocortisone from cyclodextrin solutions. J. Pharm. Pharmacol. 50, 635–640 (1998)
Nonaka, N., Farr, S.A., Kageyama, H., Shioda, S., Banks, W.A.: Delivery of galanin-like peptide to the brain: targeting with intranasal delivery and cyclodextrins. J. Pharmacol. Exp. Ther. 325, 513–519 (2008)
Uekama, K., Otagiri, M., Sakai, A., Irie, T., Matsuo, N., Matsuoka, Y.: Improvement in the percutaneous absorption of beclomethasone dipropionate by γ-cyclodextrin complexation. J. Pharm. Pharmacol. 37, 532–535 (1985)
Lengyel, M.T., Szejtli, J.: Menadione-γ-cyclodextrin inclusion complex. J. Incl. Phenom. 3, 1–8 (1985)
Argenziano, M., Haimhoffer, A., Bastiancich, C., Jicsinszky, L., Caldera, F., Trotta, F., Castagnoli, C.: In vitro enhanced skin permeation and retention of imiquimod loaded in β-cyclodextrin nanosponge hydrogel. Pharmaceutics. 11, 138 (2019)
Kim, J.K., Kim, M.S., Park, J.S., Kim, C.K.: Thermo-reversible flurbiprofen liquid suppository with HP-β-CD as a solubility enhancer: improvement of rectal bioavailability. J. Incl. Phenom. Macrocycl. Chem. 64, 265–272 (2009)
Kondo, T., Irie, T., Uekama, K.: Combination effects of α-cyclodextrin and xanthan gum on rectal absorption and metabolism of morphine from hollow-type suppositories in rabbits. Biol. Pharm. Bull. 19, 280–286 (1996)
Masahiko, K., Fumitoshi, H., Kaneto, U.: Improvement of oral and rectal bioavailabilities of carmofur by methylated β-cyclodextrin complexations. Int. J. Pharm. 38, 191–198 (1987)
Uekama, K., Imai, T., Maeda, T., Irie, T., Hirayama, F., Otagiri, M.: Improvement of dissolution and suppository release characteristics of flurbiprofen by inclusion complexation with heptakis (2, 6-di-O-methyl)-β-cyclodextrin. J. Pharm. Sci. 74, 841–845 (1985)
Arima, H., Kondo, T., Irie, T., Hirayama, F., Uekama, K., Miyaji, T., Inoue, Y.: Use of water-soluble beta-cyclodextrin derivatives as carriers of anti-inflammatory drug biphenylylacetic acid in rectal delivery. Yakugaku Zasshi 112, 65–72 (1992)
Arima, H., Kondo, T., Irie, T., Uekama, K.: Enhanced rectal absorption and reduced local irritation of the anti-inflammatory drug ethyl 4-biphenylylacetate in rats by complexation with water-soluble β-cyclodextrin derivatives and formulation as oleaginous suppository. J. Pharm. Sci. 81, 1119–1125 (1992)
Arima, H., Irie, T., Uekama, K.: Differences in the enhancing effects of water soluble β-cyclodextrins on the release of ethyl 4-biphenylyl acetate, an anti-inflammatory agent from an oleaginous suppository base. Int. J. Pharm. 57(2), 107–115 (1989)
Uekama, K., Kondo, T., Nakamura, K., Irie, T., Arakawa, K., Shibuya, M., Tanaka, J.: Modification of rectal absorption of morphine hollow-type suppositories with a combination of α-cyclodextrin and viscosity-enhancing polysaccharide. J. Pharm. Sci. 84, 15–20 (1995)
Pitha, J., Harman, S.M., Michel, M.E.: Hydrophilic cyclodextrin derivatives enable effective oral administration of steroidal hormones. J. Pharm. Sci. 75, 165–167 (1986)
Hoon, T.J., Dawood, M.Y., Khan-Dawood, F.S., Ramos, J., Batenhorst, R.L.: Bioequivalence of a 17β-estradiol hydroxypropyl-β-cyclodextrin complex in postmenopausal women. J. Clin. Pharmacol. 33, 1116–1121 (1993)
Brown, G.A., Martini, E.R., Roberts, B.S., Vukovich, M.D., King, D.S.: Acute hormonal response to sublingual androstenediol intake in young men. J. Appl. Physiol. 92, 142–146 (2002)
Yoo, S.D., Yoon, B.M., Lee, H.S., Lee, K.C.: Increased bioavailability of clomipramine after sublingual administration in rats. J. Pharm. Sci. 88, 1119–1121 (1999)
Badawy, S.I.F., Ghorab, M.M., Adeyeye, C.M.: Bioavailability of danazol-hydroxypropyl-β-cylodextrin complex by different routes of administration. Int. J. Pharm. 145, 137–143 (1996)
Bilensoy, E., Hincal, A.A.: Recent advances and future directions in amphiphilic cyclodextrin nanoparticles. Exp. Opin. Drug Deliv. 6, 1161–1173 (2009)
Mori, T., Tsuchiya, R., Doi, M., Nagatani, N., Tanaka, T.: Solubilization of ultraviolet absorbers by cyclodextrin and their potential application in cosmetics. J. Incl. Phenom. Macrocycl. Chem. 93, 91–96 (2019)
Kfoury, M., Hadaruga, N. G., Hadaruga, D. I., Fourmentin, S.: Cyclodextrins as encapsulation material for flavors and aroma. In: Nanotechnology in the Agri-Food Industry, Academic Press (2016)
Ryzhakov, A., Do Thi, T., Stappaerts, J., Bertoletti, L., Kimpe, K., Couto, A.R.S., Kurkov, S.: Self-assembly of cyclodextrins and their complexes in aqueous solutions. J. Pharm. Sci. 105, 2556–2569 (2016)
Cova, T. F. G. G., Cruz, S. M., Valente, A. J., Abreu, P. E., Marques, J. M., & Pais, A. A.: Aggregation of cyclodextrins: fundamental issues and applications. Springer Nature Switzerland AG Basel. In Cyclodextrin Fundamentals, Reactivity and Analysis, pp. 45-65 (2018)
Jansook, P., Ogawa, N., Loftsson, T.: Cyclodextrins: structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 535, 272–284 (2018)
Conceiçao, J., Farto-Vaamonde, X., Goyanes, A., Adeoye, O., Concheiro, A., Cabral-Marques, H., Alvarez-Lorenzo, C.: Hydroxypropyl-β-cyclodextrin-based fast dissolving carbamazepine printlets prepared by semisolid extrusion 3D printing. Carbohydr. Polym. 221, 55–62 (2019)
Gerloczy, A., Fonagy, A., Keresztes, P., Perlaky, L., Szejtli, J.: Absorption, distribution, excretion and metabolism of orally administered 14C-beta-cyclodextrin in rat. Arzneimittelforschung 35, 1042–1047 (1985)
Duchene, D., Bachot, A., Loftsson, T.: Les cyclodextrines et leurs utilisations en pharmacie et cosmetologie. STP Pharma Pratiques. 19, 15–27 (2009)
Frank, D.W., Gray, J.E., Weaver, R.N.: Cyclodextrin nephrosis in the rat. Am. J. Pathol. 83, 367 (1976)
Perrin, J.H., Field, F.P., Hansen, D.A., Mufson, R.A., Torosian, G.: Beta-Cyclodextrin as an aid to peritoneal dialysis. Renal toxicity of beta-cyclodextrin in the rat. Res. Commun. Chem. Pathol. Pharmacol. 19, 373–376 (1978)
Gould, S., Scott, R.C.: 2-Hydroxypropyl-β-cyclodextrin (HP-β-CD): a toxicology review. Food Chem. Toxicol. 43, 1451–1459 (2005)
Van De Manakker, F., Vermonden, T., Van Nostrum, C.F., Hennink, W.E.: Cyclodextrin-based polymeric materials: synthesis, properties, and pharmaceutical/biomedical applications. Biomacromol 10, 3157–3175 (2009)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dhiman, P., Bhatia, M. Pharmaceutical applications of cyclodextrins and their derivatives. J Incl Phenom Macrocycl Chem 98, 171–186 (2020). https://doi.org/10.1007/s10847-020-01029-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-020-01029-3